Abstract
Bean common mosaic virus (BCMV) is one of the most widespread and damaging viruses of cultivated legumes in the world. In addition to serious yield reduction and germplasm decline, BCMV infection also makes legumes more vulnerable to other pathogens. Early diagnosis of the virus is particularly important in limiting its spread. Recombinase polymerase amplification (RPA) is a novel isothermic amplification technology. The whole reaction can be done outside the laboratory environment after the nucleic acid sample is obtained. In this study, we established a rapid and sensitive RPA combined with the lateral flow dipstick (LFD) assay for detection of BCMV, based on the conserved BCMV coat protein (CP) gene sequence. Specific primers and a probe were designed, which amplify ~ 150 bp CP fragments from BCMV-infected samples under a constant temperature of 37 °C for 20 min. The end-labeled amplification products were detected by high-affinity LFD within 5 min. Sensitivity of this RPA-LFD assay was 1000 times greater than that of the conventional polymerase chain reaction (PCR) assay. Furthermore, when the primers/probe were used against related potyviruses including soybean mosaic virus (SMV), bean yellow mosaic virus (BYMV), and turnip mosaic virus (TuMV), the three potyviruses were not detected, indicating that the assay was BCMV species-specific. The RPA-LFD assay was also successfully applied for the detection of seed-borne BCMV in beans. The RPA-LFD assay has great potential application in the rapid diagnosis of BCMV in the field.
Similar content being viewed by others
Background
Bean common mosaic virus (BCMV, genus Potyvirus) is a pathogen of common beans (Phaseolus vulgaris), as well as other cultivated and wild legumes (Morales 2006). Yield loss can reach 100% in severely BCMV-infected bean fields (Saqib et al. 2010; Li et al. 2014), and the virus is internationally distributed. Isolates of BCMV have been identified and characterized from different countries, including USA, India, China, Iran, South Korea, Turkey, and Australia, based on analysis of complete or partial genome sequences (Flores-Estévez et al. 2000; Saqib et al. 2005; Damayanti et al. 2008; Cui et al. 2014; Seo et al. 2015; Jang et al. 2018). BCMV is divided into strains based on host symptoms, serological relationships, high performance liquid chromatography peptide mapping, and genome sequence (Drijfhout et al. 1978; McKern et al. 1992; Mink and Silbernagel 1992; Feng et al. 2014a, 2014b; Li et al. 2016). An RT-PCR-based method combined with restriction enzyme analysis was first used to distinguish between BCMV and its closest relative, bean common mosaic necrosis virus (BCMNV) (Saiz et al. 1994). Flores-Estévez et al. (2003) used RT-PCR with primers based on the viral coat protein (CP) gene sequence to differentiate BCMV from BCMNV. A disadvantage of conventional RT-PCR-based assays for BCMV is their unsuitability for rapid detection in the field.
Recombinase polymerase amplification (RPA) (Piepenburg et al. 2006) has been explored for molecular detection of bacteria, fungi, and viruses. The RPA process employs three enzymes: a recombinase, a single stranded DNA-binding protein (SSB) and a strand-displacing polymerase (Piepenburg et al. 2006) to implement the amplification of a specific genome fragment. Unlike PCR-based methods, which require stringent temperature cycling, RPA reactions can be operated at a constant temperature of 37 °C, thereby avoiding the requirement of a thermal cycler. The reactions can be completed in 5–20 min depending on the starting copy number of the targeted template and the size of the amplicon. The RPA assay has been reported in diagnosis of several plant viruses, including those with DNA genomes such as wheat dwarf virus (Glais and Jacquot 2015), banana bunchy top virus (Kapoor et al. 2017), citrus yellow mosaic virus (Kumar et al. 2018), bean golden yellow mosaic virus, tomato mottle virus, tomato yellow leaf curl virus (Londoño et al. 2016; Wang and Yang 2019), milk vetch dwarf virus (Cao et al. 2020), and piper yellow mottle virus (Mohandas and Bhat 2020); and with RNA-based genomes such as little cherry virus-2 (Mekuria et al. 2014), plum pox virus (Zhang et al. 2014; Babujee et al. 2019), rose rosette virus (Babu et al. 2017), rice black-streaked dwarf virus (Zhao et al. 2019), cucumber mosaic virus (Srivastava et al. 2019), maize chlorotic mottle virus (Jiao et al. 2019a), apple stem pitting virus (Kim et al. 2019), cucumber green mottle mosaic virus (Jiao et al. 2019b; Zeng et al. 2019), cucurbit yellow stunting disorder virus (Kalischuk et al. 2020), and peach latent mosaic viroid (Lee et al. 2020). RPA has also been successfully applied for detection of the potyviruses plum pox virus (Zhang et al. 2014), yam mosaic virus and yam mild mosaic virus (Silva et al. 2015), potato virus Y (Glais and Jacquot 2015; Babujee et al. 2019), and chilli veinal mottle virus (Jiao et al. 2020). The type of tissue used as viral sources in RPA assays include leaves, budwood, grape mealybugs, stems, petals, pollen/anthers, whole flowers and roots, but seeds haven’t been reported for the assay. In this study, we designed specific primers based on CP gene sequences of BCMV isolates to develop an RPA assay. RPA products can be analyzed by agarose gel electrophoresis or by using a lateral flow dipstick (LFD) assay that avoids the time and equipment required for electrophoresis (MileniaBiotec, Giessen, Germany). End-labeled RPA amplification products can be detected by LFD in 5 min. The main advantage of the new RPA-LFD assay described here is that the whole reaction process can be carried out in a non-laboratory environment after the nucleic acid sample is obtained.
The objectives of this study were to (i) develop primers and probe for specific detection of BCMV by RPA-LFD; (ii) evaluate the specificity of the assay against related potyviruses; (iii) compare the sensitivity of the new developed RPA-LFD assay with conventional PCR; (iv) evaluate the assay with different BCMV-infected legume species; (v) evaluate the assay in detecting seed-borne BCMV.
Results
Establishment of RPA-LFD assay for the detection of BCMV
To evaluate the simplicity and rapidity of RPA-LFD assay for the detection of BCMV in legume plants, BCMV-infected soybean samples exhibiting severe mosaic symptoms were collected from different experimental farms in Jiangsu Province, China. The cDNA synthesized from the total RNA of diseased soybean samples was used as template for PCR and RPA-LFD assays, with cDNA samples from the uninfected (healthy) soybean plants as negative controls. PCR results demonstrated that a 150-bp product corresponding to the partial BCMV CP gene was amplified from diseased soybean plants, but not from healthy soybean plants (Fig. 1a). Similarly, the RPA-LFD assay successfully detected BCMV in the diseased plants using specific primers and a probe against the partial BCMV CP gene, and did not detect BCMV in uninfected plants (Fig. 1b), indicating that RPA-LFD can be used for the detection of BCMV in soybean plants.
Development of an RPA-LFD assay for the detection of BCMV from soybean plant tissues. a Detection of BCMV from BCMV-infected soybean plants by RT-PCR assay using BCMV-CP primers, with non-infected (healthy) plants as negative control. b Detection of BCMV from BCMV-infected soybean plants by RPA-LFD using BCMV-specific primers and probe, with non-infected healthy plants as negative control
RPA-LFD assay is more sensitive than conventional PCR in the detection of BCMV
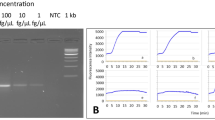
To evaluate the sensitivity of the RPA-LFD assay, the detection limit of the RPA-LFD assay was evaluated by testing 10-fold serial dilutions (starting from 4 ng/μL) of cDNA samples derived from BCMV-infected soybean, and by comparing results with those of conventional PCR. RPA-LFD successfully detected BCMV even when cDNA samples was diluted 105-fold (Fig. 2a). In contrast, the PCR assay detected BCMV in cDNA samples diluted up to 102-fold (Fig. 2b). Thus, the RPA-LFD assay was 1000 times more sensitive than the PCR assay.
Specificity of the RPA-LFD assay
Symptoms of BCMV infection can resemble those of other potyviruses that occur in legume crops. Therefore, isolates of soybean mosaic virus (SMV), bean yellow mosaic virus (BYMV) and turnip mosaic virus (TuMV), all belonging to the genus Potyvirus, were selected to test specificity of the RPA-LFD assay. Isolates of SMV, TuMV and BYMV all consistently tested negative in both the RPA-LFD-based and PCR-based assays for BCMV (Fig. 3a, b).
Specificity of RPA-LFD assay for BCMV detection. a BCMV, SMV, TuMV and BYMV samples were detected by RPA-LFD assay using BCMV-specific primers and probe. b BCMV, SMV, TuMV and BYMV samples were detected by PCR using BCMV-CP primers. In RPA-LFD and PCR assays, the cDNAs from virus-infected soybean were used as templates, with cDNA from healthy plant as negative control
Evaluation of the RPA-LFD assay using soybean and adzuki bean plants
To verify the effectiveness of the RPA-LFD method for detecting BCMV in legume plants, leaves of nine soybean (Glycine max) plants were manually-inoculated with sap from a BCMV-infected soybean plant. Untreated soybean plants served as negative controls. Two weeks later, all plants were sampled for RT, followed by PCR and RPA-LFD detection of BCMV. As expected, both RPA-LFD and PCR assay results showed that all diseased plants were positive for BCMV while all control plants were negative (Fig. 4a, b).
Application of the RPA-LFD assay in BCMV-infected legumes. a Twelve suspected BCMV-infected soybean samples were detected by RPA-LFD assay. b The same soybean samples tested in a were detected by PCR method. c Twenty-one adzuki bean samples were tested by RPA-LFD. d The same adzuki bean samples used in c were tested by PCR
To further test the effectiveness of RPA-LFD in detecting BCMV in legume plants in the field, we collected 21 adzuki bean (Phaseolus angularis) samples from several regions of Jiangsu Province, China, including 16 suspected virus-infected and five symptomless ones. These were tested by PCR and RPA-LFD for BCMV. There were 16 positive plants among the 21 tested samples, as detected by both RPA-LFD and PCR assays (Fig. 4c, d). Detailed results of the detection analyses are provided in Table 1.
Detection of seed-borne BCMV using RPA-LFD
BCMV is seed-borne in many legumes. We tested the effectiveness of the RPA-LFD assay for detection of seed-borne BCMV in adzuki beans. Total RNA was extracted from ten seeds derived from infected parental plants, and this was used as a template for RT, followed by RPA-LFD and PCR assays. Both assays detected BCMV in seeds (Fig. 5a, b). However, bands from the PCR assay were unclear when observed on an imager, because adzuki bean seeds contain large quantities of polysaccharides, proteins, secondary metabolites such as phenols and a fat content of up to 40%. Polysaccharides and these other metabolites can inhibit the activity of many enzymes and therefore may interfere with PCR-based methods for BCMV detection. By contrast, using the same samples, we found the sensitivity of RPA-LFD to detect BCMV was 1000 times higher than that of the PCR method. The results of detection are read with a test strip, which is clear and intuitive. Therefore, when detecting BCMV in adzuki bean seeds, the RPA-LFD method was more sensitive and effective than the PCR.
Detection of crude plant extract using RPA-LFD
To further test the effectiveness of the RPA-LFD in detecting BCMV from the crude extract of plant samples, eight BCMV-infected soybean samples and four healthy samples were used. In all samples, 0.1 g of fresh leaves were ground with 1 mL of buffer solution, then were centrifuged and the supernatant was used immediately to perform the RPA-LFD experiment. RT-PCR assay was performed on the corresponding samples using purified RNA extracts. The results showed that the RPA-LFD method can detect BCMV directly from crude soybean extracts and that the test results were consistent with results of RT-PCR (Fig. 6a, b). The whole RPA-LFD process can be completed within 30 min. The RPA-LFD is also sensitive to detect BCMV from crude extract of soybean leaves. It is suitable for non-professional experimenters to rapidly and simply detect BCMV infected-plants in the field.
Discussion
We developed an RPA-LFD assay to detect BCMV from legume plants and compared outcomes with a conventional PCR assay. The RPA-LFD assay was 1000 times more sensitive than the PCR, and had no cross-reactivity with three other potyviruses. Reaction time of the RPA-LFD assay was less than 20 min, compared with 150 min for the PCR assay using samples of the same target concentration. Detection of BCMV in adzuki bean leaves and seeds proved applicability of the RPA-LFD assay beyond soybean. RPA-LFD was able to detect BCMV from crude leaf extracts, without tedious extraction and purification of total RNA. This method is suitable for rapid on-site diagnosis.
Compared with other plant virus assays, the RPA-LFD method has the following advantages:
-
(1)
Primer design is simple. Compared with the loop-mediated isothermal amplification method (LAMP), a constant temperature amplification technology which requires a complex primer design, RPA-LFD detection requires only a pair of primers and one probe to complete amplification;
-
(2)
It does not require thermal cycling instruments. The RPA-LFD isothermal amplification reaction can be achieved under isothermal conditions from 37 °C–42 °C, requiring only a water heater and thermostat;
-
(3)
The reaction time is only 15–30 min;
-
(4)
RPA combined with a lateral flow dipstick (RPA-LFD) avoids a gel electrophoresis step;
-
(5)
It has high sensitivity and can directly detect BCMV in seeds. Although RT-PCR could detect BCMV in seeds, sensitivity was low. Polysaccharides, proteins and secondary metabolites in legume seeds may interfere with RNA extraction and inhibit subsequent PCR-based assays. As a result, no clear BCMV-CP gene bands were observed using gel imaging system, which may lead to false negatives. In contrast, RPA-LFD is more sensitive than PCR, and the results of detection are more clearly visualized without misjudgment;
-
(6)
It was able to detect BCMV in soybean and adzuki bean.
Although RPA has clear advantages over PCR in diagnosis of plant viruses, a limitation is the cost. Cost of reagents per sample is much higher than the cost of a PCR. The authors believe that as RPA technology becomes more widely adopted, the cost per unit will eventually be closer to that of PCR reagents.
Conclusions
The RPA-LFD method for detecting BCMV established in this study is simple, specific and highly sensitive. It can detect BCMV in dry seeds and in different legume crops including soybean and adzuki bean. We believe that with the continuous development of RPA technology, it will play a wider role in the rapid diagnosis of BCMV and other viruses of concern in agriculture and elsewhere.
Methods
Sources of plant samples
All BCMV-infected legume plants including soybean (Glycine max) and adzuki bean (Phaseolus angularis) showing severe mosaic symptom were collected from different regions of Jiangsu Province, China. The positive control for the test is the confirmed BCMV source from the laboratory and the negative control is set as healthy soybean leaves. All samples were stored at − 80 °C after PCR detection and sequence determination. The virus samples of SMV and BYMV were stored in our laboratory and TuMV full length cDNA infectious clone vector was kindly provided by Prof Aiming Wang (Agriculture and Agri-Food, Canada).
RNA extraction, cDNA synthesis
Total RNA of soybean and adzuki bean leaves and adzuki bean seeds was extracted using a Plant Total RNA extraction kit from Tiangen Biotechnology Co., Ltd.. The purity and yield of RNA extracts were measured by NanoDrop 2000C microvolume UV-Vis spectrophotometer. First-strand cDNA was synthesized from 1 μg of total RNA in a 20 μL volume using the PrimeScript™ RT Master Mix Kit (TaKaRa, Dalian, China), according to the manufacturer’s instructions. The cDNA was stored at − 20 °C.
Crude sample extraction
Approximately 100 mg of fresh soybean leaf tissue was homogenized in a centrifuge tube with 1 mL 1 × PBS buffer (pH 7.4) containing 2% Tween-20. The supernatant of crude extracts were performed reverse transcription immediately after centrifugation at 4 °C.
Detection of BCMV using RPA-LFD
RPA primers were designed to target the partial BCMV CP gene (An alignment of CPs from different strains of NCBI reference sequence) following the manufacturer’s specific parameters (TwistDx, Cambridge, UK). RPA was carried out using the materials and protocol (TwistDx, Cambridge, UK) from the TwistAmp® nfo kit. The RPA reaction was performed in a 50-μL reaction volume containing 1 μL of BCMV-infected soybean plant cDNA, 29.5 μL rehydration buffer, 2.1 μL of 10 mM forward primer (RPA-BCMV-F:5′-TTAGATCATCTGTTGGATTACAAGCCAGAACA-3′), 2.1 μL of 10 mM reverse primer (RPA-BCMV-R:5′-CACCACACCATAAAGCCATTCATCACAATTGA -3′), 0.6 μL of 10 mM probe (BCMV-nfo-probe: [5′FAM] ACAAAGATGCAGTTTGAAATGTGGTACAAT [THF] CTGTGAAGGAAGAGTA [3′BLOCK]) and 2.5 μL of 280 mM magnesium acetate. All RPA samples were incubated at 37 °C for 20 min. For lateral flow analysis, with reference to the kit operation (Milenia Genline HybriDetect test strip, Germany), 5 μL of RPA-amplified products were mixed with 100 μL of assay buffer (HybriDetect assay Buffer) in a new reaction tube. Then the LFD strip was immersed into the mixture and incubated for 5 min at room temperature. A band with one visible line in the control area was considered as negative, and a band with two visible lines in both the control area and test area was considered as positive.
Detection of BCMV using PCR
The PCR reactions were performed using 10 μL 2 × Tap Master Mix (Dye Plus) (Vazyme Biothe, Nanjing, China), 0.2 μM each of forward primer (BCMV-CP-F:5′-TTAGATCATCTGTTGGATTGG-3′) and reverse primer (BCMV-CP-R:5′-CACCACACCATAAAGCCATTC-3′) and 1 μL cDNA in a 20 μL reaction volume. All samples were denatured at 95 °C for 5 min. Then 30 cycles were performed (95 °C for 30 s, 58 °C for 30 s and 72 °C for 30 s), and the last step was 72 °C for 10 min. The obtained PCR product was separated on a 3% agarose gel. After staining with ethidium bromide, images were observed using a Bio-Rad Molecular Imager gel DOCXR system (Bio-Rad, Hercules, CA, USA).
Specificity and sensitivity assay
The specificity of the designed primers/ probe was tested using RPA-LFD, against other potyviruses infecting legume or no host-pathogen. A positive control is the cDNA from the BCMV-infected soybean samples and the negative control is the cDNA from healthy soybean plant. For the sensitivity assay, a series of 10-fold dilutions (to 105) of cDNA from 4 ng were prepared in diethyl pyrocarbonate (DEPC)-treated water for RPA-LFD and PCR analysis using the RPA primers.
Availability of data and materials
Not applicable.
Change history
09 February 2021
There is an extra envelope superscript next to the sixth author's name. The article has been updated to rectify the errors.
Abbreviations
- BCMNV:
-
Bean common mosaic necrotic virus
- BCMV:
-
Bean common mosaic virus
- BYMV:
-
Bean yellow mosaic virus
- cDNA:
-
Complementary DNA
- CP:
-
Coat protein
- LAMP:
-
Loop-mediated isothermal amplification method
- LFD:
-
Lateral flow dipsticks
- PBS:
-
Phosphate buffer saline
- PCR:
-
Polymerase chain reaction
- RPA:
-
Recombinase polymerase amplification
- RPA-LFD:
-
Recombinase polymerase amplification combined with the lateral flow dipstick
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- SMV:
-
Soybean mosaic virus
- TuMV:
-
Turnip mosaic virus
References
Babu B, Washburn BK, Miller SH, Poduch K, Sarigul T, Knox GW, et al. A rapid assay for detection of rose rosette virus using reverse transcription-recombinase polymerase amplification using multiple gene targets. J Virol Methods. 2017;240:78–84.
Babujee L, Witherell RA, Mikami K, Aiuchi D, Charkowski AO, Rakotondrafara AM, et al. Optimization of an isothermal recombinase polymerase amplification method for real-time detection of potato virus Y O and N types in potato. J Virol Methods. 2019;267:16–21.
Cao Y, Yan D, Wu X, Chen Z, Lai Y, Lv L, et al. Rapid and visual detection of milk vetch dwarf virus using recombinase polymerase amplification combined with lateral flow strips. Virol J. 2020;17:102.
Cui XY, Shen L, Yuan XX, Gu HP, Chen X. First report of bean common mosaic virus infecting mungbean (Vigna radiata (Linn.) Wilczek.) in China. Plant Dis. 2014;98:1590.
Damayanti TA, Susilo D, Nurlaelah S, Sartiami D, Okuno T, Mise K. First report of bean common mosaic virus in yam bean [Pachyrhizus erosus (L.) urban] in Indonesia. J Gen Plant Pathol. 2008;74:438–42.
Drijfhout E, Slibernagel MJ, Burke DW. Differentiation of strains of bean common mosaic virus. Neth J Plant Pathol. 1978;84:13–26. https://doi.org/10.1007/BF01978099.
Feng X, Poplawsky AR, Karasev AV. A recombinant of bean common mosaic virus induces temperature-insensitive necrosis in an I gene-bearing line of common bean. Phytopathology. 2014b;104:1251–7.
Feng X, Poplawsky AR, Nikolaeva OV, Myers JR, Karasev AV. Recombinants of bean common mosaic virus (BCMV) and genetic determinants of BCMV involved in overcoming resistance in common bean. Phytopathology. 2014a;104:786–93.
Flores-Estévez N, Acosta-Gallegos JA, Silva-Rosales L. Bean common mosaic virus and bean common mosaic necrosis virus in Mexico. Plant Dis. 2003;87:21–5.
Flores-Estévez N, Silva-Rosales L, Acosta-Gallegos JA. First report of bean common mosaic necrotic virus infecting bean plants in Aguascalientes and Veracruz. Mexico Plant Dis. 2000;84:923.
Glais L, Jacquot E. Detection and characterization of viral species/subspecies using isothermal recombinase polymerase amplification (RPA) assays. In: Lacomme C, editor. Plant pathology. Methods in molecular biology, vol. 1302. New York: Humana Press; 2015. p. 207–25. https://doi.org/10.1007/978-1-4939-2620-6_16.
Jang YW, Jo Y, Cho WK, Choi H, Yoon YN, Lim SM, et al. First report of bean common mosaic necrosis virus infecting soybean in Korea. Plant Dis. 2018;102:2051.
Jiao Y, Jiang J, An M, Xia Z, Wu Y. Recombinase polymerase amplification assay for rapid detection of maize chlorotic mottle virus in maize. Arch Virol. 2019a;164:2581–4.
Jiao Y, Jiang J, Wu Y, Xia Z. Rapid detection of cucumber green mottle mosaic virus in watermelon through a recombinase polymerase amplification assay. J Virol Methods. 2019b;270:146–9.
Jiao Y, Xu C, Li J, Gu Y, Xia C, Xie Q, et al. Characterization and a RT-RPA assay for rapid detection of chilli veinal mottle virus (ChiVMV) in tobacco. Virol J. 2020;17:33.
Kalischuk ML, Roberts PD, Paret ML. A rapid fluorescence-based real-time isothermal assay for the detection of cucurbit yellow stunting disorder virus in squash and watermelon plants. Mol Cell Probes. 2020;53:101613.
Kapoor R, Srivastava N, Kumar S, Saritha RK, Sharma SK, Jain RK, et al. (2017). Development of a recombinase polymerase amplification assay for the diagnosis of banana bunchy top virus in different banana cultivars. Arch Virol. 2017;162:2791–6.
Kim NY, Lee HJ, Jeong RD. A portable detection assay for apple stem pitting virus using reverse transcription-recombinase polymerase amplification. J Virol Methods. 2019;274:113747.
Kumar PV, Sharma SK, Rishi N, Ghosh DK, Baranwal VK. An isothermal based recombinase polymerase amplification assay for rapid, sensitive and robust indexing of citrus yellow mosaic virus. Acta Virol. 2018;62:104–8.
Lee HJ, Kim HJ, Lee K, Jeong RD. Rapid detection of peach latent mosaic viroid by reverse transcription recombinase polymerase amplification. Mol Cell Probes. 2020;53:101627.
Li Y, Cao Y, Fan Z, Wan P. Identification of a naturally occurring bean common mosaic virus recombinant isolate infecting azuki bean. J Plant Pathol. 2016;98:129–33.
Li YQ, Liu ZP, Yang K, Li YS, Zhao B, Fan ZF, et al. First report of bean common mosaic virus infecting azuki bean (Vigna angularis Ohwi Ohashi) in China. Plant Dis. 2014;98:1017.
Londoño MA, Harmon CL, Polston JE. Evaluation of recombinase polymerase amplification for detection of begomoviruses by plant diagnostic clinics. Virol J. 2016;13:48.
McKern NM, Mink GI, Barnett OW, Mishra A, Whittaker LA, Silbernagel MJ, et al. Isolates of bean common mosaic virus comprising two distinct potyviruses. Phytopathology. 1992;82:923–9.
Mekuria TA, Zhang S, Eastwell KC. Rapid and sensitive detection of little cherry virus 2 using isothermal reverse transcription-recombinase polymerase amplification. J Virol Methods. 2014;205:24–30.
Mink GI, Silbernagel MJ. Serological and biological relationships among viruses in the bean common mosaic virus group. Arch Virol. 1992;5(Suppl 5):397–406. https://doi.org/10.1007/978-3-7091-6920-9_42.
Mohandas A, Bhat AI. Recombinase polymerase amplification assay for the detection of piper yellow mottle virus infecting black pepper. Virus Dis. 2020;31:38–44.
Morales FJ. Common beans. In: Loebenstein G, Carr JP, editors. Natural resistance mechanisms of plants to viruses. Dordrecht: Springer; 2006. p. 367–82.
Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLoS Biol. 2006;4(7):e204.
Saiz M, Castro S, De Blas C, Romero J. Serotype-specific detection of bean common mosaic potyvirus in bean leaf and seed tissue by enzymatic amplification. J Virol Methods. 1994;50:145–54.
Saqib M, Jones RAC, Cayford B, Jones MGK. First report of bean common mosaic virus in Western Australia. Plant Pathol. 2005;54:563.
Saqib M, Nouri S, Cayford B, Jones RAC, Jones MGK. Genome sequences and phylogenetic placement of two isolates of bean common mosaic virus from Macroptilium atropurpureum in north-West Australia. Australas Plant Pathol. 2010;39:184–91.
Seo JK, Kang M, Shin OJ, Kwak HR, Kim MK, Choi HS, et al. First report of bean common mosaic virus in Cudrania tricuspidata in Korea. Plant Dis. 2015;99:292.
Silva G, Bömer M, Nkere C, Kumar PL, Seal SE. Rapid and specific detection of yam mosaic virus by reverse-transcription recombinase polymerase amplification. J Virol Methods. 2015;222:138–44.
Srivastava N, Kapoor R, Kumar R, Kumar S, Saritha RK, Kumar S, et al. Rapid diagnosis of cucumber mosaic virus in banana plants using a fluorescence-based real-time isothermal reverse transcription-recombinase polymerase amplification assay. J Virol Methods. 2019;270:52–8.
Wang TM, Yang JT. Visual DNA diagnosis of tomato yellow leaf curl virus with integrated recombinase polymerase amplification and a gold-nanoparticle probe. Sci Rep. 2019;9:15146.
Zeng R, Luo J, Gao S, Xu L, Song Z, Dai F, et al. Rapid detection of cucumber green mottle mosaic virus by reverse transcription recombinase polymerase amplification. Mol Cell Probes. 2019;43:84–5.
Zhang S, Ravelonandro M, Russell P, McOwen N, Briard P, Bohannon S, et al. Rapid diagnostic detection of plum pox virus in Prunus plants by isothermal AmplifyRP (R) using reverse transcription-recombinase polymerase amplification. J Virol Methods. 2014;207:114–20.
Zhao C, Sun F, Li X, Lan Y, Du L, Zhou T, et al. Reverse transcription-recombinase polymerase amplification combined with lateral flow strip for detection of rice black-streaked dwarf virus in plants. J Virol Methods. 2019;263:96–100.
Acknowledgements
We thank Prof. Daolong Dou (Nanjing Agricultural University) and Dr. Feng Sun (Jiangsu Academy of Agricultural Sciences) for their valuable suggestions on this work, and Prof. Stephen J Wylie (Murdoch University, Australia) for critical reading of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31101411), National Key Research and Development Project (2016YFE0203800, 2018YFE0112200), the Key R & D project of Jiangsu Province (BE2019376), National edible bean industry technology system (CARS-08-G15) and Jiangsu Agricultural Industry Technology System (JATS[2019]399). The research was carried out in the joint lab of China-AAFC for legume research in JAAS.
Author information
Authors and Affiliations
Contributions
XYC conceived the project. XYC and XHC supervised the work. JCQ performed most of the experiments with assistance from ZY, XC, DYS and HTC. All authors analyzed the data. JCQ and XYC wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, J., Yin, Z., Shen, D. et al. Development of a recombinase polymerase amplification combined with lateral flow dipstick assay for rapid and sensitive detection of bean common mosaic virus. Phytopathol Res 3, 3 (2021). https://doi.org/10.1186/s42483-021-00080-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42483-021-00080-3