Abstract
Pulmonary vein isolation is an well-established treatment strategy for atrial fibrillation (AF), and it is especially effective for patients with paroxysmal AF. However, the success rate is limited for patients with persistent AF, because non-pulmonary vein triggers which increase AF recurrence are frequently found in these patients. The major non-pulmonary vein triggers are from the left atrial posterior wall, left atrial appendage, ligament of Marshall, coronary sinus, superior vena cava, and crista terminalis, but other atrial sites can also generate AF triggers. All these sites have been known to contain atrial myocytes with potential arrhythmogenic electrical activity. The prevalence and clinical characteristics of these non-pulmonary vein triggers are well studied; however, the clinical outcome of catheter ablation for persistent AF is still unclear. Here, we reviewed the current ablation strategies for persistent AF and the clinical implications of major non-pulmonary vein triggers.
Similar content being viewed by others
Current ablation strategies for persistent atrial fibrillation
Catheter ablation is an effective treatment for atrial fibrillation (AF) because it prolongs the maintenance of sinus rhythm or reduces the number of acute episodes, thereby improving the quality of life [1]. In addition, it is well known that catheter ablation lowers the risk of mortality, stroke, and heart failure hospitalization [2,3,4]. The main goal of the ablation procedure is to remove all possible AF triggers with a minimum ablation amount [5].
Circumferential pulmonary vein isolation (PVI) is the most important technique of catheter ablation for AF [6]. Most triggers of paroxysmal AF arise from the pulmonary veins; thus, the procedure involves creating circumferential ablation lesions around the PVs to electrically isolate them from the left atrial body. According to the previous study by Ouyang et al. [7], the recurrence rate after the first ablation procedure for paroxysmal AF is 46.6%, and the AF-free survival was increased up to 79.5% with repeated procedures.
Catheter ablation for persistent AF (PeAF) is more challenging than paroxysmal AF treatment, and clinical outcomes are not favorable despite technical advancement in catheter ablation [8,9,10]. After multiple procedures, 45% of long-standing persistent AF (PeAF) patients were in sinus rhythm during follow-up [9]. In PeAF, circumferential PVI alone does not ensure favorable clinical outcome; therefore, non-PV triggers have important roles in the disease initiation and progression [11]. To improve outcomes, atrial substrate modification is often performed with PVI. However, the STAR AF II randomized controlled trial revealed that empirical complex fractionated atrial electrogram ablation or linear ablation in addition to PVI did not reduce the AF recurrence rate in PeAF patients [10].
Nevertheless, the identification and ablation of non‐PV triggers are of paramount importance in preventing AF recurrence and improving long‐term clinical outcomes in PeAF. Several groups have reported the distribution and clinical characteristics of non-PV triggers, as well as the improved clinical outcome after complete elimination in PeAF [12, 13]. Recent findings demonstrated that AF recurrence decreased with empirical ablation of non-PV sites even in long-standing PeAF population [12, 14].
According to the 2020 ESC guidelines, more extensive ablation has been advocated particularly for PeAF and long-standing PeAF. This may include the linear ablation in the left atria, the left atrial appendage (LAA) isolation, superior vena cava (SVC) isolation, targeted ablation of potentially arrhythmogenic atrial sites [15, 16]. However, the benefit of additional ablation lesions beyond PVI is not well established.
Here, we review the characteristics of non-PV triggers and their clinical implications.
Definition/prevalence/distribution of non-PV triggers
Increasing evidence has shown that sites outside the PVs may harbor arrhythmogenic triggers, which are responsible for the atrial tachyarrhythmia initiation, thus predisposing to arrhythmia recurrence [17]. The prevalence of non-PV triggers reported to be higher in patients with Persistent than paroxysmal form of AF, and the patients who have specific risk factors such as old age, female sex, sleep apnea, obesity, atrial structural remodeling, presence of heart failure, other cardiomyopathy, or valvular heart disease [17, 18]. Non-PV triggers are ectopic beats initiating sustained/non-sustained of atrial tachyarrhythmias, which are harbored in various anatomical regions in atria. Especially, some atrial structures are well-known sources of non-PV triggers contributing to atrial tachyarrhythmia, including the left atrial posterior wall (LAPW), interatrial septum, crista terminalis, LAA, and vessels connecting to atriums. The left SVC or its fetal remnant, the ligament of Marshall are also common extra-PV sites. Recently, uncommon atrial tachycardia from the non-coronary cusp has been reported as an uncommon non-PV trigger in AF patients with an overall prevalence of 0.08% in AF ablation cases [19]. In addition, atrioventricular nodal reentrant tachycardia or atrioventricular reentrant tachycardia may play a role as a non-PV trigger in 2% of PeAF patients [16, 20].
As there are no standard induction protocol and no standard definition of clinically significant non-PV triggers, the prevalence is variable across studies. In previous studies, the reported prevalence of non-PV triggers varies from 3 to 47% [13, 21,22,23,24,25,26,27,28]. From the study by Santangeli et al. [16] in 2016, non-PV triggers were found in 11% of PeAF patients in their large prospective study. The overall prevalence was similar to that observed among patients with paroxysmal AF or long-standing PeAF [16]. However, in another study, the prevalence was higher in PeAF than in paroxysmal AF [24]. In a large multicenter cohort study of de novo catheter ablation in PeAF, clinically significant non-PV triggers initiating AF were reported in more than 10%, and non-PV triggers were found in more than 75% of all PeAF patient cases [18].
The LA walls are made up of thin muscular layers which extend up to 20 mm inside the PVs [29]. At the connection level between atrium and venous structure, some muscular sleeves are known to constitute an arrhythmogenic substrate. Except for PV, there is controversy over where is the most common site of non-PV triggers in PeAF. Santangeli and the colleagues reported the most common site was reported to be the crista terminalis/Eustachian ridge region [16]. Others reported that extra-PV triggered activity was found most commonly at the CS and LAA [18]. Tohhoku et al. [30] showed that the SVC and LAPW were the two most popular sites.
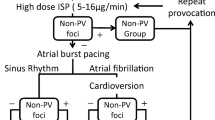
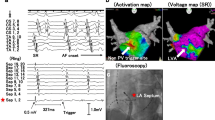
Several techniques have been proposed to provoke and localize the non-PV triggers foci. Santangeli et al. [5] proposed the following provocation protocol: isoproterenol infusion and cardioversion of AF induced by atrial pacing with or without isoproterenol infusion at 3–6 mg/min. During the trigger protocol, several groups suggested that catheters were positioned following setup for detection of triggers [5, 17, 18]: (1) mapping catheters for simultaneously recording electrical activity from the left superior PV, recording the far-field potential of left atrial appendage; (2) positioning ablation catheter inside the right superior PV to record the far-field potential of a interatrial septum; (3) positioning multipolar catheters for right atrial side and CS signals. Figure 1 shows an example of catheter setup during provocation test and examples of activation pattern of PACs [17].
Example of the catheter setup during isoproterenol provocation test and examples of activation pattern of PACs. A Duodecapolar catheter with electrodes spanning from the SVC, right atrium/CT [blue] to the CS [green], ablation catheter in the right superior PV recording the far-field IAS [violet], 10-pole circular mapping catheter in the left superior PV recording the far-field LAA activity [red]. B Activation pattern during sinus rhythm. C Example of activation pattern PACs from the CS. The earliest activation (red star) is recorded in the dipole CS 7–8 of the distal segment of the duodecapolar catheter (inside the CS). D Example of activation pattern of PACs from the LAA. The earliest activation (red star) is recorded by the circular mapping catheter inside the left superior PV recording the far-field from the LAA. (Della Rocca, Di Biase et al. 2021)
Major non-PV trigger sites and their role in PeAF
Posterior wall (PW)
The LAPW is well known to be a common non-PV trigger site, and the additional benefit of PW isolation beyond PVI in PeAF patients has been frequently reported in previous studies. From an embryological and anatomical view, the LAPW should be considered as an extension of the PVs. As the LA wall develops and expands, the PVs with multiple bifurcations are progressively incorporated into the LA wall. At this point, four separate openings are present at the posterior part of LA; this area includes the LAPW and the four PVs. The LAPW harbors myocytes and has distinct electrophysiological characteristics. Studies using in vitro and in vivo models showed that cardiomyocytes in the LAPW have a shorter action potential duration and a shorter refractory period. Also, the degree of fibrous fatty infiltration is higher in the PW than in other structures, which is associated with non‐PV triggers and unidirectional anatomical block. Therefore, the LAPW can be regarded as a perpetuator for AF as well as a source of triggers, suggesting that its isolation may be a potentially useful approach for rhythm control in all types of AF. Electrical isolation of the LAPW has been reported to be beneficial [31, 32]. However, a recent randomized study investigating the effect of additional LAPW isolation for PeAF patients showed no improvement in rhythm outcomes [33]. Discrepancy of results of clinical trials can be explained by the extent of antral versus wide antral pulmonary vein isolation, verification of electrical isolation of the posterior wall. One of the main limitations of clinical trials that evaluate additional treatment beyond pulmonary vein isolation is that pulmonary vein reconnection is not rare in the current state-of-the-art PVI and common in patients without AF recurrence.
For electrical isolation of the LAPW, several strategies are suggested [34, 35]. A schematic illustration of PW isolation strategies and example of LAPW trigger is shown in Fig. 2. This approach includes wide PV antral isolation followed by inferior and roof linear ablation to achieve complete posterior wall isolation. In the single ring approach, a large circumferential ablation lesion encloses the four pulmonary veins and posterior wall. The potential esophageal injury risk may be reduced with the single ring approach. However, it may lead to reconnection in both set of PVs and PW.
LA appendage (LAA)
Embryologically, the LA appendage (LAA) is a remnant of the primordial left atrial tissue. Considering its complex morphology, several studies have investigated its role in AF initiation and maintenance. In 2005, Takahashi et al. [36] reported that multiple foci were found inside the LAA after PVI in a paroxysmal AF ablation case. Natele et al. [37] reported in 2010 that the LAA was responsible for triggering atrial tachyarrhythmias in 27% of patients with redo AF procedures. The BELIEF randomized controlled trial compared the standard ablation strategy (PVI with extra ablation as needed) with standard ablation plus empirical LAA electrical isolation [38] and reported that at the 12-month follow-up, about half (56%) of patients with empirical LAA isolation and 28% of patients with standard ablation alone were free of AF recurrence. A previous observational study reported 73% of patients had durable isolation of LAA verified in a subsequent procedure [39]. However, some studies reported complications after empirical LAA isolation, such as LAA thrombus, stroke, or LAA dysfunction [40]. In a propensity score-matched analysis conducted by Romero et al. [41], empirical LAA isolation showed significantly lower AF recurrence in both PeAF and long-standing PeAF patients without increasing the acute procedural complication rate; however, there appears to be a higher stroke in the LAA isolation group among patients who discontinue oral anticoagulation. Therefore, a stroke prevention strategy after LAA isolation, such as life-long un-interrupted oral anticoagulation or LAA occlusion, is mandatory to guarantee the benefits of LAA isolation.
Since LAA has a thin wall and is easily perforated, it is critical to perform LAA isolation by transferring high-frequency energy at the level of LAA ostium, which has a relatively thick wall. High-output pacing (20 mA/) at the LAA posterior side before ablation may be helpful to avoid left phrenic nerve injury [41]. Radiofrequency settings typically include power from 40 to 45 W while maintaining a temperature of 42 °C for a maximum of 30 s per each ablation site [42]. For the thicker part of LAA (anterior and superior edge), longer ablation duration (more than 30 s, up to 60 secs) might be required [41].
Superior vena cava (SVC)
The SVC, which arises between the right atrium and the sinus venosus, is one of the important non-PV foci of AF [43]. SVC contains atrial myocytes with automaticity [44]. The myocardial sleeves over the SVC share certain electrical properties with the sinoatrial node including a stronger propensity for automaticity in addition to triggered activity. The SVC musculature is involved in the sustaining of AF as well [43]. In most AF ablation cases, SVC triggers are usually identified following adenosine injection, continuous isoproterenol infusion, or cardioversion [13, 43]. The role of the SVC in PeAF is less well studied. Two meta-analyses of SVC isolation showed conflicting results. Li et al. compared “empirical” (all patients underwent SVC isolation) versus “conventional” (only those with SVC triggers underwent SVC isolation). This meta-analysis found a significant improvement in the 12-month outcome in the empirical SVC isolation group (16% vs. 29%) [45]. Conversely, Sharma et al. [46] found no benefit to SVC isolation plus PVI over PVI alone. Nevertheless, SVC isolation might have therapeutic potential, especially for those with SVC triggers.
A useful strategy for the isolation of SVC is the segmental approach, in which the area adjacent to the arrhythmogenic focus is targeted. During SVC isolation, increases in the sinus node automaticity can be considered to indicate the risk of sinus node damage. During isoproterenol infusion, ablation should not be performed in order to monitor sinus node dysfunction. Prior to radiofrequency delivery at the posterolateral segment of the SVC High output pacing (> 20 mA) should be done to avoid collateral injury of the phrenic nerve [5].
Coronary sinus (CS)
Non‐PV triggers from the CS are common, especially in patients with PeAF [12]. It is known that the muscle layer surrounding the CS can generate rapid electrical activity. The muscular bundles enveloping the CS run with different orientations, connecting to both atria. While those muscular bundles usually end where the great cardiac vein begins, they sometimes extend over the vein by > 10 mm. Several studies reported that the CS plays a key role in the electrical connection of both atria [47, 48]. Therefore, CS isolation may contribute to the AF ablation success in two different ways. First, the abolition of all electrical potentials inside CS means the elimination of an important source of abnormal electrical activity, which is likely associated with the initiation of AF [49]. Second, CS isolation can interfere with the electrical connection between the two atria, thereby preventing AF perpetuation [50].
To isolate the CS completely, both the endocardial and the epicardial sides of the vessel should be targeted, to eliminate whole CS potentials (Fig. 3A). It is important to maintain the ablation catheter tip direction facing the atrial aspect of the CS. It is not necessary to perform ablation at the ventricular aspect of the CS to avoid the risk of coronary arterial injury [5].
3D electro-anatomical map showing the ablation inside of the coronary sinus. A Epicardial ablation inside the CS was performed for CS isolation. B Atrial tachycardia originated from vein of Marshall was induced during rapid atrial pacing. Epicardial ablation inside of the coronary sinus was performed
Ligament or vein of Marshall (LOM or VOM)
The vein of Marshall (VOM) is located in the LA ridge and the posterior mitral isthmus [51]. The VOM can be retrogradely cannulated from CS at the level of the valve of Vieussens. The ligament of Marshall (LOM) is the vestigial fold containing the remnant of the left SVC, i.e., the VOM, and plays an important role in atrial arrhythmogenesis [52]. As it is insulated by fat tissue, perfect ablation of the VOM by radiofrequency catheter ablation (Fig. 3B) is highly challenging [53]. Intravascular ethanol injections combined with radiofrequency ablation synergistically improve the possibility of left PV isolation and elimination of the occasional direct LOM-PV connections [54]. Liu et al. [55] reported that VOM ethanol injection was a useful strategy for maintaining AF free period during follow-up. Valderrábano et al. [56] recently published the results of the VENUS trial, which showed that VOM ethanol infusion decreased atrial tachyarrhythmias recurrence in PeAF ablation.
Crista terminalis (CT)
The CT is an elongated muscular prominence between the SVC and IVC in the posterolateral part of the right atrium. The arrhythmogenicity of CT may be associated with the vicinity with the Bachmann's bundle, propensity to fibrosis, and peculiar muscular anisotropic conduction [57,58,59]. Several studies demonstrated the role of CT in the development and maintenance of AF [60,61,62].
Persistent left superior vena cava (PLSVC)
The PLSVC, which results from the persistent patency of the left cardinal vein, is an uncommon, but most common congenital anomaly of thoracic venous system, which arise from the junction of the left subclavian and internal jugular veins, passes along the left side of the mediastinum [63], and drains into coronary sinus which is usually dilated [27]. Like other thoracic veins, a PLSVC also present arrhythmogenic activity initiating AF [64, 65], and its isolation is important to reduce the AF recurrence [27, 66, 67]. Because complete isolation of PLSVC may be difficult to achieve in some cases due to proximity to the left phrenic nerve or the risk of esophageal injury, segmental isolation may be sufficient for reducing AF recurrence [5, 66].
Future perspectives and conclusion
There are some possible explanations for the low success rate of various ablation strategies for treating PeAF. First, appropriate targets for ablation outside of the PVs have not been identified. Second, RF lesions may not be durable enough to ensure long-term outcomes [67, 68]. Third, targeting only the left atrium is not sufficient to treat PeAF. Furthermore, the accurate mapping of non-PV triggers is sometimes difficult. The electrograms obtained by the reference catheters are often insufficient to localize the focus.
Considering that identifying and successfully ablating extra-PV triggers in each patient with optimal lesion formation are still challenging, many researchers continue to conduct studies on PeAF. However, it is still controversial whether the elimination of inducible repetitive PACs improves AF success rate. In the review by Natale et al. [17], they suggest that empirical ablation of critical areas should be performed. However, no randomized clinical trial has demonstrated the benefit of additional ablation.
In conclusion, compared with paroxysmal AF, PeAF is still more challenging to treat with less efficacy. Adjunctive ablation strategies targeting extra-PV sites can be performed for patients with PeAF; however, the best approach and additional benefit still remain unclear. Many substrate modification techniques have been developed to obtain better clinical outcomes (e.g., LAA isolation, ablation of abnormal atrial signals, creating linear lesions); however, these strategies are controversial and not uniformly performed [69]. In the near future, considerable progress in PeAF treatment can be expected with a better understanding of the disease and the continuous development of ablation technology.
Availability of data and materials
The datasets used and/or analyzed in the current review are available from the corresponding author upon reasonable request.
Abbreviations
- AF:
-
Atrial fibrillation
- CS:
-
Coronary sinus
- CT:
-
Crista terminalis
- LAA:
-
Left atrial appendage
- LAPW:
-
Left atrial posterior wall
- LOM:
-
Ligament of Marshall
- PACs:
-
Premature atrial contractions
- PeAF:
-
Persistent AF
- PV:
-
Pulmonary vein
- PVI:
-
Pulmonary vein isolation
- PW:
-
Posterior wall
- RA:
-
Right atrium
- SVC:
-
Superior vena cava
- VOM:
-
Vein of Marshall
References
Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the cabana randomized clinical trial. JAMA. 2019;321:1275–85. https://doi.org/10.1001/jama.2019.0692.
Friberg L, Tabrizi F, Englund A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J. 2016;37:2478–87. https://doi.org/10.1093/eurheartj/ehw087.
Jin MN, Kim TH, Kang KW, Yu HT, Uhm JS, Joung B, Lee MH, Kim E, Pak HN. Atrial fibrillation catheter ablation improves 1-year follow-up cognitive function, especially in patients with impaired cognitive function. Circ Arrhythm Electrophysiol. 2019;12:e007197. https://doi.org/10.1161/circep.119.007197.
Park JW, Yang PS, Bae HJ, Yang SY, Yu HT, Kim TH, Uhm JS, Joung B, Lee MH, Pak HN. Five-year change in the renal function after catheter ablation of atrial fibrillation. J Am Heart Assoc. 2019;8:e013204. https://doi.org/10.1161/jaha.119.013204.
Santangeli P, Marchlinski FE. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm. 2017;14:1087–96. https://doi.org/10.1016/j.hrthm.2017.02.030.
Pappone C, Augello G, Sala S, Gugliotta F, Vicedomini G, Gulletta S, Paglino G, Mazzone P, Sora N, Greiss I, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48:2340–7. https://doi.org/10.1016/j.jacc.2006.08.037.
Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T, Neven K, Köktürk B, Konstantinidou M, Metzner A, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368–77. https://doi.org/10.1161/circulationaha.110.946806.
Parkash R, Verma A, Tang AS. Persistent atrial fibrillation: current approach and controversies. Curr Opin Cardiol. 2010;25:1–7. https://doi.org/10.1097/HCO.0b013e3283336d52.
Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, Hsu LF, Sanders P. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm. 2010;7:835–46. https://doi.org/10.1016/j.hrthm.2010.01.017.
Verma A, Mantovan R, Macle L, De Martino G, Chen J, Morillo CA, Novak P, Calzolari V, Guerra PG, Nair G, et al. Substrate and trigger ablation for reduction of atrial fibrillation (STAR AF): a randomized, multicentre, international trial. Eur Heart J. 2010;31:1344–56. https://doi.org/10.1093/eurheartj/ehq041.
Tilz RR, Rillig A, Thum AM, Arya A, Wohlmuth P, Metzner A, Mathew S, Yoshiga Y, Wissner E, Kuck KH, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg sequential ablation strategy. J Am Coll Cardiol. 2012;60:1921–9. https://doi.org/10.1016/j.jacc.2012.04.060.
Della Rocca DG, Mohanty S, Mohanty P, Trivedi C, Gianni C, Al-Ahmad A, Burkhardt JD, Gallinghouse GJ, Hranitzky P, Sanchez JE, et al. Long-term outcomes of catheter ablation in patients with longstanding persistent atrial fibrillation lasting less than 2 years. J Cardiovasc Electrophysiol. 2018;29:1607–15. https://doi.org/10.1111/jce.13721.
Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, Huang JL, Yu WC, Yang SP, Ding YA, et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–83. https://doi.org/10.1161/01.Cir.0000074206.52056.2d.
Romero J, Michaud GF, Avendano R, Briceño DF, Kumar S, Carlos Diaz J, Mohanty S, Trivedi C, Gianni C, Della Rocca D, et al. Benefit of left atrial appendage electrical isolation for persistent and long-standing persistent atrial fibrillation: a systematic review and meta-analysis. Europace. 2018;20:1268–78. https://doi.org/10.1093/europace/eux372.
Shah D, Haissaguerre M, Jais P, Hocini M. Nonpulmonary vein foci: do they exist? Pacing Clin Electrophysiol. 2003;26:1631–5. https://doi.org/10.1046/j.1460-9592.2003.t01-1-00243.x.
Santangeli P, Zado ES, Hutchinson MD, Riley MP, Lin D, Frankel DS, Supple GE, Garcia FC, Dixit S, Callans DJ, et al. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart Rhythm. 2016;13:374–82. https://doi.org/10.1016/j.hrthm.2015.10.023.
Della Rocca DG, Tarantino N, Trivedi C, Mohanty S, Anannab A, Salwan AS, Gianni C, Bassiouny M, Al-Ahmad A, Romero J, et al. Non-pulmonary vein triggers in nonparoxysmal atrial fibrillation: implications of pathophysiology for catheter ablation. J Cardiovasc Electrophysiol. 2020;31:2154–67. https://doi.org/10.1111/jce.14638.
Della Rocca DG, Di Biase L, Mohanty S, Trivedi C, Gianni C, Romero J, Tarantino N, Magnocavallo M, Bassiouny M, Natale VN, et al. Targeting non-pulmonary vein triggers in persistent atrial fibrillation: results from a prospective, multicentre, observational registry. Europace. 2021;23:1939–49. https://doi.org/10.1093/europace/euab161.
Cha M-J, Kim J, Park YJ, Cho MS, Park H-S, Kwon S, Lee YS, Ahn J, Choi H-O, Park J-S, et al. Prevalence and characteristics of atrial tachycardia from noncoronary aortic cusp during atrial fibrillation catheter ablation. Korean Circ J. 2022;52:513.
Sauer WH, Alonso C, Zado E, Cooper JM, Lin D, Dixit S, Russo A, Verdino R, Ji S, Gerstenfeld EP, et al. Atrioventricular nodal reentrant tachycardia in patients referred for atrial fibrillation ablation: response to ablation that incorporates slow-pathway modification. Circulation. 2006;114:191–5. https://doi.org/10.1161/CIRCULATIONAHA.106.621896.
Chen SA, Tai CT, Yu WC, Chen YJ, Tsai CF, Hsieh MH, Chen CC, Prakash V, Ding YA, Chang MS. Right atrial focal atrial fibrillation: electrophysiologic characteristics and radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 1999;10:328–35.
Chen S-A, Hsieh M-H, Tai C-T, Tsai C-F, Prakash V, Yu W-C, Hsu T-L, Ding Y-A, Chang M-S. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–86.
Hwang C, Wu T-J, Doshi RN, Peter CT, Chen P-S. Vein of Marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503–5.
Della Rocca DG, Mohanty S, Trivedi C, Di Biase L, Natale A. Percutaneous treatment of non-paroxysmal atrial fibrillation: a paradigm shift from pulmonary vein to non-pulmonary vein trigger ablation? Arrhythm Electrophysiol Rev. 2018;7:256–60. https://doi.org/10.15420/aer.2018.56.2.
Tai CT, Hsieh MH, Tsai CF, Lin YK, Yu WC, Lee SH, Ding YA, Chang MS, Chen SA. Differentiating the ligament of Marshall from the pulmonary vein musculature potentials in patients with paroxysmal atrial fibrillation: electrophysiological characteristics and results of radiofrequency ablation. Pacing Clin Electrophysiol. 2000;23:1493–501.
Katritsis D, Ioannidis JP, Anagnostopoulos CE, Sarris GE, Giazitzoglou E, Korovesis S, Camm AJ. Identification and catheter ablation of extracardiac and intracardiac components of ligament of Marshall tissue for treatment of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2001;12:750–8.
Elayi CS, Fahmy TS, Wazni OM, Patel D, Saliba W, Natale A. Left superior vena cava isolation in patients undergoing pulmonary vein antrum isolation: impact on atrial fibrillation recurrence. Heart Rhythm. 2006;3:1019–23.
Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–53.
Margulescu AD, Mont L. Persistent atrial fibrillation vs paroxysmal atrial fibrillation: differences in management. Expert Rev Cardiovasc Ther. 2017;15:601–18. https://doi.org/10.1080/14779072.2017.1355237.
Tohoku S, Fukunaga M, Nagashima M, Korai K, Hirokami J, Yamamoto K, Takeo A, Niu H, Ando K, Hiroshima K. Clinical impact of eliminating nonpulmonary vein triggers of atrial fibrillation and nonpulmonary vein premature atrial contractions at initial ablation for persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32:224–34. https://doi.org/10.1111/jce.14830.
Kumagai K, Muraoka S, Mitsutake C, Takashima H, Nakashima H. A new approach for complete isolation of the posterior left atrium including pulmonary veins for atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1047–52. https://doi.org/10.1111/j.1540-8167.2007.00911.x.
Thiyagarajah A, Kadhim K, Lau DH, Emami M, Linz D, Khokhar K, Munawar DA, Mishima R, Malik V, O’Shea C, et al. Feasibility, safety, and efficacy of posterior wall isolation during atrial fibrillation ablation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2019;12:e007005. https://doi.org/10.1161/circep.118.007005.
Lee JM, Shim J, Park J, Yu HT, Kim TH, Park JK, Uhm JS, Kim JB, Joung B, Lee MH, et al. The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin Electrophysiol. 2019;5:1253–61. https://doi.org/10.1016/j.jacep.2019.08.021.
Sugumar H, Thomas SP, Prabhu S, Voskoboinik A, Kistler PM. How to perform posterior wall isolation in catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:345–52. https://doi.org/10.1111/jce.13397.
Tahir KS, Mounsey JP, Hummel JP. Posterior wall isolation in atrial fibrillation ablation. J Innov Card Rhythm Manag. 2018;9:3186–94. https://doi.org/10.19102/icrm.2018.090602.
Takahashi Y, Sanders P, Rotter M, Haïssaguerre M. Disconnection of the left atrial appendage for elimination of foci maintaining atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16:917–9. https://doi.org/10.1046/j.1540-8167.2005.40804.x.
Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton R, Gallinghouse GJ, Bailey SM, Zagrodzky JD, Santangeli P, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–18. https://doi.org/10.1161/CIRCULATIONAHA.109.928903.
Di Biase L, Burkhardt JD, Mohanty P, Mohanty S, Sanchez JE, Trivedi C, Gunes M, Gokoglan Y, Gianni C, Horton RP, et al. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation: BELIEF trial. J Am Coll Cardiol. 2016;68:1929–40. https://doi.org/10.1016/j.jacc.2016.07.770.
Reissmann B, Rillig A, Wissner E, Tilz R, Schluter M, Sohns C, Heeger C, Mathew S, Maurer T, Lemes C, et al. Durability of wide-area left atrial appendage isolation: results from extensive catheter ablation for treatment of persistent atrial fibrillation. Heart Rhythm. 2017;14:314–9. https://doi.org/10.1016/j.hrthm.2016.11.009.
Rillig A, Tilz RR, Lin T, Fink T, Heeger CH, Arya A, Metzner A, Mathew S, Wissner E, Makimoto H, et al. Unexpectedly high incidence of stroke and left atrial appendage thrombus formation after electrical isolation of the left atrial appendage for the treatment of atrial tachyarrhythmias. Circ Arrhythm Electrophysiol. 2016;9:e003461. https://doi.org/10.1161/CIRCEP.115.003461.
Romero J, Di Biase L, Mohanty S, Trivedi C, Patel K, Parides M, Alviz I, Diaz JC, Natale V, Sanchez J, et al. Long-term outcomes of left atrial appendage electrical isolation in patients with nonparoxysmal atrial fibrillation: a propensity score-matched analysis. Circ Arrhythm Electrophysiol. 2020;13:e008390. https://doi.org/10.1161/CIRCEP.120.008390.
Panikker S, Jarman JW, Virmani R, Kutys R, Haldar S, Lim E, Butcher C, Khan H, Mantziari L, Nicol E, et al. Left atrial appendage electrical isolation and concomitant device occlusion to treat persistent atrial fibrillation: a first-in-human safety, feasibility, and efficacy study. Circ Arrhythm Electrophysiol. 2016. https://doi.org/10.1161/circep.115.003710.
Miyazaki S, Takigawa M, Kusa S, Kuwahara T, Taniguchi H, Okubo K, Nakamura H, Hachiya H, Hirao K, Takahashi A, et al. Role of arrhythmogenic superior vena cava on atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:380–6. https://doi.org/10.1111/jce.12342.
Kholová I, Kautzner J. Morphology of atrial myocardial extensions into human caval veins: a postmortem study in patients with and without atrial fibrillation. Circulation. 2004;110:483–8. https://doi.org/10.1161/01.Cir.0000137117.87589.88.
Li JY, Jiang JB, Zhong GQ, Ke HH, He Y. Comparison of empiric isolation and conventional isolation of superior vena cava in addition to pulmonary vein isolation on the outcome of paroxysmal atrial fibrillation ablation. Int Heart J. 2017;58:500–5. https://doi.org/10.1536/ihj.16-460.
Sharma SP, Sangha RS, Dahal K, Krishnamoorthy P. The role of empiric superior vena cava isolation in atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. J Interv Card Electrophysiol. 2017;48:61–7. https://doi.org/10.1007/s10840-016-0198-2.
Antz M, Otomo K, Arruda M, Scherlag BJ, Pitha J, Tondo C, Lazzara R, Jackman WM. Electrical conduction between the right atrium and the left atrium via the musculature of the coronary sinus. Circulation. 1998;98:1790–5.
Di Biase L, Romero J, Briceno D, Valderrabano M, Sanchez JE, Della Rocca DG, Mohanty P, Horton R, Gallinghouse GJ, Mohanty S. Evidence of relevant electrical connection between the left atrial appendage and the great cardiac vein during catheter ablation of atrial fibrillation. Heart Rhythm. 2019;16:1039–46.
Yin X, Zhao Z, Gao L, Chang D, Xiao X, Zhang R, Chen Q, Cheng J, Yang Y, Xi Y. Frequency gradient within coronary sinus predicts the long-term outcome of persistent atrial fibrillation catheter ablation. J Am Heart Assoc. 2017;6:e004869.
Haïssaguerre M, Hocini M, Takahashi Y, O’neill MD, Pernat A, Sanders P, Jonsson A, Rotter M, Sacher F, Rostock T. Impact of catheter ablation of the coronary sinus on paroxysmal or persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:378–86.
Valderrábano M. Improving ablation results in persistent AF: is ethanol the answer? J Cardiovasc Electrophysiol. 2019;30:1229–30.
Hwang C, Chen PS. Ligament of Marshall: why it is important for atrial fibrillation ablation. Heart Rhythm. 2009;6:S35-40. https://doi.org/10.1016/j.hrthm.2009.08.034.
Kamakura T, Derval N, Duchateau J, Denis A, Nakashima T, Takagi T, Ramirez FD, André C, Krisai P, Nakatani Y, et al. Vein of Marshall ethanol infusion: feasibility, pitfalls, and complications in over 700 patients. Circ Arrhythm Electrophysiol. 2021;14:e010001. https://doi.org/10.1161/circep.121.010001.
Lador A, Valderrábano M. Atrial fibrillation ablation using vein of marshall ethanol infusion. Methodist Debakey Cardiovasc J. 2021;17:52–5. https://doi.org/10.14797/zqme8581.
Liu CM, Lo LW, Lin YJ, Lin CY, Chang SL, Chung FP, Chao TF, Hu YF, Tuan TC, Liao JN, et al. Long-term efficacy and safety of adjunctive ethanol infusion into the vein of Marshall during catheter ablation for nonparoxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2019;30:1215–28. https://doi.org/10.1111/jce.13969.
Valderrábano M, Peterson LE, Swarup V, Schurmann PA, Makkar A, Doshi RN, DeLurgio D, Athill CA, Ellenbogen KA, Natale A, et al. Effect of catheter ablation with vein of Marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: the VENUS randomized clinical trial. JAMA. 2020;324:1620–8. https://doi.org/10.1001/jama.2020.16195.
Liu TY, Tai CT, Huang BH, Higa S, Lin YJ, Huang JL, Yuniadi Y, Lee PC, Ding YA, Chen SA. Functional characterization of the crista terminalis in patients with atrial flutter: implications for radiofrequency ablation. J Am Coll Cardiol. 2004;43:1639–45. https://doi.org/10.1016/j.jacc.2003.11.057.
Proietti R, Hadjis A, AlTurki A, Thanassoulis G, Roux JF, Verma A, Healey JS, Bernier ML, Birnie D, Nattel S, et al. A systematic review on the progression of paroxysmal to persistent atrial fibrillation: shedding new light on the effects of catheter ablation. JACC Clin Electrophysiol. 2015;1:105–15. https://doi.org/10.1016/j.jacep.2015.04.010.
Morris GM, Segan L, Wong G, Wynn G, Watts T, Heck P, Walters TE, Nisbet A, Sparks P, Morton JB, et al. Atrial tachycardia arising from the crista terminalis, detailed electrophysiological features and long-term ablation outcomes. JACC Clin Electrophysiol. 2019;5:448–58. https://doi.org/10.1016/j.jacep.2019.01.014.
Yamada T, Murakami Y, Okada T, Murohara T. Focal atrial fibrillation associated with multiple breakout sites at the crista terminalis. Pacing Clin Electrophysiol. 2006;29:207–10. https://doi.org/10.1111/j.1540-8159.2006.00320.x.
Liu TY, Tai CT, Chen SA. Treatment of atrial fibrillation by catheter ablation of conduction gaps in the crista terminalis and cavotricuspid isthmus of the right atrium. J Cardiovasc Electrophysiol. 2002;13:1044–6. https://doi.org/10.1046/j.1540-8167.2002.01044.x.
Fynn SP, Morton JB, Deen VR, Kistler PM, Vohra JK, Sparks PB, Kalman JM. Conduction characteristics at the crista terminalis during onset of pulmonary vein atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:855–61. https://doi.org/10.1046/j.1540-8167.2004.03467.x.
Azizova A, Onder O, Arslan S, Ardali S, Hazirolan T. Persistent left superior vena cava: clinical importance and differential diagnoses. Insights Imaging. 2020;11:110. https://doi.org/10.1186/s13244-020-00906-2.
Liu H, Lim KT, Murray C, Weerasooriya R. Electrogram-guided isolation of the left superior vena cava for treatment of atrial fibrillation. Europace. 2007;9:775–80. https://doi.org/10.1093/europace/eum118.
Hsu LF, Jaïs P, Keane D, Wharton JM, Deisenhofer I, Hocini M, Shah DC, Sanders P, Scavée C, Weerasooriya R, et al. Atrial fibrillation originating from persistent left superior vena cava. Circulation. 2004;109:828–32. https://doi.org/10.1161/01.Cir.0000116753.56467.Bc.
Turagam MK, Atoui M, Atkins D, Di Biase L, Shivkumar K, Jared Bunch T, Mohanty S, Gianni C, Natale A, Lakkireddy D. Persistent left superior vena cava as an arrhythmogenic source in atrial fibrillation: results from a multicenter experience. J Interv Card Electrophysiol. 2019;54:93–100. https://doi.org/10.1007/s10840-018-0444-x.
Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8. https://doi.org/10.1161/circep.109.859116.
Cappato R, Negroni S, Pecora D, Bentivegna S, Lupo PP, Carolei A, Esposito C, Furlanello F, De Ambroggi L. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003;108:1599–604. https://doi.org/10.1161/01.Cir.0000091081.19465.F1.
Terricabras M, Piccini JP, Verma A. Ablation of persistent atrial fibrillation: challenges and solutions. J Cardiovasc Electrophysiol. 2020;31:1809–21. https://doi.org/10.1111/jce.14311.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SYY and MJC collected data and wrote a manuscript. HJO, MSC, JK, GBN, KJC reviewed and revised manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient/parent/guardian/relative of the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, S.Y., Cha, MJ., Oh, H.J. et al. Role of non-pulmonary vein triggers in persistent atrial fibrillation. Int J Arrhythm 24, 7 (2023). https://doi.org/10.1186/s42444-023-00088-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42444-023-00088-0