Abstract
Background
In patients with non-paroxysmal AF (atrial fibrillation), various ablation strategies have been attempted to target non-pulmonary vein (PV) foci or to achieve substrate modification beyond pulmonary vein isolation. The efficacy of empirical ablation of the SVC, one of the most common non-PV foci, is unclear. The aim of this study was to investigate the efficacy and safety of additional superior vena cava (SVC) isolation in patients with non-paroxysmal AF undergoing thoracoscopic surgical ablation.
Methods/results
A total of 191 patients with persistent or long-standing persistent AF was enrolled. All patients underwent total thoracoscopic surgical ablation for AF, and half of them also received empirical SVC isolation. We compared the atrial tachyarrhythmia (ATa)-free survival rate and procedure-related complications in the two groups of patients. The 3-year ATa-free survival rate was 53% in the SVC isolation group and 52% in the no-SVC isolation group (p = 0.644). There were no differences between the two groups with respect to AF type or LA size. Procedure-related complications occurred in 12 patients (6%). Pacemakers were implanted only in three patients from the SVC isolation group. The only factor influencing recurrence of ATa was LA diameter.
Conclusions
Empirical SVC isolation during thoracoscopic ablation for persistent AF did not improve patient outcomes.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Pulmonary vein isolation (PVI) is the cornerstone of atrial fibrillation (AF) treatment [1]. However, despite complete PVI, some patients, especially non-paroxysmal AF patients, experience recurrence of AF [2, 3]. Many investigators have postulated that both substrate modification and trigger isolation are necessary for effective treatment of persistent or long-standing AF, and various ablation strategies, such as non-PV trigger, linear line, complex fractionated atrial electrogram (CFAE), or rotor ablation, have been assessed [4, 5].
Non-PV trigger sources are present in approximately 11% of patients with persistent and long-standing persistent AF [6, 7]. The superior vena cava (SVC) is one of the most common non-PV AF trigger sites, along with the coronary sinus, vein of Marshall, left atrial posterior wall, interatrial septum and crista terminalis. The SVC acts as both an initiator and perpetuator of AF [1, 8]. Previous studies have shown that arrhythmogenic SVC or empirical SVC isolation in combination with PVI improves clinical outcomes in paroxyamal AF patients [9,10,11]. However, the efficacy of SVC isolation in patients with persistent or chronic atrial fibrillation remains controversial [9].
Thoracoscopic surgical ablation has been advanced as an alternative for non-paroxysmal AF patients. This approach allows durable PVI through a direct epicardial approach, with the added benefit of ablating epicardial structures such as the ganglion plexus (GP) or Marshall's ligament, while excluding the left atrial appendage and limiting the risk of phrenic nerve injury [12]. However, in thoracoscpic ablation, non-PV trigger cannot be tested and only empirical ablation is available. To date, there have been no studies assessing the efficacy and safety of additional empirical SVC isolation in thoracoscopic surgical ablation for AF. The aim of this study was to investigate the effect of empirical SVC isolation in thoracoscopic surgical ablation of patients with non-paroxysmal AF.
Methods
Study population
We retrospectively analyzed patients who underwent thoracoscopic ablation for persistent or long-standing persistent atrial fibrillation at a single center from January 2012 to December 2018. Thoracoscopic ablation was indicated in patients with a high risk of catheter ablation failure such as long-standing persistent AF, persistent AF large LA size or long duration, or who failed prior catheter ablation. We screened 293 consecutive patients, and the following patients were excluded: (1) previous catheter or surgical ablation (n = 20), (2) incomplete thoracoscopic ablation due to thoracic adhesions (n = 15) and (3) hybrid approach with epicardial and endocardial ablation during index hospitalization (n = 67). A total of 191 patients were selected for this study, with 51% (n = 98) in the empirical SVC isolation group and 49% (n = 93) in the no-SVC isolation group. (Fig. 1). All data were collected through a review of medical records. This study was approved by the Institutional Review Board of Samsung Medical Center, and informed consent was waived (IRB No. 2020-06-159).
Surgical procedures
Total thoracoscopic ablation (TTA) refers to a video-assisted thoracoscopic surgical ablation technique using bipolar radiofrequency energy without the assistance of the Da Vinci system (Intuitive Surgical, Sunnyvale, CA, USA) or cardiopulmonary bypass. First, three ports were introduced on the right side. One 5-mm port was inserted into the fourth intercostal space, and CO gas was injected to expand the chest cavity and secure the surgical field. The remaining two ports were placed in the third intercostal space at the anterior axillary line and the sixth intercostal space at the midaxillary line. After pericardial tenting, a lighted dissector (AtriCure® Lumitip, Atricure, Inc., Cincinnati, OH, USA) was used to pass a rubber band under the antrum of the pulmonary vein (PV) through the oblique sinus. An AtriCure® Isolator® transpolar clamp was positioned around the PV antrum through a connection to the rubber band. Bipolar radiofrequency energy was delivered to the clamp six times to achieve PV isolation. Superior and inferior lines connecting the PV isolation lines were created using a linear pen device (MLP, Atricure, Inc.). Ganglionated plexuses were subsequently ablated with bipolar energy under high-frequency pacing. The procedure was performed in the same way on the left side. The endpoint of each ablation is the confirmation of bidirectional acute conduction block across pulmonary veins isolation defined as the absence of sensed atrial potentials in the PVs and pacing conduction to the atria from PVs in patients in sinus rhythm. A high-frequency stimulation is used at ablation, and its response is defined as ≥ 50% increase in the R-R interval. Using a bipolar ablation pen (Isolator Transpolar pen), the high-frequency stimulation is delivered (cycle length 60 ms, 16 Hz, pulse width 1.0 ms) with output increments from 1 to 25 mA. When the high-frequency stimulation does not evoke a vagal response, ablation is performed on the basis of anatomic landmarks. After PV and ganglionated plexus ablation, the ligament of Marshall was dissected and ablated. Once ablations were completed and conduction block was confirmed, the left atrial auricle was removed with an endoscopic stapling device. In patients in the SVC group, a clamp was placed above the SVC and right atrial junctions, and ablation was performed using the bipolar clamp previously used for pulmonary vein isolation. To avoid damage to the phrenic nerve, the phrenic nerve was excluded and circular ablation was performed only around the SVC. Because the ablation was made between the clamps, contact outside the clamps did not damage the surrounding tissue. Circular ablation of the SVC was performed twice following an exit block test. All procedures were performed by one experienced cardiac surgeon (DSJ). (Additional file 1: Fig. S1).
Follow-up
After surgery, all patients were monitored in the intensive care unit for the first 24 h. After confirming that there was no bleeding or pericardial effusion, heparin infusion was started 4 h after the operation; on the day after the operation, the heparin infusion was discontinued and the patient was switched to oral anticoagulation (non-vitamin K antagonist or warfarin). Antiarrhythmic drugs were continued after surgery. All patients had follow-up evaluations at 2 weeks, 3 months and 6 months postoperatively and every 6 months thereafter. At every visit, 12-lead electrocardiography (ECG) and 24-h Holter monitoring were performed. After a 3-month blanking period, recurrence was defined as the detection of any atrial tachyarrhythmias (ATa) including AF, atrial flutter (AFL) or atrial tachycardia (AT) occurring for a duration of more than 30 s. Cardioversion was performed as needed during the blanking period. In patients with recurrence of ATa, additional interventions, such as antiarrhythmic drugs, catheter ablation, or DC cardioversion, were performed in consideration of the patient's status. In general, antiarrhythmic drugs (AADs) were discontinued after 3 months if there was no evidence of recurrence. Oral anticoagulants (OACs) were continued postoperatively; in patients with no history of stroke, low CHA2DS2-VASc score and well-maintained sinus rhythm, OACs were discontinued on the decision of the attending physician. During all follow-up periods, complications were defined as events requiring additional interventions, medications, or that lengthened the duration of hospital stay.
Statistical analyses
Categorical variables are reported as numbers and percentages, while continuous variables are presented as the mean ± standard deviation (SD). For comparison between the two groups based on SVC isolation, a chi-square test or Fisher's exact test was used for categorical variables, and an independent sample T-test was used for continuous variables. The ATa-free survival rate was analyzed using the Kaplan–Meier method, and the survival rates of the two groups were compared using a log-rank test. Factors associated with ATa recurrence were identified using univariate and stepwise multivariate analyses in Cox regression. All variables with p values < 0.10 in the univariate analyses were entered into the multivariate analyses. A two-sided p value < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS® software (version 23.0; IBM, Armonk, NY).
Results
Baseline patient characteristics
Of the 191 total patients, 98 (51%) received SVC isolation (SVC isolation group), and 93 (49%) did not (no-SVC isolation group). The baseline patient characteristics are presented in Table 1. The mean age was 57 ± 8 years, and 89% were male. There were no significant difference in comorbidities between the two groups. The mean AF duration was 44 ± 44 months (IQR 10–68 months). Persistent AF and long-standing persistent AF accounted for 55% and 45% of the patient diagnoses, respectively. The SVC isolation group contained a significantly greater percentage of patients with long-standing persistent AF (53%) than did the no-SVC isolation group (36%, p = 0.015), and the LA size was larger (LA diameter 49 ± 6 vs. 46 ± 7 mm, p = 0.002). The mean left ventricular ejection fraction was 59 ± 8%.
Surgical procedure and complications
All patients underwent PVI. More than 90% received roof and posterior line ablation or ganglion plexus (GP) ablation, and there were no significant differences between the two groups with respect to the use of these procedures. Twenty-one patients (11%) also underwent additional linear ablation from the SVC to IVC. Marshall ligament division was performed in a lower percentage of patients in the no-SVC than in the SVC isolation group. Left atrial appendage (LAA) removal was performed in all but two patients (Table 2).
Procedure-related complications occurred in 12 patients (6%), and there were no differences between the two groups (7% vs. 6%, p = 0.925). There were no patients with phrenic nerve injury in either group. Pacemakers were implanted only in three patients from the SVC isolation group: Two of these patients had pre-existing sinus node dysfunction before ablation, and the other had a newly discovered arrhythmia after the procedure and had a pacemaker implanted. One patient died of an unknown cause one month after surgical ablation (Table 3).
Recurrence of atrial tachyarrhythmia
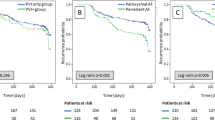
The overall mean follow-up duration was 32 ± 17 months. The 1-year and 3-year ATa-free survival rates were 68% and 53% in the SVC isolation group and 75% and 52% in the no-SVC isolation group, respectively, and there were no significant differences between the two groups (p = 0.644, Fig. 2). Of the 98 patients who underwent SVC isolation, 26 patients underwent redo catheter ablation due to AF recurrence. Incomplete SVC isolation was confirmed in 7 patients (27%) and additional ablation was performed, and complete SVC isolation was confirmed in the remaining 19 patients (73%). In subgroup analysis, there were also no differences according to AF type: At 3 years, no ATa recurrence was reported in 62% and 67% (p = 0.207) of patients with persistent AF and 43% and 38% (p = 0.211) of those with long-standing persistent AF in the SVC isolation and control groups, respectively (Fig. 3A, B). There were also no differences in ATa-free survival according to LA diameter (Fig. 3C, D). Table 4 presents the results of the univariate and multivariate analyses to identify determinants of ATa recurrence. The only factor influencing the recurrence of ATa was LA diameter in the multivariate analyses (hazard ratio 1.079, 95% confidence interval 1.040–1.121, p < 0.001).
Discussion
In this study, we investigated the efficacy and safety of additional SVC isolation when performing thoracoscopic surgical ablation in patients with non-paroxysmal AF. To our knowledge, this is the first study to evaluate the efficacy and safety of additional SVC isolation in surgical ablation for patients with AF. Our study demonstrated that empirical SVC isolation did not improve outcomes in patients with non-paroxysmal AF. A larger LA size was the only independent predictor of ATa recurrence, which was consistent with the results of previous studies. Complications occurred in 6% of patients. There were no patients with phrenic nerve injury, but three patients in the SVC isolation group required pacemaker implantation.
The SVC is one of the most common non-PV AF trigger sites. The SVC has been shown to act not only as an initiator, but also as a perpetuator of AF [8, 13]. During embryogenesis, the SVC originates from the sinus venosus, which is also the origin of the sinoatrial node [14]. This can explain the arrhythmogenicity of this structure. There have been many studies of SVC isolation using catheter ablation. In paroxysmal AF, empirical SVC isolation with PVI has been shown to improve the outcome of AF ablation in de novo or redo procedure. However, it did not improve outcomes in persistent AF [9, 11, 15, 16]. In a prospective randomized study, empirical SVC isolation improved Ata-free survival rate for paroxysmal AF (77% vs. 90%, p = 0.04), but did not significantly improve outcomes for patients with persistent or permanent AF (74 vs. 80%, p = 0.52 and 69 vs. 67%, p = 0.77, respectively) [9]. These results are also consistent with our findings using thoracoscopic ablation in patients with persistent AF. Xu et al. [17] investigated the role of SVC in patients with long-standing persistent AF who underwent extensive endocardial ablation. The arrhythmogenicity of the SVC was confirmed in only 1 of the patients (0.98%). Both AF duration and LA diameter were determinants of AF recurrence. Similarly, in another study, the prevalence of arrhythmogenic SVC in persistent and long-standing persistent AF was very rare, at 1.9% and 1.3%, respectively [18]. These results suggest that empirical SVC ablation has no additional benefit in patients with persistent AF. On the contrary, in paroxysmal AF patients, arrhythmogenic SVC were identified in 9–12% [9, 11]. Small LA size and typical atrial flutter were reported to be independent predictors of arrhythmogenic SVC. Atrial fibrillation is a progressive disease characterized by chronic atrial structural remodeling, including cellular hypertrophy, fibroblast proliferation and tissue fibrosis. Left atrial enlargement is a surrogate marker of elevated left ventricular filling pressure and the result of atrial remodeling. These contradictory results suggest that the mechanism of AF in patients with arrhythmogenic SVC differs from the typical course of AF progression. Additionally, in this study, the LA diameter was larger in the SVC isolation group than in the no-SVC isolation group. The bigger LA in SVC isolation group could affect the results. So it needs to be considered in interpreting the results.
More extensive atrial ablation compared to catheter ablation, such as thoracoscopic epicardial ablation or COX MAZE III or IV operation, may increase the risk of sinus node through direct or indirect injury [19,20,21]. It may directly induce postoperative sinus node dysfunction due to atrial scarring, cause local inflammation and edema and also damage the sinus node or sinus artery [21]. In particular, in about 40% of patients whose sinus node artery originates from the left circumflex artery, the nodal artery passes through the ablated field as it winds around the PV. In this study, three patients were implanted permanent pacemakers. Two patients were diagnosed with sinus node dysfunction prior to procedure, and one patient was diagnosed postoperatively. Other studies that performed thoracoscopic epicardial ablation for lone AF also showed similar results [19]. Transient or permanent sinus node dysfunction occurred after procedure in 7%, and a pacemaker was implanted in 2.5% of patients. Although this outcome is rarer than MAZE operation, which is reported at 8–9%, it is a significantly higher incidence compared to never reported or less than 1% in previous studies with catheter ablation [9, 11, 15, 20, 21].
In the present study, we found an ATa-free survival rate of 67% at 12 months for persistent or long-standing persistent AF patients who underwent thoracoscopic surgical ablation. This outcome was better than that reported in a previous study in which catheter ablation was performed on patients with similar LA diameter, an independent predictor of AF recurrence (43% ATa-free survival at 12 months) [17]. These findings suggest that epicardial ablation is an alternative treatment option for patients with advanced AF with larger LA size.
Limitations
Our study has several limitations. This study was retrospective in design and reports the findings from a single center. As a retrospective study, there were significant differences in baseline characteristics between the two groups. However, sub-analysis was performed to overcome these differences. Since epicardial ablation was performed using a thoracoscopic approach, the arrhythmogenicity of SVC could not be evaluated. In addition, the ATa recurrence rate might have been underestimated when using 12-lead ECG or Holter ECG rather than continuous monitoring. Further larger and randomized studies are required to support our results.
Conclusions
In patients with persistent AF, empirical SVC isolation did not improve Ata-free survival rate. Even extensive atrial ablation increases the risk of sinus node dysfunction. Empirical SVC isolation seems unnecessary during thoracoscopic ablation in patients with persistent AF.
Abbreviations
- AF:
-
Atrial fibrillation
- PV:
-
Pulmonary vein
- PVI:
-
Pulmonary vein isolation
- SVC:
-
Superior vena cava
- ATa:
-
Atrial tachycardia
- CFAE:
-
Complex fractionated atrial electrogram
- GP:
-
Ganglion plexus
- TTA:
-
Total thoracoscopic ablation
- AFL:
-
Atrial flutter
- OAC:
-
Oral anticoagulant
- IVC:
-
Inferior vena cava
- LA:
-
Left atrium
References
Haissaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Eng J Med. 1998;339(10):659–66.
Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F Jr, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Eng J Med. 2006;354(9):934–41.
Haïssaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Caridovasc Electrophysiol. 2005;16(11):1138–47.
Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cariol. 2004;43(11):2044–53.
Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller JMJ. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cariol. 2012;60(7):628–360.
Santangeli P, Zado ES, Hutchinson MD, Riley MP, Lin D, Frankel DS, et al. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart Rhythm. 2016;13(2):374–82.
Gökoğlan Y, Mohanty S, Güneş MF, Trivedi C, Santangeli P, Gianni C, et al. Pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: more than a decade of follow-up. Circ Arrhythm Electrophysiol. 2016;9(5):e003660.
Miyazaki S, Takigawa M, Kusa S, Kuwahara T, Taniguchi H, Okubo K, et al. Role of arrhythmogenic superior vena cava on atrial fibrillation. J Caridovasc Electrophysiol. 2014;25(4):380–6.
Corrado A, Bonso A, Madalosso M, Rossillo A, Themistoclakis S, Di Biase L, et al. Impact of systematic isolation of superior vena cava in addition to pulmonary vein antrum isolation on the outcome of paroxysmal, persistent, and permanent atrial fibrillation ablation: results from a randomized study. J Caridovasc Electrophysiol. 2010;21(1):1–5.
Overeinder I, Osório TG, Călburean P-A, Bisignani A, Bala G, Sieira J, et al. Comparison between superior vena cava ablation in addition to pulmonary vein isolation and standard pulmonary vein isolation in patients with paroxysmal atrial fibrillation with the cryoballoon technique. J Interv Card Electrophysiol. 2021;62:1–8.
Ejima K, Kato K, Iwanami Y, Henmi R, Yagishita D, Manaka T, et al. Impact of an empiric isolation of the superior vena cava in addition to circumferential pulmonary vein isolation on the outcome of paroxysmal atrial fibrillation ablation. Am J Cardiol. 2015;116(11):1711–6.
Vos LM, Kotecha D, Geuzebroek GS, Hofman FN, van Boven WJP, Kelder J, et al. Totally thoracoscopic ablation for atrial fibrillation: a systematic safety analysis. Europace. 2018;20(11):1790–7.
Higuchi K, Yamauchi Y, Hirao K, Sasaki T, Hachiya H, Sekiguchi Y, et al. Superior vena cava as initiator of atrial fibrillation: factors related to its arrhythmogenicity. Heart Rhythm. 2010;7(9):1186–91.
Gianni C, Sanchez JE, Mohanty S, Trivedi C, Della Rocca DG, Al-Ahmad A, et al. Isolation of the superior vena cava from the right atrial posterior wall: a novel ablation approach. Europace. 2018;20(9):e124–32.
Arruda M, Mlcochova H, Prasad SK, Kilicaslan F, Saliba W, Patel D, et al. Electrical isolation of the superior vena cava: an adjunctive strategy to pulmonary vein antrum isolation improving the outcome of AF ablation. J Cardiovasc Electrophysiol. 2007;18(12):1261–6.
Zhang T, Wang Y, Liang Z, Zhao H, Han Z, Wang Y, et al. Effect of combined pulmonary vein and superior vena cava isolation on the outcome of second catheter ablation for paroxysmal atrial fibrillation. Am J Cardiol. 2020;125(12):1845–50.
Xu K, Wang Y, Wu S, Zhou L, Zhao L, Jiang W, et al. The role of superior vena cava in catheter ablation of long-standing persistent atrial fibrillation. Europace. 2017;19(10):1670–5.
Miyazaki S, Taniguchi H, Kusa S, Ichihara N, Nakamura H, Hachiya H, et al. Factors predicting an arrhythmogenic superior vena cava in atrial fibrillation ablation: insight into the mechanism. Heart Rhythm. 2014;11(9):1560–6.
Neefs J, Ons SA, Berger WR, Krul SP, van den Berg NW, Piersma FR, et al. Clinical course of sinus node dysfunction after thoracoscopic surgery for atrial fibrillation—analysis of the Atrial Fibrillation Ablation and Autonomic Modulation via Thoracoscopic Surgery (AFACT) study. J Interv Card Electrophysiol. 2021;60:185–93.
Weimar T, Schena S, Bailey MS, Maniar HS, Schuessler RB, Cox JL, et al. The cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol. 2012;5(1):8–14.
Kulikov AA, Bokeria LAJ. Assessment of sinoatrial node function in patients with persistent and long-standing persistent forms of atrial fibrillation after maze III procedure combined with mitral valve operation. J Atr Fibrillation. 2016;9(1):1408.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HJK, DSJ and YKO were a major contributor analyzing data and writing the manuscript. KMP and JSK involved in creating concept of study. KMP, YKO and SJP reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Samsung Medical Center which waived the requirement of informed consent (IRB No. 2020-06-159).
Consent of publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Lesion set of thoracoscopic surgical ablation. CS, coronary sinus; GP, ganglion plexus; IVC, inferior vena cava; LAA, left atrial appendage; LLPV, left lower pulmonary vein; LoM, ligament of Marshall; LUPV, left upper pulmonary vein; PVI, pulmonary vein isolation; RLPV, right lower pulmonary vein; RUPV, right upper pulmonary vein; SVC, superior vena cava.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwon, HJ., Jeong, D.S., Park, SJ. et al. The effect of empirical superior vena cava isolation during total thoracoscopic ablation in patients with persistent atrial fibrillation. Int J Arrhythm 24, 23 (2023). https://doi.org/10.1186/s42444-023-00105-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42444-023-00105-2