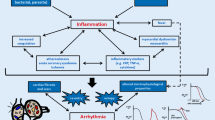
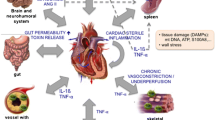
Abstract
Background
A biological mechanism called inflammation is necessary for reacting to damaging stimuli, but it can also, ironically, play a role in the formation of arrhythmias, or the group of disorders known as arrhythmogenesis. This review delves into the intricate relationship that exists between localized and systemic inflammation and the electrophysiological changes that result in abnormal heart rhythms.
Main body
Through oxidative stress, autonomic dysfunction, electrolyte imbalances, and coagulation activation, systemic inflammation may impact arrhythmogenicity. Similar to neuropathic alterations, direct cellular damage, and structural remodeling, localized heart inflammation also predisposes to arrhythmias. Studies demonstrating the impact of cytokines on ion channel expression and function, along with clinical associations between inflammatory indicators and arrhythmia incidence, offer the molecular insights. Immune cells like macrophages that alter cardiac conduction are involved in the interaction between inflammation and electrophysiology, which further complicates the situation. Clinical ramifications include the use of anti-inflammatory drugs to treat arrhythmic episodes and the possible adjustment of arrhythmia treatment based on inflammatory condition. Even yet, further thorough study is necessary to fully understand the efficacy of these medicines.
Conclusions
Arrhythmogenesis and inflammation are inherently linked by a number of mechanisms that change the electrical characteristics of the heart. Arrhythmia treatment and prevention may benefit from therapeutic approaches that reduce inflammatory processes. The difficulties that still exist in applying this information to clinical settings highlight the necessity of continuing studies to better comprehend the subtleties of inflammation-induced electrical alterations. Progress in identifying biomarkers of inflammation and developing tailored therapies will be crucial in enhancing the prognosis of individuals suffering from arrhythmogenic disorders that are aggravated by inflammation.
Similar content being viewed by others
Background
The body uses inflammation as a crucial biological reaction to fight against dangerous stimuli like infections, cellular damage, or poisonous chemicals [1]. Immune system activation and the production of many mediators, including chemokines, reactive oxygen species, and cytokines, are involved in this complex process [2]. Positive or negative outcomes from inflammation depend on the particular circumstances and the ratio of pro- to anti-inflammatory factors [2].
An increased risk of deadly infections has been linked to systemic inflammation, which affects the entire body [3]. Moreover, it has been connected to a number of cancers, including as gastric cancer, colorectal cancer, head and neck cancer, oral squamous cell carcinoma, and high-grade glioma [4]. On the other hand, pericardial disease, arrhythmias, myocardial infarction, atherosclerosis, valvular heart disorders, cardiomyopathies, and heart failure are among the cardiovascular diseases (CVDs) in which localized cardiac inflammation plays a crucial role [5].
Bradycardia and tachycardia are examples of arrhythmogenesis, the process that results in arrhythmias or disturbances in cardiac rhythm [6,7,8]. Tachycardia and bradycardia involve improperly decreasing or increasing the heart rate [8]. The atrioventricular node (AV node) and sinoatrial node dysfunction, as well as other illnesses, diseases, or disorders, may be the cause of these anomalies [8]. Arrhythmias can be caused by external causes like stress, drugs, or electrolyte imbalances, as well as internal heart disorders such structural, electrical, or metabolic difficulties [7, 9]. Palpitations, chest pain, dyspnea, or syncope are some of the symptoms that may occur; specific arrhythmias raise the risk of stroke, heart failure, or sudden cardiac death [9].
Automaticity is one of the etiologies of arrhythmias, resulting from changes in cellular ion exchange systems that impact the action potential pattern [7]. Electrical impulses that go around an impediment produce a reentrant channel, which facilitates reentry [7]. Abnormal impulses are triggered by arrhythmias, which are infrequent events caused by medicines or diseases that change cell action potentials [7].
Common arrhythmia atrial fibrillation (AF) is associated with increased rates of morbidity and mortality; it is frequently brought on by inflammatory conditions such myocarditis, autoimmune disorders, infections, and cardiac surgery [10]. A potentially fatal ventricular arrhythmia called ventricular tachycardia (VT) can cause sudden cardiac death [11]. A slowing heartbeat, termed bradyarrhythmia, can cause syncope or cardiac arrest [12].
William Osler first identified the link between inflammation and arrhythmogenesis in 1896 when he saw cases of AF after rheumatic fever, a systemic inflammatory disease brought on by a streptococcal infection [9].
An increasing body of research indicates that inflammation and arrhythmogenesis are influenced by one another and affect one another's trajectory. Arrhythmias can be initiated or exacerbated by inflammation, which can change the electrophysiological properties of cardiac cells, such as ion channel expression, membrane potential, calcium handling, and gap junction coupling [13]. Apoptosis, hypertrophy, and fibrosis are examples of structural changes that inflammation may cause in cardiac tissue [14]. It can also affect the autonomic nervous system, which can have an influence on the neural regulation of heart rhythm [15].
On the other hand, arrhythmias may cause or worsen inflammation by injuring the heart through mechanical strain, ischemia–reperfusion, or oxidative stress [16]. Arrhythmias can also impact the brain, kidneys, lungs, and other organs in addition to the heart, which can impact inflammatory pathways [17]. Moreover, arrhythmias may delay the release of inflammatory mediators from the circulation, so extending their systemic effects [15].
The aim of this narrative review is to clarify the intricate connection between inflammation and arrhythmogenesis, highlighting the numerous ways that both localized and systemic inflammation can affect the onset and course of different cardiac arrhythmias. The purpose of the review is to highlight prospective treatment strategies and emphasize the significance of taking inflammation into account as a critical aspect in the management of arrhythmias by examining the relationship between inflammatory mediators and cardiac electrophysiology.
Main body
Inflammation and arrhythmogenesis: mechanistic insights
There are several causes of systemic inflammation, including sepsis, trauma, surgery, and long-term illnesses. There are many ways in which systemic inflammation might raise the risk of arrhythmias.
First, oxidative stress and cardiac cell damage from systemic inflammation might result in decreased electrical conduction and heightened vulnerability to reentry circuits [10]. Oxidative stress is the result of an imbalance between the body's generation of reactive oxygen species (ROS) and antioxidants' capacity to neutralize the detrimental effects of ROS [10, 18]. This oxidative stress can lead to inflammation and damage to the heart's tissue, which can accelerate the development of heart failure [10, 18].
Second, the autonomic nervous system (ANS) might have its equilibrium upset by systemic inflammation [19, 20]. Arrhythmias may be brought on or made worse by this imbalance [19, 20]. Through its sympathetic and parasympathetic branches, the ANS controls both innate and adaptive immunity. An imbalance in this system can lead to an altered inflammatory response, which is commonly seen in long-term illnesses such systemic autoimmune disorders [20].
Third, since potassium, calcium, and magnesium are necessary for a proper ventricular action potential, systemic inflammation may have an impact on these electrolyte levels [13]. The most prevalent intracellular cation in the body, potassium keeps muscle and nerve cells excitable [21]. Variations in potassium concentrations can impact an action potential's ability to conduct, which, in severe cases, can result in ventricular tachycardia [21].
Fourth, systemic inflammation has the potential to trigger the coagulation system and raise the risk of thromboembolism, both of which can result in myocardial infarction and ischemia [13]. Increased thrombin production, improved platelet activation, downregulation of anticoagulant regulatory proteins, and elevation of procoagulant factors such as tissue factor and cellular adhesion molecules can all result from inflammation [22]. This may lead to the creation of a blood clot, which may cause ischemia, or the limitation of oxygen and blood flow, if it breaks off and plugs an artery [22].
Systemic inflammatory markers were found to have high correlations with the development of atrial fibrillation/flutter (AF), ventricular arrhythmia (VA), and bradyarrhythmia in a research including 478,524 participants from the UK Biobank cohort [10] (Table 1). C-reactive protein (CRP) levels were found to have an almost linear positive connection with the incidence of different arrhythmias after correcting for all possible confounding factors [10]. The greatest correlation was seen with VA, and then AF and bradyarrhythmia in that order [10].
Specific infections include bacterial, fungal, or viral infections as well as autoimmune disorders can result in localized inflammation. Arrhythmias can result from localized inflammation through a number of mechanisms:
First, there is a chance that localized inflammation will cause structural remodeling in cardiovascular tissue, in addition to processes like necrosis, apoptosis, and fibrosis [5, 23]. These changes result in heterogeneous and anisotropic regions by affecting the electrical connection and propagation between cardiac cells [5, 23]. Increased dispersion in conduction velocity (CV) and steeper CV restitution slopes are two key outcomes of this structural remodeling that affect the stability of reentry [5, 23]. The accumulation of extra fibrous connective tissue within an organ is known as fibrosis. This may cause arrhythmias, alter the heart's natural architecture, and hinder its functionality [24].
Second, localized inflammation may result in inflammation of the ganglia or cardiac nerves, which may change how the heart's autonomic nervous system regulates itself [10]. It may have an impact on contractility, arrhythmogenicity, heart rate, and conduction velocity [25]. Neuropathic pain, which can result from inflammation, can also set off cardiac arrhythmias and reflexes [25].
Third, localized inflammation may directly harm the heart's cells, impairing their ability to handle calcium, boosting their automaticity, or making them more susceptible to catecholamines [10]. A vital component of cardiac muscle cell contraction is calcium. Increased sarcoplasmic reticulum (SR) calcium leak and reduced SR calcium absorption brought on by inflammatory cytokines can both lead to cytosolic calcium excess [26]. This capacity to induce spontaneous calcium waves and delayed after-depolarizations (DADs) may lead to arrhythmias [27].
Fourth, localized inflammation may compress or infiltrate the cardiac conduction system, including the His-Purkinje system, the atrioventricular node, and the sinoatrial node, which may result in heart block or bradyarrhythmias [28].
Conditions like endocarditis, myocarditis, or pericarditis may result from localized inflammation that targets specific heart components [29]. Endocarditis can cause valve destruction, leakage, or constriction. It is usually caused by bacterial or fungal infections that travel through the circulation and cling to the valves. These changes interfere with the heart's electrical impulses as well as blood flow [30, 31]. Furthermore, endocarditis can result in vegetation or blood clots, which are masses made of bacteria and cells that can separate and spread to vital organs like the brain or lungs and cause dangerous consequences [32]. Myocarditis can also result from autoimmune diseases, allergic responses, poisons, or parasites [33]. It is frequently caused by viral infections that damage the heart. Myocarditis can also weaken the heart muscle, impairing its ability to contract and relax, which can affect blood pressure and the electrical activity of the heart [34]. Arrhythmias may result from the obstruction or slowing down of electrical impulses caused by scar tissue growth in the heart as a result of myocarditis [34]. Although viral infections are usually the cause of pericarditis, other causes include renal failure, rheumatoid arthritis, inflammatory disorders, trauma, radiation, or drugs [35]. One of the effects of pericarditis is a buildup of fluid in the pericardial region, which puts pressure on the heart and reduces its functionality [36]. Additionally, pericarditis may cause the pericardium to thicken and harden, which would limit the heart's motion and disrupt electrical impulses [37].
Here are a few instances of both localized and systemic cardiac inflammation, along with the corresponding arrhythmias:
When the body's reaction to an infection results in widespread inflammation and organ malfunction, it can lead to sepsis, a potentially fatal illness [38]. Myocardial depression, or a reduction in the heart's capacity to pump blood, and systemic inflammatory response syndrome (SIRS), a condition of hyperinflammation that can harm heart tissue, are two ways that sepsis can impact the heart [38]. In addition, hypoxia, hypotension, acid–base imbalances, and electrolyte abnormalities can all result from sepsis and disrupt the heart's regular electrical activity, leading to arrhythmias [38]. An autoimmune condition that damages and inflames joints is rheumatoid arthritis (RA). The heart is among the numerous organs that RA can impact [39]. Because RA can induce inflammation of the heart's muscle (myocarditis), lining (pericarditis), or electrical system (conduction system), it can raise the risk of arrhythmias, or irregular heartbeats [39]. In addition, RA can raise the risk of heart failure and coronary artery disease by causing atherosclerosis, or the accumulation of plaque in the arteries [39]. Another autoimmune condition that can impact several organs, such as the skin, kidneys, lungs, and heart, is systemic lupus erythematosus (SLE) [40]. Heart muscle, pericardium, blood arteries, and heart valves can all become inflamed and damaged as a result of SLE [40]. Anti-phospholipid syndrome, a disorder that makes blood clot more readily, and an increased risk of heart attack and stroke can also be brought on by SLE [40]. People with SLE may have arrhythmias as a result of these causes [40].
Other complex and varied physiological and molecular mechanisms that link arrhythmogenesis and inflammation include:
Cardiac ion channels, which include sodium, potassium, calcium, and chloride, are impacted by inflammation and control cardiac action potentials (APs) [41]. Inflammatory cytokines such as interleukin-1 beta (IL-1-beta) and tumor necrosis factor alpha (TNF-alpha) play a key role in altering the expression and functionality of these ion channels [41, 42]. Specifically, these cytokines can reduce the expression and function of sodium, calcium, and potassium channels, resulting in a shorter action potential (AP) duration and greater repolarization dispersion [41, 42]. The impact extends to the facilitation of early after-depolarizations (EADs) and the occurrence of reentrant arrhythmias [43]. Pro-inflammatory cytokines such TNF-α, IL-1β, and IL-6 also contribute to longer action potential duration, decreased excitability, and greater dispersion of repolarization in addition to their direct effects on ion channels [41, 42]. These cytokines also cause reactive oxygen species to be produced, which exacerbates ion channel dysfunction and results in oxidative stress [41, 42]. They also have an impact on how other cytokines, such as interleukin-10 (IL-10), which has anti-inflammatory and anti-arrhythmic qualities, are expressed and activated [41, 42].
Another study that used a computational model of the human ventricle showed that arrhythmogenic changes in the action potential and the pseudo-electrocardiogram, such as prolonged QT interval, early after-depolarizations, and reentry, could be caused by the effects of TNF-α, IL-1β, and IL-6 on the ion channels [13]. In patients with a history of myocardial infarction and increased C-reactive protein, the suppression of inflammation may lower the risk of cardiovascular events, including arrhythmias, according to a randomized controlled trial utilizing canakinumab, a monoclonal antibody that targets IL-1β [44].
The expression of connexin 43 (Cx43), a crucial part of gap junctions that mediate intercellular communication, can also be increased by inflammation [9]. Inflammatory kinases including protein kinase C (PKC) and p38 mitogen-activated protein kinase (MAPK) have the ability to phosphorylate Cx43, altering gap junction function and distribution [9]. This may have an impact on the electrical synchronization and coupling of heart cells [2].
The role of cardiac bitter taste receptors (TAS2Rs) in the pathogenesis of heart diseases is discussed in a study titled “Cellular mechanisms and molecular pathways linking bitter taste receptor signalling to cardiac inflammation, oxidative stress, arrhythmia, and contractile dysfunction in heart diseases” [43]. According to the study by Menizibeya et al. [43], abnormal TAS2R signaling may put people at risk for developing cardiac inflammatory and oxidative stress diseases, which are marked by arrhythmia and contractile dysfunction. According to the same study, cardiac TAS2Rs serve as gateway surveillance systems that monitor and identify toxins or pathogens, including microbial components, and then launch reactions that eventually result in the host being protected against aggression [43].
Swelling in inflammation: implications for cardiac function
A coordinated reaction to tissue damage or infection, inflammatory swelling is characterized by elevated blood flow and enhanced capillary permeability [45]. White blood cells, proteins, and fluid from the circulation may migrate into the interstitial space thanks to this well planned mechanism, which aids in infection prevention and promotes healing. On the other hand, edema, or an abnormal buildup of fluid in tissue spaces, can result from excessive fluid leakage from capillaries [46].
Through a variety of processes, edema can then affect the electrolyte balance as well as the mechanical and electrical activities of the heart [47]. Edema's modification of the distribution and concentration of electrolytes in bodily fluids can lead to either an excess or a shortage of certain electrolytes, such as hyponatremia (low sodium levels) or hyperkalemia (high potassium levels), which can affect the heart and other nerve and muscle functions [47].
Additionally, the electrical conduction system of the heart, which controls heart rate and rhythm, may be hampered by edema [48]. Hypoxia can result from edema's buildup of fluid in the lungs, which can hinder gas exchange [48]. In consequence, hypoxia reduces the oxygen delivery to cardiac cells, which modifies their excitability and membrane potential [48]. Furthermore, hypoxia can increase reactive oxygen species (ROS) generation, which can harm cardiac cells and promote arrhythmias [7, 48].
Arrhythmias can be facilitated by heterogeneity caused by edema-induced changes in plasma volume and osmolarity, which can also affect blood and cardiac cells' electrolyte levels [7, 49]. Stretching cardiac cells mechanically triggers mechanosensitive ion channels and receptors [7, 50]. These elements alter intracellular calcium levels, which has an impact on cardiac cells' excitability and contractility [7, 50]. Pro-arrhythmic effects are further enhanced by the release of neurohormones and cytokines generated by stretching [7, 50]. Stretch also modifies extracellular matrix and gap junctions, affecting heart tissue's structural integrity and electrical coupling [7, 50].
Edema affects not only the cellular and electrical components of cardiac function but also the mechanical functions of the heart by raising heart rate and effort [51]. Heart failure, a disease in which the heart is unable to pump enough blood to fulfill the body's demands, can be caused by or result from this increased strain [51, 52]. Heart failure symptoms include exhaustion, swelling, and shortness of breath. It can also present as a fluid buildup in the legs and abdomen (peripheral edema) or in the lungs (pulmonary edema) [51, 52].
Role of immune responses
Macrophages are a key player in the complex regulation of immune cells in the setting of inflammation and arrhythmogenesis. They are particularly important in regulating the electrical characteristics of cardiomyocytes, which are the main cells in charge of cardiac contraction and conduction [6, 53]. Based on their origin, phenotype, and function, cardiac macrophages are a heterogeneous population that may be categorized as resident or recruited [6, 53]. Originating from embryonic and fetal progenitors, resident macrophages regulate cardiac electrophysiology by preserving tissue homeostasis in steady-state settings [53, 54]. On the other hand, when the heart is injured or infected, recruited macrophages that are sourced from circulating monocytes enter the heart and aid in tissue healing and inflammation [53, 54].
Myocardial infarction (MI), where a blood supply stoppage results in tissue damage and necrosis, is a powerful example of the sequential activation of immune cells in the setting of inflammation and arrhythmogenesis [6]. Damaged cardiomyocytes after MI produce signals of danger, which trigger the innate immune system and start an inflammatory response [6]. The first immune cells to reach the infarcted region are neutrophils, which phagocytose dead cells and debris. This process releases reactive oxygen species and proteases, which worsen tissue damage [6]. Granule protein production during neutrophil degranulation has been linked to a number of outcomes throughout the MI process [55]. Notably, cardiac neutrophils raised the risk of arrhythmia in a mouse model of ventricular tachycardia following MI, indicating a connection between their presence and a pro-arrhythmic milieu [56].
After that, monocytes infiltrate the tissue and differentiate into macrophages, which polarize into pro- or anti-inflammatory (M1) phenotypes depending on the tissue [6]. By producing pro-inflammatory cytokines, M1 macrophages lead to tissue damage, fibrosis, and compromised heart function [6, 57]. On the other hand, by generating growth factors and anti-inflammatory cytokines, M2 macrophages aid in tissue regeneration and repair [6, 57]. For infarcted tissue repair and inflammation resolution, the delicate balance between M1 and M2 macrophages is essential [6, 57].
Following MI, the remodeling and inflammatory processes affect the electrical characteristics of the heart as well, resulting in a pro-arrhythmic substrate that increases the risk of ventricular arrhythmias [58]. Immune cells may affect arrhythmogenesis through a variety of mechanisms, some of which are direct (via gap junctions), indirect (through cytokines and chemokines changing the function of ion channels in cardiomyocytes), and macrophage-mediated modulation of cardiac sympathetic nervous system (SNS) activity [6, 53, 57, 59].
An association between increased neutrophil, basophil, and lymphocyte counts and atrial fibrillation was shown in a Mendelian randomization research [60]. More specifically, an increased incidence of atrial fibrillation was linked to genetically predicted elevations in CD4 + T cell numbers [60]. These results emphasize even more the complex interplay of immune cells, inflammation, and arrhythmogenesis in a range of heart diseases.
Clinical implications
Arrhythmia management and therapy may be impacted by inflammation in a number of different ways.
First, inflammation can alter how the body reacts to anti-arrhythmic medications or device therapy, including catheter ablation or implanted cardioverter defibrillators (ICDs) [2]. For instance, in specific circumstances, such as atrial fibrillation (AF) or postoperative arrhythmias, some anti-inflammatory medications, such as corticosteroids or colchicine, may have anti-arrhythmic benefits [2]. However, several anti-arrhythmic medications, like amiodarone and sotalol, have the potential to promote inflammation or interact with inflammatory markers [9]. In addition, inflammation could make it more likely that device implantation or ablation procedures would result in problems or infections [2].
Second, arrhythmias can be diagnosed using inflammation as a biomarker. It has been demonstrated that a number of inflammatory markers, including C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-alpha), and monocyte chemoattractant protein-1 (MCP-1), are linked to the occurrence or recurrence of arrhythmias, including atrial fibrillation (AF), ventricular tachycardia (VT), and bradyarrhythmia [61]. These indicators can be tested in blood samples or found using imaging methods like magnetic resonance imaging (MRI) or positron emission tomography (PET) [62]. Inflammatory indicators can aid in identifying individuals who are at a high risk for arrhythmias or help with therapy selection.
Third, new treatment approaches for arrhythmias may address inflammation. For the prevention or treatment of arrhythmias, a number of anti-inflammatory medications, including statins, omega-3 fatty acids, colchicine, corticosteroids, and biologics, have undergone clinical trials [63].
More research is required to identify the ideal timing, dosage, duration, and safety of these therapies because the outcomes thus far have been contradictory or inconclusive.
Clinical consequences of inflammation-induced ECG alterations include:
Heightened mortality and cardiovascular disease (CVD) risk
Atherosclerosis, endothelial dysfunction, and left ventricular dysfunction are more common in patients with chronic inflammatory illnesses. These conditions can result in coronary heart disease, stroke, heart failure, and sudden cardiac death [64].
Diagnostic challenges
In patients with chest discomfort and increased cardiac biomarkers, in particular, inflammation-induced alterations in the ECG may resemble acute coronary syndromes [65, 66]. Therefore, it's critical to employ further testing, such as echocardiography, cardiac magnetic resonance imaging, or coronary angiography, to distinguish between ischemia and non-ischemic causes of ECG alterations [65, 66].
Therapeutic implications
To lessen cardiac inflammation and stop more damage, inflammation-induced alterations in ECG may point to the need for anti-inflammatory therapy with corticosteroids, non-steroidal anti-inflammatory medications, or biologic agents [67]. Furthermore, intensive care of classic CVD risk factors, such as dyslipidemia, hypertension, smoking, and diabetes, may be beneficial for individuals with chronic inflammatory illnesses [67].
The following are the consequences of inflammation-induced arrhythmogenesis in the electrocardiogram (ECG):
Prolonged QT interval
Inflammation can impact cardiomyocytes' ion channel function, resulting in a delayed repolarization and a prolonged action potential duration (APD) [9, 13]. An ECG manifestation of this might be a longer QT interval, which raises the possibility of torsades de pointes (TdP), a kind of ventricular tachycardia [68].
Enhanced heterogeneity
Inflammation can also result in transmural and regional variations in the electrical characteristics of the heart tissue, including dispersion of repolarization, refractoriness, and conduction velocity [9, 13]. As a common cause of arrhythmias, this may enhance the heterogeneity of the heart tissue and encourage the creation and maintenance of reentrant circuits [68].
Reduced adaptability
When there is inflammation, the heart's tissue may be less able to adjust to variations in heart rate, which may occur during stress or exercise [13]. This may lead to a decreased rate adaptation of the QT and APD intervals, which may make a person more vulnerable to arrhythmias in either a physiological or pathological setting [68].
Therapeutic interventions
Atrial fibrillation (AF), ventricular tachycardia (VT), and sudden cardiac death (SCD) are a few arrhythmias that can be prevented or treated by reducing inflammation. Several of these strategies include:
Anti-inflammatory drugs
These consist of corticosteroids, NSAIDs, colchicine, statins, and biologics, among others. They may be used to treat inflammatory cardiomyopathies that increase the risk of arrhythmias, such as cardiac sarcoidosis, myocarditis, and rheumatic heart disease [16]. By modifying the electrophysiological characteristics of cardiac cells, such as ion channel function, calcium handling, and gap junction coupling, they may also have anti-arrhythmic effects [69]. Anti-inflammatory medicine effectiveness and safety for the prevention or treatment of arrhythmias, however, are only partially and inconsistently supported by data. Following cardiac surgery, corticosteroids or colchicine have been demonstrated in certain trials to be helpful in preventing AF [69], whereas NSAIDs or biologics have been linked to an increased risk of AF [69, 70]. Anti-inflammatory medications can also have negative side effects, including bleeding, infections, and metabolic disturbances [69].
Lifestyle modifications
These include of reducing body weight, working out, making dietary adjustments, quitting smoking, and managing stress. They could assist in lowering blood pressure and cholesterol levels, improving endothelial function and autonomic balance, and reducing inflammation and oxidative stress [20, 70]. The prevalence and burden of AF in obese people can be decreased with lifestyle changes, according to several research [70, 71]. Additionally, they could enhance the results of catheter ablation for AF or VT [70, 71]. However, lifestyle changes need long-term compliance and behavioral adjustments, which may be difficult for certain individuals. For best effects, they might also need to be paired with pharmacological or interventional therapy [70, 71].
Other interventions
These include implanted cardioverter defibrillators (ICDs), catheter ablation, cardiac resynchronization treatment (CRT), and anti-arrhythmic medications. The underlying causes or initiators of arrhythmias, including as ischemia, scar tissue, reentry circuits, or ectopic foci, may be treated with them [69,70,71]. By changing the generation of cytokines, immune cell infiltration, or neuro-hormonal activation, they may also modify the inflammatory response [69]. These procedures could be restricted by procedural issues, device issues, infections, recurrent arrhythmias, or pro-arrhythmic side effects [69,70,71]. They might also be affected by the level of inflammation or its presence. By increasing atrial fibrosis or electrical heterogeneity, inflammation, for instance, may reduce the effectiveness of catheter ablation or anti-arrhythmic medications [69].
The kind, etiology, and severity of the arrhythmias, as well as the patient's features and comorbidities, may all affect how effective certain treatment techniques are. As a result, it's critical to customize the treatment plan based on the risk–benefit analysis and patient preferences. Several general ideas are:
Anti-inflammatory medications
They may be helpful for individuals with inflammatory cardiomyopathies or postoperative AF in terms of arrhythmia prevention or therapy [72]. In individuals who may be at risk for drug interactions or for whom there are contraindications, they should be taken with caution [72]. Additionally, they need to be watched for any negative effects and stopped as necessary [71].
Lifestyle changes
The prevention or treatment of arrhythmias in people with obesity, hypertension, diabetes mellitus, or metabolic syndrome may be aided by lifestyle changes [73]. However, they must be used in conjunction with a thorough program to lower cardiovascular risk, which may also include, if needed, pharmacological or interventional therapy [73]. Additionally, behavioral therapy and follow-up should be provided [70].
Other interventions
In patients with structural heart disease, ischemic heart disease, heart failure, or refractory arrhythmias, they may be beneficial in preventing or treating arrhythmias [74]. However, they should be chosen in accordance with evidence-based recommendations and guidelines that take patient eligibility requirements and procedure results into account [74]. Inflammatory indicators and modifiable risk factors that might reduce their effectiveness should also be addressed [70, 71].
In the area of cardiac electrophysiology, there are a number of difficulties and constraints in applying research findings from fundamental science to clinical practice. Among them are:
Lack of relevant animal models [69, 75, 76]
Animal models are frequently employed to investigate the workings of inflammation and how it affects arrhythmogenesis. They might not, however, accurately reflect human physiology, illness, or genetics. They could also react differently to treatments or medications. Therefore, it's possible that the conclusions drawn from research on animals cannot be applied to or generalized to patients who are human.
Lack of standardized definitions and measurements [69, 75, 76]
The process of inflammation is intricate and multifaceted, including several cellular and molecular elements, pathways, and interactions. On how to define, measure, or quantify inflammation in connection to arrhythmias, there is no agreement, though. Inflammation may be measured using various biomarkers, imaging techniques, or criteria depending on the study. As a result, the findings from various research might not be comparable or reliable.
Lack of large-scale clinical trials [69, 75, 76]
To assess the effectiveness and safety of therapy strategies for arrhythmias, clinical trials are crucial. However, there aren't many extensive clinical studies that particularly discuss how inflammation plays a part in managing arrhythmias. The majority of currently conducted studies are modest, observational, or retrospective. Additionally, they could contain methodological flaws such bias in selection, confounding variables, or heterogeneity in interventions or results.
Lack of multidisciplinary collaboration and communication [75, 77]
Collaboration and communication between researchers from several fields, including fundamental science, clinical medicine, epidemiology, biostatistics, and bioinformatics, are essential for translational research. The knowledge transfer, feedback, or integration across different fields may, however, face obstacles or gaps. The involvement or engagement of doctors, patients, or other stakeholders in the study process may also be lacking.
Future directions and research gaps
Areas that require further study to broaden our comprehension include:
The ways in which cardiomyopathies and inflammatory diseases cause cardiac electrical remodeling and arrhythmogenesis [2, 54].
Modulation of cardiac ion channels, gap junctions, calcium handling, and fibrosis by certain inflammatory cytokines, chemokines, and immune cells [15, 54]; connections between inflammation and other arrhythmia risk factors, including oxidative stress, metabolic abnormalities, genetic mutations, and autonomic imbalance [9, 54]; and the ongoing impacts of anti-inflammatory medications on the detection and management of arrhythmias in patients with inflammatory heart diseases [2, 54].
Future research approaches and possible directions include:
Identifying biomarkers of inflammation and arrhythmia risk in individuals with diverse inflammatory heart diseases is the goal of prospective cohort studies [2, 9].
Randomized controlled studies to assess the effectiveness and safety of new anti-inflammatory medications, including colchicine, statins, glucocorticoids, and biologics, in lowering arrhythmia burden and improving outcomes in patients with inflammatory heart diseases [2].
Preclinical animal models to examine novel treatment approaches and unravel the molecular and cellular underpinnings of inflammation-induced arrhythmogenesis [15, 54].
The effects of inflammation and anti-inflammatory therapies on cardiac electrophysiology may be predicted using computational models that include multi-scale data from research on the genomic, proteomic, metabolomic, and electrophysiological levels [9].
Addressing these deficiencies will have the following effects on improving cardiac care:
Utilizing innovative biomarkers and imaging tools to enhance the diagnosis of inflammatory cardiac disorders and the risk classification of patients with arrhythmias [2, 9].
Creating individualized and focused anti-inflammatory treatments that can control the inflammatory pathways implicated in arrhythmogenesis without endangering the immune system or having negative effects [2].
Improving knowledge of the intricate connection between arrhythmogenesis and inflammation as well as the potential advantages of anti-inflammatory treatments for other cardiovascular illnesses such atherosclerosis, heart failure, and stroke [9, 54].
Conclusions
As a result, this narrative review has compiled a plethora of information about inflammation and its complex connection to arrhythmogenesis. Through a number of pathways, inflammation—whether systemic or localized—has a significant impact on cardiac electrophysiology. Arrhythmogenic events can be more likely as a result of oxidative stress-induced damage to cardiac cells, changes to the balance of the autonomic nervous system, imbalances in electrolyte levels, and activation of the coagulation system.
The review has also brought attention to the crucial role that immune cells—particularly macrophages—play in regulating the heart's electrical characteristics, offering fresh perspectives on the cellular and molecular mechanisms that support the link between inflammation and irregular heartbeats. It emphasized the importance of cytokine profile disruptions, including IL-1β, TNF-α, and IL-6, which can modify cardiac ion channel function and connexin expression, which in turn shapes the ventricular action potential and increases the risk of arrhythmia.
It is clear from clinical implications to therapeutic treatments that inflammation plays a critical role in the onset, development, and control of many arrhythmias, including as bradyarrhythmias, VT, and AF. In order to reduce arrhythmogenesis, this study highlights the necessity of increased clinical awareness and all-encompassing techniques for managing inflammation. Although they have been somewhat successful, anti-inflammatory medicines have demonstrated potential, which emphasizes the need for greater research into more specialized and effective treatments.
Furthermore, the results imply that improved patient outcomes may result from taking anti-inflammatory therapies into account and include inflammatory markers in risk assessment. A better understanding of inflammation's function in arrhythmias might lead to both a decrease in the frequency of arrhythmias and an improved method of treating those that already exist.
By creating standardized techniques for assessing inflammation, carrying out extensive clinical trials with sound methodology, and encouraging multidisciplinary partnerships that can convert lab results into clinical practice, future research should try to close the existing gaps. By doing this, we might potentially usher in a new age of cardiac treatment, one that better protects patient health by efficiently combating the widespread effects of inflammation on the heart’s rhythm.
Availability of data and materials
All data and materials used in this research are freely available in electronic databases of PubMed, Google scholar and Elsevier. References have been provided.
Abbreviations
- CVDs:
-
Cardiovascular diseases
- AF:
-
Atrial fibrillation
- VT:
-
Ventricular tachycardia
- AV node:
-
Atrioventricular node
- ROS:
-
Reactive oxygen species
- ANS:
-
Autonomic nervous system
- CRP:
-
C-reactive protein
- MI:
-
Myocardial infarction
- CV:
-
Conduction velocity
- SR:
-
Sarcoplasmic reticulum
- DADs:
-
Delayed after-depolarizations
- SIRS:
-
Systemic inflammatory response syndrome
- RA:
-
Rheumatoid arthritis
- SLE:
-
Systemic lupus erythematosus
- APs:
-
Action potentials
- IL-1β:
-
Interleukin 1 beta
- TNF-α:
-
Tumor necrosis factor alpha
- IL-6:
-
Interleukin 6
- IL-10:
-
Interleukin 10
- Cx43:
-
Connexin 43
- PKC:
-
Protein kinase C
- MAPK:
-
Mitogen-activated protein kinase
- TAS2Rs:
-
Bitter taste receptors
- ICDs:
-
Implanted cardioverter defibrillators
- CRT:
-
Cardiac resynchronization therapy
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- TdP:
-
Torsades de pointes
- VEGF:
-
Vascular endothelial growth factor
- IGF-1:
-
Insulin-like growth factor 1
- TGF-β:
-
Transforming growth factor beta
- SNS:
-
Sympathetic nervous system
References
Harvard Health Publishing. Understanding acute and chronic inflammation. Harvard Health. 2020 (https://www.health.harvard.edu/staying-healthy/understanding-acute-and-chronic-inflammation). https://www.health.harvard.edu/staying-healthy/understanding-acute-and-chronic-inflammation. Accessed 5 Dec 2023.
Whayne TF Jr, Morales GX, Darrat YH. Clinical aspects of systemic inflammation and arrhythmogenesis, especially atrial fibrillation. Angiology. 2018;69(4):281–5. https://doi.org/10.1177/0003319717721399.
Drozd M, Pujades-Rodriguez M, Morgan AW, Lillie PJ, Witte KK, Kearney MT, Cubbon RM. Systemic inflammation is associated with future risk of fatal infection: an observational cohort study. J Infect Dis. 2022;226(3):554–62. https://doi.org/10.1093/infdis/jiac186.
Zhu M, Chen L, Kong X, Wang X, Fang Y, Li X, Wang J. The systemic inflammation response index as an independent predictor of survival in breast cancer patients: a retrospective study. Front Mol Biosci. 2022;28(9):856064. https://doi.org/10.3389/fmolb.2022.856064.
Boyalla V, Gallego-Colon E, Spartalis M. Immunity and inflammation in cardiovascular disorders. BMC Cardiovasc Disord. 2023;23(1):148. https://doi.org/10.1186/s12872-023-03185-z.
Xia R, Tomsits P, Loy S, Zhang Z, Pauly V, Schüttler D, Clauss S. Cardiac macrophages and their effects on arrhythmogenesis. Frontiers in Physiology. 2022:1227. https://doi.org/10.3389/fphys.2022.900094
Karpawich PP. Pathophysiology of cardiac arrhythmias: arrhythmogenesis and types of arrhythmias. Pathophysiology and Pharmacotherapy of Cardiovascular Disease. 2015:1003–14. https://doi.org/10.1007/978-3-319-15961-4_47
Jones CA, Wallace MJ, Bandaru P, Woodbury ED, Mohler PJ, Wold LE. E-cigarettes and arrhythmogenesis: a comprehensive review of pre-clinical studies and their clinical implications. Cardiovasc Res. 2023;119(12):2157–64. https://doi.org/10.1093/cvr/cvad113.
Yalta T, Yalta K. Systemic inflammation and arrhythmogenesis: a review of mechanistic and clinical perspectives. Angiology. 2018;69(4):288–96. https://doi.org/10.1177/0003319717709380.
Yang X, Zhao S, Wang S, Cao X, Xu Y, Yan M, Pang M, Yi F, Wang H. Systemic inflammation indicators and risk of incident arrhythmias in 478,524 individuals: evidence from the UK Biobank cohort. BMC Med. 2023;21(1):76. https://doi.org/10.1186/s12916-023-02770-5.
Lionte C, Sorodoc V, Haliga RE, Bologa C, Ceasovschih A, Petris OR, Coman AE, Stoica A, Sirbu O, Puha G, Constantin M. Inflammatory and cardiac biomarkers in relation with post-acute COVID-19 and mortality: what we know after successive pandemic waves. Diagnostics. 2022;12(6):1373. https://doi.org/10.3390/diagnostics12061373.
Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(7):e51-156. https://doi.org/10.1016/j.jacc.2018.10.044.
Bi X, Zhang S, Jiang H, Ma W, Li Y, Lu W, Yang F, Wei Z. Mechanistic insights into inflammation-induced arrhythmias: a simulation study. Front Physiol. 2022;13:843292. https://doi.org/10.3389/fphys.2022.843292.
Rao M, Wang X, Guo G, Wang L, Chen S, Yin P, Chen K, Chen L, Zhang Z, Chen X, Hu X. Resolving the intertwining of inflammation and fibrosis in human heart failure at single-cell level. Basic Res Cardiol. 2021;116:1–9. https://doi.org/10.1007/s00395-021-00897-1.
Cheng WL, Li SJ, Lee TI, Lee TW, Chung CC, Kao YH, Chen YJ. Sugar fructose triggers gut dysbiosis and metabolic inflammation with cardiac arrhythmogenesis. Biomedicines. 2021;9(7):728. https://doi.org/10.3390/biomedicines9070728.
Karki R, Janga C, Deshmukh AJ. Arrhythmias associated with inflammatory cardiomyopathies. Curr Treat Opt Cardiovasc Med. 2020;22:1–5. https://doi.org/10.1007/s11936-020-00871-5.
Shirakabe A, Matsushita M, Shibata Y, Shighihara S, Nishigoori S, Sawatani T, Kiuchi K, Asai K. Organ dysfunction, injury, and failure in cardiogenic shock. J Intensive Care. 2023;11(1):26. https://doi.org/10.1186/s40560-023-00676-1.
Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S, Emdin M, Giannoni A. Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur J Prev Cardiol. 2020;27(5):494–510. https://doi.org/10.1177/2047487319870344.
Wang J, Liu W, Chen H, Liu C, Wang M, Chen H, Zhou H, Liu Z, Zhang S, Yu Z, Duan S. Novel insights into the interaction between the autonomic nervous system and inflammation on coronary physiology: a quantitative flow ratio study. Front Cardiovasc Med. 2021;8:700943. https://doi.org/10.3389/fcvm.2021.700943.
Bellocchi C, Carandina A, Montinaro B, Targetti E, Furlan L, Rodrigues GD, Tobaldini E, Montano N. The interplay between autonomic nervous system and inflammation across systemic autoimmune diseases. Int J Mol Sci. 2022;23(5):2449. https://doi.org/10.3390/ijms23052449.
Schupp T, Bertsch T, von Zworowsky M, Kim SH, Weidner K, Rusnak J, Barth C, Reiser L, Taton G, Reichelt T, Ellguth D. Prognostic impact of potassium levels in patients with ventricular tachyarrhythmias. Clin Res Cardiol. 2020;109:1292–306. https://doi.org/10.1007/s00392-020-01624-x.
Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;6:142. https://doi.org/10.3389/fped.2018.00142.
Banville I, Gray RA. Effect of action potential duration and conduction velocity restitution and their spatial dispersion on alternans and the stability of arrhythmias. J Cardiovasc Electrophysiol. 2002;13(11):1141–9. https://doi.org/10.1046/j.1540-8167.2002.01141.x.
Sutanto H, Heijman J. Integrative computational modeling of cardiomyocyte calcium handling and cardiac arrhythmias: current status and future challenges. Cells. 2022;11(7):1090. https://doi.org/10.3390/cells11071090.
Krishnappa D, Brignole M, Benditt DG. Cardioneuroablation for cardioinhibitory vasovagal syncope. syncope: an evidence-based approach. 2020:307–17. https://doi.org/10.1007/978-3-030-44507-2_26
Zhang XD, Thai PN, Lieu DK, Chiamvimonvat N. Model systems for addressing mechanism of arrhythmogenesis in cardiac repair. Curr Cardiol Rep. 2021;23(6):72. https://doi.org/10.1007/s11886-021-01498-z.
Antzelevitch C. Cellular, molecular, and pharmacologic mechanisms underlying drug-induced cardiac arrhythmogenesis. In: Cardiac safety of noncardiac drugs: practical guidelines for clinical research and drug development. Humana Press. 2005 pp. 37–66. https://doi.org/10.1007/978-1-59259-884-7_3
Lazzerini PE, Laghi-Pasini F, Boutjdir M, Capecchi PL. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat Rev Immunol. 2019;19(1):63–4. https://doi.org/10.1038/s41577-018-0098-z.
Zhang S, Lu W, Wei Z, Zhang H. Air pollution and cardiac arrhythmias: from epidemiological and clinical evidences to cellular electrophysiological mechanisms. Front Cardiovasc Med. 2021;8:736151. https://doi.org/10.3389/fcvm.2021.736151.
Rajani R, Klein JL. Infective endocarditis: a contemporary update. Clin Med. 2020;20(1):31. https://doi.org/10.7861/clinmed.cme.20.1.1.
McDonald EG, Aggrey G, Aslan AT, Casias M, Cortes-Penfield N, Dong MQ, Egbert S, Footer B, Isler B, King M, Maximos M. Guidelines for diagnosis and management of infective endocarditis in adults: a WikiGuidelines group consensus statement. JAMA Netw Open. 2023;6(7):e2326366. https://doi.org/10.1001/jamanetworkopen.2023.26366.
Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: a review. JAMA. 2018;320(1):72–83. https://doi.org/10.1001/jama.2018.7596.
Rroku A, Kottwitz J, Heidecker B. Update on myocarditis–What we know so far and where we may be heading. Eur Heart J Acute Cardiovasc Care. 2021;10(4):455–67. https://doi.org/10.1177/2048872620910109.
Pillay J, Gaudet L, Wingert A, Bialy L, Mackie AS, Paterson DI, Hartling L. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review. BMJ. 2022;378. https://doi.org/10.1136/bmj-2021-069445
Lazarou E, Tsioufis P, Vlachopoulos C, Tsioufis C, Lazaros G. Acute pericarditis: update. Curr Cardiol Rep. 2022;24(8):905–13. https://doi.org/10.1007/s11886-022-01710-8.
Hoit BD. Pericardial effusion and cardiac tamponade pathophysiology and new approaches to treatment. Curr Cardiol Rep. 2023;25(9):1003–14. https://doi.org/10.1007/s11886-023-01920-8.
Sohal S, Mathai SV, Lipat K, Kaur A, Visveswaran G, Cohen M, Waxman S, Tiwari N, Vucic E. Multimodality imaging of constrictive pericarditis: pathophysiology and new concepts. Curr Cardiol Rep. 2022;24(10):1439–53. https://doi.org/10.1007/s11886-022-01758-6.
Habimana R, Choi I, Cho HJ, Kim D, Lee K, Jeong I. Sepsis-induced cardiac dysfunction: a review of pathophysiology. Acute Crit Care. 2020;35(2):57–66. https://doi.org/10.4266/acc.2020.00248.
Jagpal A, Navarro-Millán I. Cardiovascular co-morbidity in patients with rheumatoid arthritis: a narrative review of risk factors, cardiovascular risk assessment and treatment. BMC rheumatology. 2018;2(1):1–4. https://doi.org/10.1186/s41927-018-0014-y.
Morand EF, Fernandez-Ruiz R, Blazer A, Niewold TB. Advances in the management of systemic lupus erythematosus. BMJ. 2023;383. https://doi.org/10.1136/bmj-2022-073980
Delisle BP, Aromolaran AS. New insights into cardiac ion channel regulation 2.0. Int J Mol Sci. 2023;24(5):4999. https://doi.org/10.3390/ijms24054999.
Takeuchi T. Cytokines and cytokine receptors as targets of immune-mediated inflammatory diseases—RA as a role model. Inflamm Regener. 2022;42(1):1–2. https://doi.org/10.1186/s41232-022-00221-x.
Welcome MO, Dogo D, Mastorakis NE. Cellular mechanisms and molecular pathways linking bitter taste receptor signalling to cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction in heart diseases. Inflammopharmacology. 2023;31(1):89–117. https://doi.org/10.1007/s10787-022-01086-9.
Pari B, Babbili A, Kattubadi A, Thakre A, Thotamgari S, Gopinathannair R, Olshansky B, Dominic P. COVID-19 vaccination and cardiac arrhythmias: a review. Curr Cardiol Rep. 2023;25(9):925–40. https://doi.org/10.1007/s11886-023-01921-7.
Mayo Clinic. Edema - Symptoms and causes. Mayo Clinic. 2021. https://www.mayoclinic.org/diseases-conditions/edema/symptoms-causes/syc-20366493. Accessed 9 Dec 2023.
Cleveland Clinic. Capillary Leak Syndrome. Cleveland Clinic. 2020. https://my.clevelandclinic.org/health/diseases/22712-capillary-leak-syndrome. Accessed 10 Dec 2023.
Rodriguez-Sanchez N, Galloway SD. A randomised trial to assess fluid and electrolyte balance responses following ingestion of different beverages in young and older men. Eur J Appl Physiol. 2023. https://doi.org/10.1007/s00421-023-05241-0.
Okada Y, Paton JF, López-Barneo J, Wilson RJ, Marina N, Pokorski M. Hypoxia and cardiorespiratory control. Front Physiol. 2021;12:820815. https://doi.org/10.3389/fphys.2021.820815.
Dull RO, Hahn RG. Hypovolemia with peripheral edema: What is wrong? Crit Care. 2023;27(1):1. https://doi.org/10.1186/s13054-023-04496-5.
Münch J, Abdelilah-Seyfried S. Sensing and responding of cardiomyocytes to changes of tissue stiffness in the diseased heart. Front Cell Dev Biol. 2021;9:403. https://doi.org/10.3389/fcell.2021.642840.
Cleveland Clinic. Electrolyte imbalance. Cleveland Clinic. 2020. https://my.clevelandclinic.org/health/symptoms/24019-electrolyte-imbalance. Accessed 12 Dec 2023.
Abassi Z, Khoury EE, Karram T, Aronson D. Edema formation in congestive heart failure and the underlying mechanisms. Front Cardiovasc Med. 2022;9:933215. https://doi.org/10.3389/fcvm.2022.933215.
Medical News Today. How does the immune system power inflammation? Medical News Today. 2023. https://www.medicalnewstoday.com/articles/247459#1. Accessed 12 Dec 2023.
Lin YN, Ibrahim A, Marbán E, Cingolani E. Pathogenesis of arrhythmogenic cardiomyopathy: role of inflammation. Basic Res Cardiol. 2021;116(1):39. https://doi.org/10.1007/s00395-021-00877-5.
Zhang N, Aiyasiding X, Li WJ, Liao HH, Tang QZ. Neutrophil degranulation and myocardial infarction. Cell Commun Signal. 2022;20(1):1–23. https://doi.org/10.1186/s12964-022-00824-4.
Lim GB. Macrophages and neutrophils modulate arrhythmia risk after myocardial infarction. Nat Rev Cardiol. 2022;19(9):573. https://doi.org/10.1038/s41569-022-00758-x.
Chen M, Li X, Wang S, Yu L, Tang J, Zhou S. The role of cardiac macrophage and cytokines on ventricular arrhythmias. Front Physiol. 2020;11:1113. https://doi.org/10.3389/fphys.2020.01113.
Frantz S, Hundertmark MJ, Schulz-Menger J, Bengel FM, Bauersachs J. Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies. Eur Heart J. 2022;43(27):2549–61. https://doi.org/10.1093/eurheartj/ehac223.
Carnagarin R, Kiuchi MG, Ho JK, Matthews VB, Schlaich MP. Sympathetic nervous system activation and its modulation: role in atrial fibrillation. Front Neurosci. 2019;12:1058. https://doi.org/10.3389/fnins.2018.01058.
Feng Y, Liu X, Tan H. Causal association of peripheral immune cell counts and atrial fibrillation: a Mendelian randomization study. Front Cardiovasc Med. 2023;9:1042938. https://doi.org/10.3389/fcvm.2022.1042938.
Zeppenfeld K, Tfelt-Hansen J, De Riva M, Winkel BG, Behr ER, Blom NA, Charron P, Corrado D, Dagres N, De Chillou C, Eckardt L. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2022;43(40):3997–4126. https://doi.org/10.1093/eurheartj/ehac262.
Pereira H, Niederer S, Rinaldi CA. Electrocardiographic imaging for cardiac arrhythmias and resynchronization therapy. https://doi.org/10.1093/europace/euaa165
Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79(3):495–502. https://doi.org/10.1253/circj.CJ-15-0138.
Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. 2015;36(8):482–9. https://doi.org/10.1093/eurheartj/ehu403.
Xanthopoulos A. and Skoularigis J. Diagnosis of acute pericarditis. E-Journal of Cardiology Practice. 2017. https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-15/Diagnosis-of-acute-pericarditis. Accessed 15 Dec 2023.
Shorikova DV and Shorikov EI. COVID-19 and acute coronary syndrome: emphasis on ACS without atherothrombosis1. E-Journal of Cardiology Practice. 2021. https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-21/covid-19-and-acute-coronary-syndrome-emphasis-on-acs-without-atherothrombosis. Accessed 15 Dec 2023.
Reina-Couto M, Pereira-Terra P, Quelhas-Santos J, Silva-Pereira C, Albino-Teixeira A, Sousa T. Inflammation in human heart failure: major mediators and therapeutic targets. Front Physiol. 2021;12:746494. https://doi.org/10.3389/fphys.2021.746494.
Ihara K, Sasano T. Role of inflammation in the pathogenesis of atrial fibrillation. Front Physiol. 2022;13:862164. https://doi.org/10.3389/fphys.2022.862164.
Vonderlin N, Siebermair J, Kaya E, Köhler M, Rassaf T, Wakili R. Critical inflammatory mechanisms underlying arrhythmias. Herz. 2019;44(2):121–9. https://doi.org/10.1007/s00059-019-4788-5.
Zhou X, Dudley SC Jr. Evidence for inflammation as a driver of atrial fibrillation. Front Cardiovasc Med. 2020;7:62. https://doi.org/10.3389/fcvm.2020.00062.
Barría PRM. The gap remains: the challenge of translating research into policies for the health care of people and communities. Investig Educ Enferm. 2017;35(2):129–30. https://doi.org/10.17533/udea.iee.v35n2a01.
Li KH, Tse G, Liu T, Yan GX. Anti-arrhythmic effects of non-anti-arrhythmic drugs or therapies. Management of Cardiac Arrhythmias. 2020:597–619. https://doi.org/10.1007/978-3-030-41967-7_26
Patel KH, Reddy RK, Sau A, Sivanandarajah P, Ardissino M, Ng FS. Obesity as a risk factor for cardiac arrhythmias. BMJ Med. 2022. https://doi.org/10.1136/bmjmed-2022-000308.
Steinberg DH, Staubach S, Franke J, Sievert H. Defining structural heart disease in the adult patient: current scope, inherent challenges and future directions. Eur Heart J Suppl. 2010;12(suppl_E):E2-9. https://doi.org/10.1093/eurheartj/suq012.
Diptyanusa A, Hasanbasri M. Lost in translation: barriers and progress in harnessing basic medical science into community practice in Indonesia. Transl Med Commun. 2020;5(1):1–5. https://doi.org/10.1186/s41231-020-00070-1.
Marques-Vidal P. Comparison of lifestyle changes and pharmacological treatment on cardiovascular risk factors. Heart. 2020;106(11):852–62. https://doi.org/10.1136/heartjnl-2019-316252.
Lau DH, Volders PG, Kohl P, Prinzen FW, Zaza A, Kaeaeb S, Oto A, Schotten U. Opportunities and challenges of current electrophysiology research: a plea to establish ‘translational electrophysiology’curricula. EP Europace. 2015;17(5):825–33. https://doi.org/10.1093/europace/euu301.
Acknowledgements
Not applicable.
Funding
This research has not received any financial support.
Author information
Authors and Affiliations
Contributions
AT presented the idea and planned its design and direction, then collected, organized and analyzed various data, and performed writing and final review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that she has no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taghdiri, A. Inflammation and arrhythmogenesis: a narrative review of the complex relationship. Int J Arrhythm 25, 4 (2024). https://doi.org/10.1186/s42444-024-00110-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42444-024-00110-z