Abstract
Introduction
The WHO estimates that each year 5 million people are left permanently disabled after stroke. Adjuvant treatments to promote the effects of rehabilitation are urgently needed. Cortical excitability and neuroplasticity can be enhanced by non-invasive brain stimulation but evidence from sufficiently powered, randomized controlled multi-center clinical trials is absent.
Methods
Neuroregeneration enhanced by transcranial direct current stimulation (tDCS) in stroke (NETS) tested efficacy and safety of anodal tDCS to the primary motor cortex of the lesioned hemisphere in the subacute phase (day 5–45) after cerebral ischemia. Stimulation was combined with standardized rehabilitative training and repeatedly applied in 10 sessions over a period of 2 weeks in a planned sample of 120 patients. Primary outcome parameter was upper-extremity function at the end of the 2-weeks intervention period of active treatment or placebo (1:1 randomization), measured by the upper-extremity Fugl-Meyer assessment. Sustainability of the treatment effect was evaluated by additional follow-up visits after 30 and 90 days. Further secondary endpoints included metrics of arm and hand function, stroke impact scale, and the depression module of the patient health questionnaire.
Perspective
NETS was aimed at providing evidence for an effective and safe adjuvant treatment for patients after stroke.
Trial registration: ClinicalTrials.gov Identifier NCT00909714. Registered May 28, 2009.
Similar content being viewed by others
Introduction
Stroke causes the vast majority of disability-adjusted life years among neurological diseases, > 50% of stroke survivors suffer from long-term deficits. Among these, upper extremity (UE) dysfunction is a major problem.
On hospital admission, 50–80% of stroke patients exhibit UE dysfunction. Validated and responsive scoring instruments to describe upper extremity function include upper extremity Fugl-Meyer assessment (UEFMA), action research arm test (ARAT), nine-hole-peg test (NHPT), box-and-block test (BBT), or simply grip force measured with a dynamometer.
An ischemic stroke lesion is followed by extensive changes of brain metabolism, functional activation, neuronal excitability, and structure. While pattern and extent of plasticity and reorganization depend on the amount and localization of initial tissue damage, some key findings point to target areas which could contribute crucially to the recovery of function. For UE motor function, these areas include primary motor cortex (M1), ventral and dorsal premotor cortex, supplementary motor area, primary somatosensory cortex, and posterior parietal cortex of the lesioned and, to some extent, the contralesional hemisphere [13,14,15, 20].
Approaches to increase cortical plasticity include (1) excitability-enhancing drugs like serotonin-reuptake inhibitors and (2) excitability-enhancing non-invasive brain stimulation (NIBS) like anodal transcranial DC stimulation (tDCS) or high-frequency repetitive transcranial magnetic stimulation (rTMS). Recent trials on drugs to enhance neurorehabilitation have been disappointing [5]. For NIBS, large-scale, placebo-controlled randomized multicenter trials in the acute or subacute phase after stroke are absent. The NICHE trial studied 1-Hz rTMS to the contralesional M1 in 199 patients 3–12 months after stroke and was neutral [7]. Nevertheless, proof-of-principle trials suggest that NIBS, especially anodal tDCS, could improve sensorimotor function [1, 3, 9, 10].
Sample sizes of available studies range mostly from 4 to 35 patients. A meta-analysis on 29 studies (351 patients) suggested a small but significant effect for tDCS (18 of 29 studies) on fine-motor skill function when applied to patients after stroke (effect size, 0.31;95% CI 0.08–0.55; P = 0.01) [17]. However, Begg’s funnel plot was asymmetric and suggested publication bias. The most frequently applied strategy is enhancing M1 excitability in order to facilitate neuronal plasticity [4, 9, 11, 12, 19].
Methods
Aim of the trial
To test efficacy and safety of anodal tDCS to the M1 of the lesioned hemisphere, combined with standardized training, over a period of 2 weeks in the subacute phase after ischemic stroke.
Study description and study design
NETS was an investigator-initiated, interventional, randomized, placebo-controlled, parallel-assignment, international multicenter efficacy and safety study with quadruple blinding (patient, care provider, investigator, outcomes assessor). Patients were randomized 1:1 to treatment (anodal tDCS to M1 of the affected hemisphere) or placebo stimulation. The effect of treatment was assessed by comparing the UEFMA before and after the 2-weeks intervention period. The first study protocol dates back to 2009, several protocol modifications were necessary in the course of the trial. These changes are described in the section ‘Time course of the trial and changes of the study protocol’ below.
Patient population and eligibility criteria
Patients with ischemic subcortical or cortical, first clinically overt stroke confirmed by CT scan or MRI were included. Time window of inclusion was 5–45 days after stroke. As the primary interest of the study was to improve UE motor function, patients were included only if they exhibited moderate to moderately severe UE paresis, defined as UEFMA score 20–58 (inclusive) to indicate preserved basal hand function. For detailed inclusion and exclusion criteria see Tables S1 and S2 of the Additional file 1.
Clinical assessment
After registration of demographic data and stroke subtype, medical history was taken, followed by physical and neurological examination including the Edinburgh handedness inventory and a mini-mental state examination (MMSE). Clinical variables were collected by trained raters. Stroke severity was expressed as National Institutes of Health stroke scale (NIHSS) score. The Barthel index (BI) was used as a generic measure of independence in activities of daily living (ADL). UE function was quantified by UEFMA, ARAT, NHPT, BBT, muscle strength according to Medical Research Council (MRC), pinch and grip force as measured with a dynamometer. Sensory function was measured with von-Frey monofilaments. Spasticity was assessed by the Ashworth scale. As metrics of health-related quality of life (QoL), the stroke impact scale (SIS) and the short version of the patient health questionnaire were used (PHQ-9). More details on the scores are given in the SOM. All data were stored in an electronic Case Report Form. Monitoring was conducted by the Clinical Trial Centre North (Hamburg, Germany) and in compliance with E6 ICH GCP guideline.
Arms and interventions
Randomization
Eligible patients were randomized to treatment with anodal tDCS or placebo stimulation (1:1). Randomization was stratified by age (< 70 years/ ≥ 70 years) and lesion type (subcortical or strokes also involving the cortex). Randomization was performed web-based using centre-wise block stratification with variable block size.
Investigational product
For active stimulation, anodal tDCS (1 mA) was delivered for 20 min through 35 cm2 (5 cm × 7 cm) sponge-electrodes soaked with sodium-chloride solution leading to a current density of 0.03 mA/cm2 (Eldith, Neuroconn, Germany). The anode was centered at C3/4 of the international 10/20 system of EEG electrode placement (left/right hemisphere, depending on stroke location), near the hand representation area of the M1. This approach was deemed to be feasible for routine use and is sufficiently accurate given the large area of the stimulation electrodes used for tDCS. This electrode montage had been applied previously and had exerted reliable and durable effects on M1 excitability [8, 16] as well as some behavioral effects in smaller studies on chronic stroke patients [1, 9]. The cathode was placed over the contralateral supraorbital region. Current was applied with an 8 s fade-in and fade-out interval to attenuate itching sensations. For the placebo condition, anodal tDCS was limited to 40 s, a procedure demonstrated to warrant successful blinding [2, 6] (Fig. 1).
Schematic of active tDCS (solid red line) and placebo stimulation (dashed black line). For both active and placebo stimulation, current was ramped up over 8 s to 1 mA (blue shaded areas). Active stimulation remained at this level for 1200 s (20 min) followed by fade-out over 8 s from 1 to 0 mA. Placebo stimulation remained stable at 1 mA for only 40 s before fade-out over 8 s
Treatment
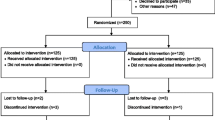
Active anodal tDCS (20 min) and placebo tDCS were applied once every workday over 2 weeks (10 working days) in combination with 45 min standardized rehabilitative training of UE function. Training started with onset of brain stimulation so that both treatments were given concurrently for 20 min in the active stimulation condition (40 s for placebo). In both conditions, the stimulator indicated after 20 min that the electrical intervention was completed. The electrodes could then be removed and the training session continued. The contents of rehabilitative training were described and illustrated in detail in a manual. All therapists and investigators participated in specific training modules by the central NETS team. These modules comprised (1) standardized rehabilitative training, (2) application of tDCS, and (3) assessment of primary and secondary outcome measures. After placement of electrodes, tDCS was applied by entering a code and thereby initiating a pre-set, masked program on the stimulator (respective code was obtained in the randomization procedure). Thus, patients, therapists, care takers and evaluators of outcome measures were blinded to the type of intervention. There was no formal requirement that participants needed to be inpatients or outpatients. See Fig. 2 for illustration of assessments.
Outcome measures
Primary endpoint
UEFMA 1–7 days after end of intervention.
Primary safety endpoint
Incidence of epileptic seizures during intervention period.
Secondary endpoints
Secondary endpoints included UEFMA 30 ± 10 days and 90 ± 20 days after intervention as well as ARAT, NHPT, BBT, muscle strength, pinch and grip force, evaluations of sensory function, spasticity, QoL and ADL at days 1–7, 30 ± 10, and 90 ± 20 after intervention. For more details see Additional file 1.
Statistical analysis
NETS was planned to show superiority of the experimental intervention compared with placebo in an intention-to-treat (ITT) population. The ITT population consists of all patients randomized who received at least one session of active or placebo stimulation. The per-protocol population (PP) includes all patients randomized who have no major protocol violation (e.g., < 9 of 10 stimulations applied or violation of inclusion or exclusion criteria). The primary analysis is based on an ANCOVA model with the difference of first post-intervention UEFMA (day 1–7 after end of treatment) to baseline UEFMA as response variable, treatment group and type of stroke as factors, and baseline UEFMA, age, and time interval between index event and baseline as covariates. The treatment effect is judged by the estimated contrast between treatment groups. Secondary endpoints are evaluated analogously to primary outcome measure. No adjustment for multiplicity is provided since these analyses are explorative. Descriptive statistics are provided for safety outcomes. In patients lost to follow-up, their last observation is carried forward (LOCF) in order to accomplish the ITT analysis. Adjusted analyses of the primary endpoint are based on the covariate severity of stroke as measured by the baseline NIHSS score. Pre-planned subgroup analyses include (1) subcortical stroke versus stroke involving cortex, (2) younger versus older patients (< median age versus ≥ median age of study population), (3) male vs. female, (4) mild versus moderate and severe stroke (NIHSS < 5 versus ≥ 5), (5) mild versus moderate and severe UE dysfunction (UEFMA ≥ 43 versus UEFMA < 43), and (6) smoker versus non-smoker.
Sample size calculation
Sample size calculation was performed using PASS 2008. Given a probability for a type-one error of 5%, an effective sample size of 100 patients per group was initially considered necessary in order to detect a clinically relevant difference of 5 points in the UEFMA [18] with an expected SD of 12.5 UEFMA points and a power of 80% with a two-sample, two-sided t-test. An early drop-out rate of 20% was expected, so the planned sample size was increased by 25%, leading to a cohort of n = 250.
Blinded re-assessment of the sample size was originally planned after 80% of the patients have been recruited or if cessation of funding before completion of recruitment could be anticipated. Because of slow recruitment, this analysis was carried out after inclusion of 76 patients (August 2018) and revealed a residual variance of 67.8, corresponding to a SD of 8.2 rather than the initially assumed 12.5 points of UEFMA based on literature. Hence, we were able to assume that even a sample size of 2 × 40 patients (80 complete data sets) would provide us with a statistical power > 80% to detect a 5-point difference in UEFMA. Based on this but also considering drop-outs and potentially incomplete data sets, the final sample size was set to n = 120.
Data safety monitoring
An independent data safety monitoring board (DSMB; see Additional file 1) was informed about adverse events and trial progress. Adverse events were adjudicated by two trialists (CG, SW). The trial would have been stopped if there had been a medically relevant increase in major, unexpected adverse events (such as seizures) with anodal tDCS compared with placebo stimulation.
Study organization and funding
NETS was funded by the Deutsche Forschungsgemeinschaft (GE 844/4-1) and conducted in 11 recruiting centers in 3 European countries (ClinicalTrials.gov Identifier NCT00909714).
Time course of the trial and changes of the study protocol
NETS was approved by the local ethics committee in February 2009. The first patient was randomized on November 17, 2009. Here, we report the final version of the protocol which is also the basis of the main analysis. NETS has faced multiple challenges causing slow recruitment. Several measures were taken in order to improve recruitment, which resulted in a series of protocol changes during the ongoing trial. The most prominent changes were (1) reduction of sample size to 120 patients (based on interim blinded re-assessment of residual variance); (2) extension of time window after stroke from 21 to 45 days (allowing inclusion of more patients in rehabilitation centers); (3) changing the time point of primary outcome evaluation from 12 months to right after the end of intervention (in order to reduce the number of patients lost to follow-up). The full history of protocol changes is available at https://clinicaltrials.gov/ct2/history/NCT00909714.
Perspective
NETS is an investigator-initiated, interventional, randomized, placebo-controlled, multicenter efficacy and safety study with quadruple blinding. It adds clinically relevant information to the topic of adjuvant non-invasive brain stimulation after stroke to enhance motor recovery.
Availability of data and materials
After publication of the trial results the data shall be made available on request.
Abbreviations
- ADL:
-
Activities of daily living
- ARAT:
-
Action research arm test
- BBT:
-
Box-and-block test
- BI:
-
Barthel index
- DSMB:
-
Data safety monitoring board
- ITT:
-
Intention-to-treat
- LOCF:
-
Last observation is carried forward
- M1:
-
Primary motor cortex
- MMSE:
-
Mini-mental state examination
- MRC:
-
Medical Research Council
- NETS:
-
Neuroregeneration enhanced by tDCS in stroke
- NHPT:
-
Nine-hole-peg test
- NIBS:
-
Non-invasive brain stimulation
- NIHSS:
-
National Institutes of Health stroke scale
- PHQ-9:
-
Patient health questionnaire
- PP:
-
Per-protocol
- QoL:
-
Quality of life
- rTMS:
-
Repetitive transcranial magnetic stimulation
- SIS:
-
Stroke impact scale
- SOM:
-
Supplementary online material
- tDCS:
-
Transcranial direct current stimulation
- UE:
-
Upper-extremity
- UEFMA:
-
Upper-extremity Fugl-Meyer assessment
References
Allman, C., Amadi, U., Winkler, A. M., Wilkins, L., Filippini, N., Kischka, U., Stagg, C. J., & Johansen-Berg, H. (2016). Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Science Translational Medicine, 8(330), 330re331. https://doi.org/10.1126/scitranslmed.aad5651
Bikson, M., Brunoni, A. R., Charvet, L. E., Clark, V. P., Cohen, L. G., Deng, Z. D., Dmochowski, J., Edwards, D. J., Frohlich, F., Kappenman, E. S., Lim, K. O., Loo, C., Mantovani, A., McMullen, D. P., Parra, L. C., Pearson, M., Richardson, J. D., Rumsey, J. M., Sehatpour, P., … Lisanby, S. H. (2018). Rigor and reproducibility in research with transcranial electrical stimulation: An NIMH-sponsored workshop. Brain Stimulation, 11(3), 465–480. https://doi.org/10.1016/j.brs.2017.12.008
Boggio, P. S., Nunes, A., Rigonatti, S. P., Nitsche, M. A., Pascual-Leone, A., & Fregni, F. (2007). Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restorative Neurology and Neuroscience, 25(2), 123–129.
Brown, J. A., Lutsep, H. L., Weinand, M., & Cramer, S. C. (2006). Motor cortex stimulation for the enhancement of recovery from stroke: A prospective, multicenter safety study. Neurosurgery, 58(3), 464–473.
Ford, G. A., Bhakta, B. B., Cozens, A., Hartley, S., Holloway, I., Meads, D., Pearn, J., Ruddock, S., Sackley, C. M., Saloniki, E. C., Santorelli, G., Walker, M. F., & Farrin, A. J. (2019). Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): A randomised, double-blind, placebo-controlled trial. The Lancet Neurology, 18(6), 530–538. https://doi.org/10.1016/S1474-4422(19)30147-4
Gandiga, P. C., Hummel, F. C., & Cohen, L. G. (2006). Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology, 117(4), 845–850.
Harvey, R. L., Edwards, D., Dunning, K., Fregni, F., Stein, J., Laine, J., Rogers, L. M., Vox, F., Durand-Sanchez, A., Bockbrader, M., Goldstein, L. B., Francisco, G. E., Kinney, C. L., Liu, C. Y., Ryan, S., Morales-Quezada, L., Worthen-Chaudhari, L., Labar, D., Schambra, H., … Pratt, W. (2018). Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke: The NICHE trial. Stroke, 49(9), 2138–2146. https://doi.org/10.1161/STROKEAHA.117.020607
Heise, K. F., Niehoff, M., Feldheim, J. F., Liuzzi, G., Gerloff, C., & Hummel, F. C. (2014). Differential behavioral and physiological effects of anodal transcranial direct current stimulation in healthy adults of younger and older age. Frontiers in Aging Neuroscience, 6, 146. https://doi.org/10.3389/fnagi.2014.00146
Hummel, F., Celnik, P., Giraux, P., Floel, A., Wu, W. H., Gerloff, C., & Cohen, L. G. (2005). Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain, 128(Pt 3), 490–499. https://doi.org/10.1093/brain/awh369
Ilic, N. V., Dubljanin-Raspopovic, E., Nedeljkovic, U., Tomanovic-Vujadinovic, S., Milanovic, S. D., Petronic-Markovic, I., & Ilic, T. V. (2016). Effects of anodal tDCS and occupational therapy on fine motor skill deficits in patients with chronic stroke. Restorative Neurology and Neuroscience, 34(6), 935–945. https://doi.org/10.3233/RNN-160668
Jefferson, S. C., Clayton, E. R., Donlan, N. A., Kozlowski, D. A., Jones, T. A., & Adkins, D. L. (2016). Cortical stimulation concurrent with skilled motor training improves forelimb function and enhances motor cortical reorganization following controlled cortical impact. Neurorehabilitation and Neural Repair, 30(2), 155–158. https://doi.org/10.1177/1545968315600274
Kim, S. Y., Hsu, J. E., Husbands, L. C., Kleim, J. A., & Jones, T. A. (2018). Coordinated plasticity of synapses and astrocytes underlies practice-driven functional vicariation in peri-infarct motor cortex. The Journal of Neuroscience, 38(1), 93–107. https://doi.org/10.1523/JNEUROSCI.1295-17.2017
Koch, P. J., Park, C. H., Girard, G., Beanato, E., Egger, P., Evangelista, G. G., Lee, J., Wessel, M. J., Morishita, T., Koch, G., Thiran, J. P., Guggisberg, A. G., Rosso, C., Kim, Y. H., & Hummel, F. C. (2021). The structural connectome and motor recovery after stroke: Predicting natural recovery. Brain, 144(7), 2107–2119. https://doi.org/10.1093/brain/awab082
Krakauer, J. W. (2006). Motor learning: Its relevance to stroke recovery and neurorehabilitation. Current Opinion in Neurology, 19(1), 84–90.
Lotze, M., Markert, J., Sauseng, P., Hoppe, J., Plewnia, C., & Gerloff, C. (2006). The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. Journal of Neuroscience, 26(22), 6096–6102. https://doi.org/10.1523/JNEUROSCI.4564-05.2006
Nitsche, M. A., Seeber, A., Frommann, K., Klein, C. C., Rochford, C., Nitsche, M. S., Fricke, K., Liebetanz, D., Lang, N., Antal, A., Paulus, W., & Tergau, F. (2005). Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. The Journal of Physiology, 568, 291–303.
O’Brien, A. T., Bertolucci, F., Torrealba-Acosta, G., Huerta, R., Fregni, F., & Thibaut, A. (2018). Non-invasive brain stimulation for fine motor improvement after stroke: A meta-analysis. European Journal of Neurology, 25(8), 1017–1026. https://doi.org/10.1111/ene.13643
Page, S. J., Fulk, G. D., & Boyne, P. (2012). Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Physical Therapy, 92(6), 791–798. https://doi.org/10.2522/ptj.20110009
Teskey, G. C., Flynn, C., Goertzen, C. D., Monfils, M. H., & Young, N. A. (2003). Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurological Research, 25(8), 794–800.
Ward, N. S. (2006). The neural substrates of motor recovery after focal damage to the central nervous system. Archives of Physical Medicine and Rehabilitation, 87(12 Suppl 2), S30-35.
Acknowledgements
We thank the members of the DSMB, Michael Hennerici and Michael Nitsche, for their continuous support and the Deutsche Forschungsgemeinschaft (DFG; German Research Council) for funding this independent trial. For a complete list of all investigators, their affiliations and COI statements see Additional file 1.
Funding
Deutsche Forschungsgemeinschaft (GE 844/4-1).
Author information
Authors and Affiliations
Consortia
Contributions
The paper is published on behalf of “The NETS Trial Collaboration Group”. The manuscript was written by the members of the writing committee who are listed in alphabetical order. Christian Gerloff (CG)* drafted the first version of the manuscript, Kirstin-Friederike Heise (K-FH), Friedhelm Christoph Hummel (FCH)*, Robert Schulz (RS), Silke Wolf (SW) and Antonia Zapf (AZ) critically revised the manuscript and gave valuable advice (*both corresponding authors). All authors read and approved the final manuscript.
Ethics declarations
Ethical approval and consent to participate
The NETS trial was approved by the responsible ethics committee (Ethik-Kommission der Ärztekammer Hamburg) on February 2, 2009 (registration # PV3124). Written informed consent was requested from each patient for participation in the study.
Consent for publication
All authors have given approval to the publication of the protocol paper.
Competing interests
For a complete list of all potentially competing interests of all members of the writing committee and all investigators see Additional file 1.
Authors' information
See see Additional file 1 for a full description of The NETS Trial Collaboration Group.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Study Details.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
The NETS Trial Collaboration Group. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of transcranial direct current stimulation to the motor cortex after stroke (NETS): study protocol. Neurol. Res. Pract. 4, 14 (2022). https://doi.org/10.1186/s42466-022-00171-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42466-022-00171-2