Abstract
Background
Parkinson’s disease (PD) is a progressive, neurodegenerative disorder. In the advanced stages it can result in severe disability despite optimal treatment. Data suggests heterogeneous classification of PD stages among physicians in different countries. The purpose of the OBSERVE-PD study was to evaluate the proportion of patients with advanced PD (APD) according to physicians’ judgments in an international cohort.
Methods
A cross-sectional, observational study was conducted in 18 countries. Data were collected during a single patient visit. Demographic data, disease status, current medical treatment, and quality of life were evaluated for the German cohort and compared to the international cohort. Potential prognostic factors of physicians’ classification of APD in the German and international cohorts were identified using logistic regression.
Results
In total, 177 German and 2438 international patients were enrolled. 68.9% of the German and 50.0% of the international patients were classified by physicians as APD. Despite similar demographics and comparable disease severity, motor fluctuations (odds ratio [OR], 49.7; 95% confidence interval [CI], 8.5–291.9) and current device-aided treatment (OR 8.7; CI 5.5–13.8) showed the strongest association to physicians’ classification of APD in the German and the international cohorts, respectively. The number of different oral anti-Parkinson-medications showed opposed associations with APD-classification between the international (OR 1.19; CI 1.03–1.37) and German (OR 0.46; CI 0.18–1.18) cohort. Although 58.2% of the German patients diagnosed with APD were considered eligible for device-aided treatment, only 40.8% actually received it.
Conclusions
This study highlights the challenges in the recognition and the effective management of APD in Germany and emphasizes the necessity of complying with standard diagnostic criteria for identification of patients with APD. Therapeutic approaches differed internationally, with a tendency in Germany towards a more complex oral medication regimen for patients with APD. In view of similar quality of life and disease status in both cohorts, our findings may prompt further exploration of parameters for disease classifications, and consideration of optimal treatment strategies.
Similar content being viewed by others

Background
Parkinson’s disease (PD) is the second most common neurodegenerative disorder [1]. With increasing disease duration, PD patients may develop severe disability despite patient-specific treatment [1, 2]. The progressing degeneration of dopaminergic neurons in the substantia nigra, the pathophysiological hallmark of this disease, requires dopamine substitution for symptom control. While dopaminergic medication usually provides good symptom control in the initial stages of disease, its effectiveness may deteriorate over time, including an increased sensitivity to subtle fluctuations in the drugs’ plasma levels, which ultimately narrows their therapeutic window. In consequence, hypo- and hyperkinetic motor fluctuations and non-motor fluctuations may emerge, and have been associated with the duration of levodopa treatment [3,4,5]. Furthermore, the development of symptoms irresponsive to conventional dopaminergic treatment add to the decrease in quality of life [6, 7].
There is no universally accepted consensus on how to define stages of PD considering motor and non-motor symptoms [8], although a patient’s disease stage may be a determining factor for optimal treatment. As the disease progresses, patients may experience an increased amplitude and frequency of fluctuations between periods of good and poor symptom control. Although device-aided treatments, such as deep brain stimulation, subcutaneous apomorphine, or intestinal levodopa infusion, are efficacious options in treating fluctuations, the decision on when to recommend and initiate device-aided therapy differs according to the physician’s staging of the disease.
The purpose of the OBSERVE-PD study was to evaluate the proportion of patients with advanced PD (APD) according to physician’s judgment and to compare demographic data, current medical treatment, disease status, and quality of life between patients classified as APD versus those classified as non-APD. The study furthermore assessed the treating physician’s judgement on the eligibility for device-aided therapies. Observe-PD was conducted in 18 countries, recruiting patients at movement disorder centers and clinics (MDCs). In this work, we extracted data of the German cohort comparing demographics, disease status, and quality-of-life scores of patients classified APD versus non-APD. Our objective was to identify factors supporting physicians’ decision for classifying PD as advanced, and comparing the findings from the German cohort with the international cohort.
Methods
Study population
OBSERVE-PD was an observational, cross-sectional, non-interventional, multi-center study with 2615 patients conducted in 18 countries across different geographic regions between February 2015 and January 2016. The study design has been reported previously [9]. In brief, adult patients diagnosed with PD according to the UK Parkinson’s Disease Society Brain Bank criteria [10] who attended a routine clinical visit or were admitted to an MDC were recruited. Data were collected as part of routine care during one single visit and consisted of demographics and disease history (disease duration, motor fluctuations, referral history and disease stage), including previous and current treatments (type and number of current treatments, treatment response, form of application and eligibility for device-aided treatment). Additionally, site and physician characteristics were collected.
The study was approved by local ethics committees in all participating countries and was conducted in accordance with the ethical principles of the Declaration of Helsinki. Before inclusion, all participants signed a patient authorization or informed consent for use and disclosure of their personal health information.
Disease status and quality of life questionnaires
Patient quality of life and disease status were evaluated by physicians with the Unified Parkinson’s Diseases Rating Scale (UPDRS parts II-IV) [11], the modified Hoehn and Yahr stage [12], and the Non-Motor Symptoms Scale for Parkinson’s Disease (NMSS) questionnaire [13]. To evaluate quality of life, patients completed the 8-item Parkinson’s Disease Quality of Life questionnaire (PDQ-8) [14].
Along with their subjective assessment of the PD stage (advanced vs not advanced), physicians completed an APD questionnaire developed by an international panel of experts on movement disorders using the Delphi method [15,16,17]. This questionnaire comprises 11 questions for the assessment of APD, with patients classified as advanced when any criterion is fulfilled (cumulative classification).
Statistical analysis
All enrolled patients fulfilling the selection criteria and with a physician’s diagnosis on PD stage were included. Descriptive statistics were conducted for quantitative and qualitative variables in the German and international cohort and separately for those with and without APD.
For the German subpopulation two-sample t tests were performed to assess potential differences in disease status and quality of life scores between these subgroups. Cohen’s kappa was calculated as a measure for the alignment of the physicians’ subjective assessments with the cumulative Delphi classification as well as with the single responses to each of the 11 questions of the Delphi questionnaire.
Multivariable logistic regression models were applied for both cohorts to investigate prognostic factors (patients demographics, PD history and treatment, dichotomized Delphi criteria, physician/institution characteristics) on physicians’ APD classification. A backward selection procedure was applied, including a fivefold cross-validation to determine the average predictive performance quantified by the Area Under the Receiver Operating Characteristic (AUROC). The set of variables with the maximal average AUROC estimates across all backward selection steps was chosen. Missing data were imputed by a regression-based single imputation method.
All statistical analyses were conducted with SAS® 9.4 (SAS Institute, Cary, NC, USA).
Results
German cohort: comparison of APD and non-APD patients
In total, 177 patients were enrolled in Germany for the OBSERVE-PD study. Half of the patients were recruited from MDCs in public hospitals (50.0%), 17.1% from MDCs in university hospitals and 32.9% from MDCs in other institutions, by either general neurologists (23.2%), movement disorder specialists (33.9%) or physicians with multiple specialties (42.9%). Physicians classified more than two-thirds of the patients in an advanced stage of PD (n = 122 [68.9%]). Table 1 provides an overview of demographic data and disease characteristics of patients classified as APD and non-APD. There was a higher percentage of male patients in the non-APD versus APD group (67.3% vs 57.4%). Patients in the APD group had a longer disease duration (10.2 vs 3.1 years). The percentage of patients requiring caregiver support and experiencing motor fluctuations was higher in the APD cohort.
In the German cohort, the APD classification relying on physicians’ judgement and the APD classification based on the Delphi method showed a fair consensus (Cohen’s kappa: 0.243, Additional file 1: Table S2). The vast majority of patients reported at least one comorbidity (APD, 95.1% vs non-APD, 85.5%; Additional file 1: Table S1) with subjective cognitive dysfunction (59.8% vs 50.9%), hypertension (36.9% vs 49.1%), and depressive symptoms (23.8% vs 16.4%) being the most prevalent.
Nearly all patients received dopaminergic treatment (APD, 99.2% vs non-APD, 96.4%). In comparison to non-APD, patients classified as APD were more frequently treated with oral levodopa (93.4% vs 76.4%), oral dopamine agonists (62.3% vs 34.5%) and catechol-o-methyltransferase inhibitors (27.0% vs 3.6%).
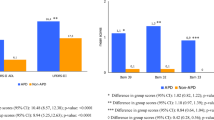
On average, UPDRS II, UPDRS III, UPDRS IV Question 32 (dyskinesia duration), and UPDRS IV Question 39 (average duration of “off” time) were significantly higher in patients with versus without APD (UPDRS II: mean: 15.9 ± SD: 6.5 vs 9.5 ± 4.9, p < 0.0001; UPDRS III: 28.1 ± 12.3 vs 19.9 ± 9.6, p < 0.0001, UPDRS IV Q32: 0.9 ± 1.0 vs 0.0 ± 0.1, p < 0.0001, UPDRS IV Q39: 0.7 ± 1.0 vs 0.0 ± 0.1, p < 0.0001). The mean total NMSS score was twice as high for patients in the APD subgroup versus the non-APD subgroup (60.7 ± 47.4 vs 30.1 ± 22.1, p = 0.0004). Significantly higher PDQ-8 scores were observed in the APD versus non-APD group (30.7 ± 17.5 vs 23.2 ± 16.0, p = 0.0072) (Fig. 1).
Disease status and quality of life scores for patients in the APD and non-APD subgroups. Disease status and quality of life scores for patients in the APD and non-APD subgroups as per a UPDRS parts II and III; b UPDRS part IV (dyskinesia duration, dyskinesia disability, and average duration of “off” time); and c the NMSS and PDQ-8. APD, advanced Parkinson’s disease; NMSS, Non-Motor Symptoms Scale for Parkinson’s Disease; PDQ-8, Parkinson’s Disease Questionnaire; Q, question; UPDRS, Unified Parkinson’s Disease Rating Scale
More than half of the APD group (58.2%) and a quarter of the non-APD group (25.5%) were deemed eligible for device-aided treatment, according to the judgment of the treating physicians (Table 2). Of these, 40.8% of the APD patients and 15.4% of the non-APD patients actually received device-aided treatment or treatment initiation was planned. The main reasons for patients not receiving or initiating a device-aided treatment were either indecisiveness or refusal of device-aided treatments altogether (Table 2).
Comparison of physicians’ APD classification in the German and international cohort
In contrast to the German cohort, most patients in the international cohort (n = 2438) were treated at university hospitals (63.7%) by movement disorder specialists (67.5%). Neither the demographics nor the disease status differed significantly between cohorts (Table 3). Furthermore, the number of patients experiencing “off” symptoms for more than 25% of the day were comparable (German cohort 78.0% vs international cohort 78.7%, p = 0.8076). Although more German patients were classified as APD than the international cohort (68.9% vs 50.0%, p < 0.0001), German patients received device-aided treatment less frequently (17.6% vs 22.6%, p = 0.1265). However, the latter difference did not reach statistical significance. Furthermore, the former were treated more frequently with ≥ 5 daily oral levodopa doses (42.6% vs 30.5%, p = 0.0008). Motor fluctuations occurred more frequently in the German cohort, although not significantly (62.1% vs 55.5%, p = 0.0834).
When applying the suggested diagnostic criteria [15], the percentage of patients with APD was higher in Germany (84.8%) than in the international cohort (69.3%) (Table 4). 69.0% of German patients with PD deemed as non-advanced according to physician judgment would have fulfilled the Delphi criteria for APD, which was higher than in the international cohort (46.8%).
Regression analyses indicated stronger effects in the occurrence of motor fluctuations on the physicians’ attribution of APD in the German (odds ratio [OR] 49.72; 95% confidence interval [CI] 8.47–291.91) than in the international cohort (OR 3.79; CI 2.78–5.17). Ongoing device-aided therapy was associated with physicians’ APD classification only in the international cohort (OR 8.68; CI 5.45–13.82) but not in the German cohort. The number of concurrently prescribed oral anti-PD medication was positively associated with assigning APD in the international cohort (OR 1.19; CI 1.03–1.37), while no such association was observed in the German cohort (OR 0.46; CI 0.18–1.18). In addition, Hoehn and Yahr stages and APD classification had a stronger association in the German (OR 5.74; CI 1.34–24.58) than in the international cohort (OR 2.04; CI 1.57–2.65).
The occurrence of non-motor symptoms showed no association with the physicians’ APD classification, neither in the international nor the German cohort. However, utilizing the Delphi criteria, criterion number 5 (non-motor symptom fluctuations) showed a significant association in the German cohort (OR 4.31; CI 1.06–17.54), and criterion number 11 (moderate or severe psychosis) in the international cohort (OR 2.61; CI 1.16–5.88).
Discussion
This study presents the German data of the observational, multi-country, cross-sectional OBSERVE-PD study [9]. We identified differences between the German physicians’ judgements and the suggested criteria for the diagnosis of APD [15]. Furthermore, our results indicate that despite the more frequent assignment of APD in Germany, advanced treatment options such as device-aided therapies were initiated less commonly. In addition, the fact that 25.5% of non-APD patients were deemed eligible for device-aided treatment, of whom two patients eventually received a therapy escalation is an intriguing observation warranting further investigation. As pointed out by Fasano et al., enhanced patient-physician guidance may facilitate transition to initiation of device-aided treatment, a factor which may also play a key role in the patient cohort in Germany [9].
APD was generally well recognized in Germany. Consensus of physicians’ judgement and APD classification based on the Delphi method was fair in the German but moderate in the international cohort (German cohort: 0.243 vs. international cohort: 0.441). In general, Cohen’s kappa coefficients of all Delphi criteria items were lower in the German than in the international cohort. However, the highest agreement with physicians’ APD classification was found in both cohorts for the same items: “moderate/severe troublesome motor fluctuations” (0.315 vs. 0.425), “at least 5 times daily oral levodopa dosing” (0.344 vs 0.410), or “moderate/severe of limitation of ADL capacity” (0242 vs. 0.440). One may speculate the later release of the Delphi study [15] resulted in a potential knowledge gap of physicians regarding current diagnostic criteria. This could have had different implications according to medical system peculiarities. The German healthcare system profits from a higher number of specialized physicians who focus on the treatment of only certain disease groups, i.e. movement disorders in contrast to the more university-focused treatment of patients with PD in the international cohort. A somehow related yet different perspective of the apparent “anosognosia” for APD in subjects deemed as non-APD may turn towards different approaches, especially in view of the German medical infrastructure allowing more frequent patient assessments. Interestingly, the high hospitalization rates and oversupply with medical products are considered traditional flaws of the German healthcare system [18]. One may thus have anticipated a larger number of patients on device-aided treatments. Contrarily, the percentage of German PD-patients on or scheduled for device-aided treatment was lower than in the international cohort. Analyses in the Swiss cohort of the OBSERVED-PD study showed similar patient characteristics and APD frequency (69.4%) of the Swiss patients in comparison to the German patients [19]. However, despite these similarities and comparable healthcare systems in both countries, consensus of physicians’ judgement and APD classification based on the Delphi method (kappa: 0.480) as well as the percentage of patients with device-aided treatment was much higher (61.3%) in the Swiss cohort.
So why do German neurologists seem to be so cautious about diagnosing APD in general and specifically about recommending device-aided treatment options even in PD-patients who are deemed advanced? A comparison of disease severity revealed equal symptom burden in both cohorts when comparing overall clinical state including motor and non-motor features, ruling out disease-specific differences between cohorts. In contrast, patients’ indecisiveness and/or refusal were identified as possible causes for not administering device-aided treatments. Moreover, the aforementioned high density of qualified physicians in Germany [18] may allow for more frequent patient visits and for tailoring a more sophisticated oral therapy regime. Simply speaking, more fine-tuning of oral medications may have delayed the consideration of device-aided treatments by physicians and patients alike. However, in addition to the characteristic differences between the healthcare systems, there seemed to be also some notable differences in the parameters that were considered relevant for diagnosis of APD. As such, in Germany, data seem to suggest that physicians put a significantly higher emphasis on non-motor and motor symptoms, as well as the symptoms according to the Hoehn & Yahr scale, than in the international cohort. Importantly, results from both the German and the international cohorts show similar levels in quality of life despite the different treatment strategies, which indicates that German PD-patients are treated equally efficacious while agreeing to a more sophisticated regimen of oral medication. These findings might highlight the need for a thorough evaluation of patients’ individual treatment expectations to facilitate individual treatment recommendations [20, 21]. At this point, we advocate for a more detailed insight for distinct therapeutic options through future studies.
The generalizability of our results is subject to certain limitations. First, the sample size of the German study population is relatively small. Secondly, the suggested criteria for the diagnosis of APD [15] were published after data collection in the OBSERVE-PD study. Hence, and as already stated, awareness of this topic might have increased in the meantime, warranting further studies. Finally, our results might not be that easily translatable to the general PD population, since German patients were recruited from MDCs, which, owing to their treatment expertise, treat a higher proportion of patients with PD in the later stages of the disease.
Conclusions
In summary, this study highlights the challenges in the recognition and the effective management of APD in Germany and emphasizes the necessity of complying with standard diagnostic criteria for identification of patients with APD. Therapeutic approaches differed internationally, with a tendency in Germany towards a more complex oral medication regimen for patients with APD. In view of similar quality of life and disease status in both cohorts, our findings may prompt further exploration of parameters for disease classifications, and consideration of optimal treatment strategies.
Availability of data and materials
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Abbreviations
- APD:
-
Advanced Parkinson’s disease
- AUROC:
-
Area under the receiver operating characteristic
- CI:
-
Confidence interval
- MDC:
-
Movement disorder center
- NMSS:
-
Non-Motor Symptoms Scale for Parkinson’s Disease
- OR:
-
Odds ratio
- PD:
-
Parkinson’s disease
- PDQ-8:
-
8-Item Parkinson’s Disease Questionnaire
- SD:
-
Standard deviation
- UPDRS:
-
Unified Parkinson’s Disease Rating Scale
References
Poewe, W., & Mahlknecht, P. (2009). The clinical progression of Parkinson’s disease. Parkinsonism & Related Disorders, 15, S28–S32.
Hoehn, M. M., & Yahr, M. D. (1998). Parkinsonism: Onset, progression, and mortality. Neurology, 50(2), 318.
Lang, A. E., & Lozano, A. M. (1998). Medical progress: Parkinson’s disease (second of two parts). New England Journal of Medicine, 339, 1130–1143.
Lang, A. E., & Lozano, A. M. (1998). Parkinson’s disease. New England Journal of Medicine, 339(15), 1044–1053.
Olanow, C. W., Watts, R. L., & Koller, W. C. (2001). An algorithm (decision tree) for the management of Parkinson’s disease (2001): Treatment guidelines. Neurology, 56(11 Suppl 5), S1-s88.
Fabbri, M., Coelho, M., Guedes, L. C., Chendo, I., Sousa, C., Rosa, M. M., Abreu, D., Costa, N., Godinho, C., Antonini, A., & Ferreira, J. J. (2017). Response of non-motor symptoms to levodopa in late-stage Parkinson’s disease: Results of a levodopa challenge test. Parkinsonism & Related Disorders, 39, 37–43. https://doi.org/10.1016/j.parkreldis.2017.02.007
Nonnekes, J., Timmer, M. H., de Vries, N. M., Rascol, O., Helmich, R. C., & Bloem, B. R. (2016). Unmasking levodopa resistance in Parkinson’s disease. Movement Disorders, 31(11), 1602–1609. https://doi.org/10.1002/mds.26712
Krüger, R., Klucken, J., Weiss, D., Tönges, L., Kolber, P., Unterecker, S., Lorrain, M., Baas, H., Müller, T., & Riederer, P. (2017). Classification of advanced stages of Parkinson’s disease: Translation into stratified treatments. Journal of Neural Transmission, 124(8), 1015–1027. https://doi.org/10.1007/s00702-017-1707-x
Fasano, A., Fung, V. S. C., Lopiano, L., Elibol, B., Smolentseva, I. G., Seppi, K., Takats, A., Onuk, K., Parra, J. C., Bergmann, L., Sail, K., Jalundhwala, Y., & Pirtosek, Z. (2019). Characterizing advanced Parkinson’s disease: OBSERVE-PD observational study results of 2615 patients. BMC Neurology, 19(1), 50. https://doi.org/10.1186/s12883-019-1276-8
Hughes, A. J., Daniel, S. E., Kilford, L., & Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery and Psychiatry, 55(3), 181–184. https://doi.org/10.1136/jnnp.55.3.181
Fahn, S., Elton, R., & Members of the UPDRS Development Committee. (1987). The Unified Parkinson's Disease Rating Scale. In S. Fahn, C. D. Marsden, D. B. Calne, & M. Goldstein (Eds.), Recent developments in Parkinson's disease (Vol. 2, pp. 153–163, 293–304). Florham Park: Macmillan Healthcare Information.
Goetz, C. G., Poewe, W., Rascol, O., Sampaio, C., Stebbins, G. T., Counsell, C., Giladi, N., Holloway, R. G., Moore, C. G., & Wenning, G. K. (2004). Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations the Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Movement Disorders, 19(9), 1020–1028.
Chaudhuri, K. R, Martinez-Martin, P, Brown, R. G., Sethi, K., Stocchi, F., Odin, P., … Schapira, A. H. (2007). The metric properties of a novel non-motor symptoms scale for Parkinson's disease: Results from an international pilot study. Movement Disorders, 22(13), 1901–1911. https://doi.org/10.1002/mds.21596
Jenkinson, C., & Fitzpatrick, R. (2007). Cross-cultural evaluation of the short form 8-item Parkinson’s Disease Questionnaire (PDQ-8): Results from America, Canada, Japan, Italy and Spain. Parkinsonism & Related Disorders, 13(1), 22–28. https://doi.org/10.1016/j.parkreldis.2006.06.006
Antonini, A., Stoessl, A. J., Kleinman, L. S., Skalicky, A. M., Marshall, T. S., Sail, K. R., Onuk, K., & Odin, P. L. A. (2018). Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: A multi-country Delphi-panel approach. Current Medical Research and Opinion. https://doi.org/10.1080/03007995.2018.1502165
Rowe, G., & Wright, G. (1999). The Delphi technique as a forecasting tool: Issues and analysis. International Journal of Forecasting, 15(4), 353–375.
Rowe, G., & Wright, G. (2001). Expert opinions in forecasting: The role of the Delphi technique. In J. S. Armstrong (Ed.), Principles of forecasting (pp. 125–144). Berlin: Springer.
Busse, R., Blumel, M., Knieps, F., & Barnighausen, T. (2017). Statutory health insurance in Germany: A health system shaped by 135 years of solidarity, self-governance, and competition. Lancet, 390(10097), 882–897. https://doi.org/10.1016/S0140-6736(17)31280-1
Möller, J. C., Baumann, C. R., Burkhard, P. R., Kaelin-Lang, A., Küng, I., Onuk, K., & Bohlhalter, S. (2021). Characterisation of advanced Parkinson’s disease: Observe-PD observational study—Results of the Swiss subgroup. Swiss Medical Weekly, 151, w20419. https://doi.org/10.4414/smw.2021.20419
Marshall, T., Pugh, A., Fairchild, A., & Hass, S. (2017). Patient preferences for device-aided treatments indicated for advanced Parkinson disease. Value in Health, 20(10), 1383–1393.
Odin, P., Chaudhuri, K. R., Slevin, J. T., Volkmann, J., Dietrichs, E., Martinez-Martin, P., Krauss, J. K., Henriksen, T., Katzenschlager, R., & Antonini, A. (2015). Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: Consensus from an international survey and discussion program. Parkinsonism & Related Disorders, 21(10), 1133–1144.
Acknowledgements
The authors would like to thank GKM Gesellschaft für Therapieforschung mbH (Munich, Germany) for support in statistical analysis and drafting the manuscript.
Funding
D.P., F.G., W.J., and L.T. received no specific funding for the executing of the study. AbbVie sponsored the study; contributed to the design; participated in the collection, analysis, and interpretation of data; in writing, reviewing, and approval of the final version. AbbVie funded the contributions of GKM Gesellschaft für Therapieforschung mbH (Munich, Germany). No honoraria or payments were made for authorship.
Author information
Authors and Affiliations
Contributions
DP acquisition, interpretation of data, drafted and critically reviewed the manuscript. FG acquisition, interpretation of data, drafted and critically reviewed the manuscript. WHJ acquisition, interpretation of data, drafted and critically reviewed the manuscript. CA drafted and critically reviewed the manuscript. KO drafted and critically reviewed the manuscript. MT acquisition, interpretation of data, drafted and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by local ethics committees and performed according to the International Conference on Harmonization and Good Clinical Practice requirements. The principles of the Declaration of Helsinki were adhered to throughout the entire trial. The local ethics committees that provided approval included those in Austria (Ethik Kommission des Landes Oberösterreich, Ethikkommission der Medizinischen Universität Innsbruck, Ethik Kommission des Bundeslandes Niederösterreichs), in Belgium (Universitair Ziekenhuis Antwerpen), in Canada (REB of Centre intégré de santé et de services sociaux de Chaudière-Appalach [MSSS pour les centres du Québec], Health Research Ethics Boards [UofA], Western University Health Science REB [HSREB], MSSS [authorized by MUHC], Ottawa Health Science Network REB, Conjoint Health Research Ethics Board of the University of Calgary, Queen’s University Health Sciences & Affiliated teaching hospitals REB [HSREB], University Health Network REB, IRB Services [Advarra], REB Horizon Health Network, MSSS [authorized by JGH], IRB Services [Advarra]), in Switzerland (Ethikkommission Ostschweiz Kantonsspital), in Germany (Ethikkommission der Universität zu Köln, Ethikkommission der Landesärztekammer Brandenburg, Ethikkommission Ärztekammer Niedersachsen, Ethikkommission der Ländesärztekammer Thüringen, Ethik-Kommission Landesärztekammer Baden-Württemberg, Ethik-Kommission Albert-Ludwigs-Universität Freiburg, Ethik-Kommission der Ärztekammer Berlin, Ethikkommission Ärztekammer Nordrhein, Ethik-Kommission der Bayerischen Landesärztekammer, Ethikkommission der Ärztekammer Hamburg, Ethikkommission Ärztekamer Niedersachsen, Ethik-Kommission Rheinische Friedrich-Wilhelms-Universität Medizinische Fakultät, Ethikkommission Ärztekammer Sachsen-Anhalt, Ethikkommission Ärztekammer Niedersachsen, Ethikkommission Ärztekammer Nordrhein), in Greece (Ethics Committee of General Hospital of Thessaloniki “G. Papanikolaou”, Ethics Committee of General Hospital of Thessaloniki “Papageorgiou”, Ethics Committee of 251 Airforce General Hospital, Ethics Committee of Naval Hospital of Athens, Ethics Committee of University General Hospital “Attikon”, Ethics Committee of University General Hospital of Heraklion, Ethics Committee of University General Hospital of Patras, Ethics Committee of Mediterraneo Hospital, Ethics Committee of University General Hospital of Alexandroupoli, Ethics Committee of HYGEIA Hospital, Ethics Committee of General Hospital of Athens “G. Gennimatas”, Ethics Commmittee of University General Hospital of Thessaloniki “Axepa”, Ethics Committee of University General Hospital of Ioannina, Ethics Committee of 417 Nursing Institution of Participial Army Fund [NIMTS]), in Ireland (Education and Research Committee St Vincent’s University Hospital, Joint Research Ethics Committee SJH/AMNCH, Clinical Research Ethics Committee of the Cork Teaching Hospitals, Galway research ethics committee), in Israel (E. Wolfson Medical Center Helsinki Committee, IRB Committee Sheba Medical Center Israel, Tel Aviv Sourasky Medical Center Institutional Review Board, Ethics committee of Rabin Medical Center), in Italy (Comitato Etico delle Aziende Sanitarie dell’Umbria di Perugia, Comitato Etico Regional [CER] delle Marche c/o AUO Ospedali Riuniti, Comitato Etico AOU di Cagliari, Comitato Etico Indipendente Azienda Ospedaliero Universitaria Policlinico Consorziale di Bari, Comitato Etico Interaziendale AOU Città della Salute e della Scienza di Torino AO Ordine Mauriziano – ASL TO1, Comitato Etico Interaziendale della Provincia di Messina AOU Policlinico “G Martino”, Comitato Etico Area Vasta Centro c/o AOU Careggi, Comitato Etico Indipendente dell’Azienda Ospedaliera Universitaria Policlinico Tor Vergata di Roma, Comitato Etico Seconda Università degli Studi di Napoli Azienda Ospedaliera Universitaria SUN-AORN “Ospedali dei Colli”), in Turkey (Kocaeli University Medical Faculty Ethics Committee), in the Czech Republic (Ethics Committee of the General Hospital of Charles University in Prague, Ethics Committee of the St Anna Hospital of Masaryk University in Brno), in Slovakia (the local legislation valid at that time of 2015 did not impose an obligation to approve epidemiological observational studies by the ethics committee; therefore, the opinions of the two ethics committees in the Czech Republic can be used to comply with the ethical principles in both countries), in Russia (Advisory council on ethics St Petersburg state budgetary healthcare institution city hospital No 40, Ethics committee of the federal state budgetary institution state research center, Independent Interdisciplinary Committee for Ethical Review of Clinical Studies [125468, Moscow, Leningradskiy prospect 51]), in Romania (National Commission of Bioethics [Comisia Nationala de Bioetica a Medicamentului si a Dispozitivelor Medicale]), in Hungary (Ethics committees of the Medical Research Council of Hungary [ETT] Egészségügyi Tudományos Tanács Klinikai Farmakológiai Etikai Bizottsága), in Slovenia (Republic of Slovenia Medical Ethics Committee, Institute of Clinical Neurophysiology, University Medical Centre Ljubljana [Komisija Republike Slovenije Za Medicinsko Etiko]), in Croatia (Drug Committee of Clinical Hospital Center Zagreb [Povjerenstvo za lijekove Kliničkog Bolničkog Centra Zagreb], Drug Committee of Clinical Hospital Center Osijek [Povjerenstvo za lijekove Kliničkog Bolničkog Centra Osijek], Drug Committee of Clinical Hospital Center Split [Povjerenstvo za lijekove Kliničkog Bolničkog Centra Split]), and Australia (Belberry Human Research Ethics Committee, Royal Brisbane and Women’s hospital human research ethics committee). All patients gave written informed consent. All authors confirm that they have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Consent for publication
Not applicable.
Competing interests
D. P. received payments as a consultant for Boston Scientific and as a speaker on symposia sponsored by Boston Scientific. The institution of D.P., not D.P. personally received funding by Boston Scientific, the Parkinson's Foundation, Dr.-Reinfried-Pohl-Stiftung and the Deutsche Parkinson Vereinigung. D.P. declare that there are no additional disclosures to report. F.G. reports honoraria from AbbVie, BIAL, Merz, and STADA outside the submitted work. W.J. has received honorarium for lectures or advisory boards from AbbVie/Allergan, Bial, Desitin, Ipsen, Merz, Stada, UCB, Zambon. L.T. received payments as a consultant for Boston Scientific, L.T. received honoraria as a speaker on symposia sponsored by UCB, Desitin, Boston Scientific, AbbVie, Novartis, GlaxoSmithKline und DIAPLAN. The institution of L.T., not L.T. personally received funding by Boston Scientific, the German Research Foundation, the German Ministry of Education and Research and the Deutsche Parkinson Vereinigung. A.C. and K.O. are AbbVie employees and may own AbbVie stock.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary Tables 1 and 2 with additional clinical information of German patients included in the observe-PD study. The two tables display the distribution of comorbidities and how many patients fullfilled the different criteria put forward at the Delphi study by Antonini and colleagues stratified for APD and non-APD subjects, respectively.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pedrosa, D.J., Gandor, F., Jost, W.H. et al. Characterization of advanced Parkinson’s disease in Germany: results of the non-interventional OBSERVE-PD study. Neurol. Res. Pract. 4, 9 (2022). https://doi.org/10.1186/s42466-022-00176-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42466-022-00176-x