Abstract
Background
Although multiple algorithms based on surface electrocardiographic criteria have been introduced to localize idiopathic ventricular arrhythmia (VA) origins from the outflow tract (OT), their diagnostic accuracy and clinical usefulness remain limited. We evaluated whether local activation time of the His bundle region could differentiate left and right ventricular OT VA origins in the early stage of electrophysiology study.
Methods
We studied 30 patients who underwent catheter ablation for OT VAs with a left bundle branch block pattern and inferior axis QRS morphology. The interval between the local V signal on the mapping catheter placed in the RVOT and His bundle region (V(RVOT)-V(HB) interval) and the interval from QRS complex onset to the local V signal on the His bundle region (QRS-V(HB) interval) were measured during VAs.
Results
The V(RVOT)-V(HB) and QRS-V(HB) intervals were significantly shorter in patients with LVOT VAs. The area under the curve (AUC) for the V(RVOT)-V(HB) interval by receiver operating characteristic analysis was 0.865. A cutoff value of ≤ 50 ms predicted an LVOT origin of VA with sensitivity, specificity, and positive and negative predictive values of 100%, 62.5%, 40%, and 100%, respectively. The QRS-V(HB) interval showed similar diagnostic accuracy (AUC, 0.840), and a cutoff value of ≤ 15 ms predicted an LVOT origin of VA with a sensitivity, specificity, and positive and negative predictive values of 100%, 70.8%, 45.2%, and 100%, respectively.
Conclusion
The V(RVOT)-V(HB) and QRS-V(HB) intervals could differentiate left from right OT origins of VA with high sensitivity and negative predictive values.
Similar content being viewed by others
Introduction
Idiopathic ventricular arrhythmia (VA) in the structurally normal heart is a distinct entity with relative benign prognosis that differs from VA associated with structural heart diseases [1,2,3,4]. The outflow tract (OT) is the most common site of idiopathic VA origin [5], and radiofrequency catheter ablation of OT VA has been established as a reliable treatment option with high efficacy and acceptable safety [6, 7].
The complex anatomy of the OT renders difficulties in the evaluation and treatment of VAs originating from this region. Analysis of the 12-lead ECG morphology of clinical arrhythmias serves as a starting point for the effective localization and ablation of VAs by reducing unnecessary invasion through additional vascular access and catheter manipulation during mapping. The OTs are located superiorly, and OT VAs will tend to have an inferior axis with negative QS complexes in leads aVR and aVL [8]. Additional leads can further refine localization on ECG, and multiple algorithms based on surface ECG have been introduced to predict the origin of OT VAs [9,10,11,12,13]. However, the diagnostic accuracy of surface ECG-based algorithms is influenced by multiple factors [14,15,16,17], and precise prediction of the origin of OT VAs remains challenging.
Parameters with intracardiac electrogram obtained in the early stage of electrophysiology study (EPS) would assist in preemptive VA localization. The temporal relationship between the activation of a specific intracardiac region and another stable anatomical reference point should be consistent. His bundle catheters are routinely placed during EPS in many laboratories, and consistency of catheter location can be confirmed by His bundle deflection and atrial and ventricular electrograms.
We hypothesized that the local V signals at the His bundle region would be recorded earlier in the cardiac cycle in cases of VAs originating from the left ventricular outflow tract (LVOT) compared with those from the right ventricular outflow tract (RVOT) and that depiction of the differences in local activation time could provide a useful criterion for regionalizing the origin of OT VAs.
Methods
Study participants
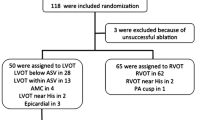
This retrospective study enrolled 30 consecutive patients with OT VA who underwent radiofrequency catheter ablation at Seoul National University Hospital between April 2009 and November 2016. All patients had clinical VAs with left bundle branch block pattern (R/S ≤ 1 in lead V1) and inferior axis QRS morphology documented with 12-lead ECGs. Transthoracic echocardiography was performed before radiofrequency catheter ablation, and patients with structural heart disease including arrhythmogenic right ventricular cardiomyopathy were excluded from the current analysis. All patients provided written informed consent for the EPS and catheter ablation procedure.
Cardiac electrophysiological study and radiofrequency catheter ablation
Antiarrhythmic drugs were discontinued for at least five half-lives before the radiofrequency catheter ablation procedure. We performed the EPS and catheter ablation in a non-sedated state. Multipolar catheters were routinely positioned in the His bundle region, right ventricular apex, and inside the coronary sinus under fluoroscopy guidance via the femoral veins (Fig. 1). All 12-lead ECG and intracardiac electrograms were recorded using a digital recording system (Prucka Cardio Lab electrophysiology system, GE Healthcare Milwaukee, WI, USA).
Electroanatomical mapping for VAs was performed using the CARTO 3 system (Biosense Webster, Inc., Diamond Bar, CA, USA) with a circular mapping catheter (Lasso, Biosense Webster) and a 7-Fr 3.5-mm open-irrigation-tip ablation catheter (Navistar Thermocool, Biosense Webster). In the case of absent or infrequent spontaneous VA during the EPS, the induction of VA was attempted by rapid pacing, programmed electrical stimulation, or isoproterenol infusion. When sustained ventricular tachycardia (VT), non-sustained VT, or premature ventricular contractions (PVCs) with the morphology of QRS complex matched to the clinical VA were induced, activation mapping was performed to identify the site of the earliest ventricular activation. Pace mapping was also performed using the distal bipolar electrodes of the mapping catheter, and paced 12-lead ECGs at the areas of interest were compared with those of the clinical VA. Mapping of VA was performed in the RVOT at first. When the RVOT mapping was not successful in identifying the earliest activation site, mapping of the LVOT was performed via transaortic approach. Intravenous heparin was administered to maintain an activated clotting time > 250 s during the left-sided procedure.
After activation and pace mapping of VA, radiofrequency energy was delivered at a power output of 30 to 40 W at sites with the earliest ventricular activation. When an acceleration or reduction in the incidence of VT or PVCs was observed during the first 10 s of application, the radiofrequency delivery was continued for 30 to 60 s. The end point of catheter ablation was the elimination and noninducibility of all clinically documented VAs.
Measured parameters
The 12-lead ECG and intracardiac electrogram recordings were retrospectively reviewed at a rate of 200 mm per second, and electrophysiological parameters were measured using the digital calipers of the Prucka Cardio Lab system. If intravenous isoproterenol was required to induce the clinical VA, measurements were performed under isoproterenol infusion. The QRS-V(HB) interval was measured from the onset of the QRS complex on a surface 12-lead ECG to the onset of the ventricular component of intracardiac His bundle electrograms during the clinical VA (Fig. 2). The onset of the QRS complex was defined as the earliest initial deflection from the isoelectric line on the 12-lead surface ECG. Additionally, the V(RVOT)-V(HB) interval was measured from the local ventricular signal on the mapping catheter placed in the RVOT region to the onset of the ventricular component of the intracardiac His bundle electrograms under fluoroscopic and electroanatomical guidance. To collect the local ventricular signals, the Lasso mapping catheter was advanced into the RVOT to the level of conus arteriosus. The cranio-caudal position of the mapping catheter was adjusted to the earliest set of endocardial activation times by visual estimation. While attempting to maintain the circular part of the catheter in a transverse position, local ventricular signals were recorded and the earliest local activation time in the RVOT (V(RVOT)) was obtained. All retrospective measurements were performed by two electrophysiologists. The inter-observer differences in measured parameter did not exceed 10 ms in any case, and the mean value of the measurements was used in the final analysis.
Representative measurements of V(RVOT)-V(HB) intervals (interval from the RVOT electrogram to the V signal in the His bundle electrogram) and QRS-V(HB) intervals (interval from the onset of the earliest QRS complex in 12-lead surface electrocardiogram to the V signal in the His bundle electrogram) in cases of ventricular arrhythmia originating from the RVOT
Statistical analysis
Baseline characteristics are presented as mean ± SD for continuous variables and number and percentage for categorical variables. Comparisons of baseline characteristics between groups with different VA origins were performed with independent-samples t tests for continuous variables and χ2 tests for categorical variables. The receiver operating characteristic (ROC) curve analysis was used to assess sensitivity and specificity. Accuracy was measured by the area under the curve (AUC) of the ROC, with a discrimination point that represented the maximum value of sensitivity and specificity. Statistical analyses were performed using SPSS Statistics 20.0 (SPSS, Inc., Chicago, IL, USA). Differences between groups were considered to be significant at a two-tailed P value < 0.05.
Results
Baseline clinical characteristics
The baseline clinical characteristic of 30 patients is summarized in Table 1. In the clinical evaluation, 28 patients had VT (18 patients had only non-sustained VT and ten patients had sustained VT in addition to non-sustained VT), and two patients had symptomatic premature ventricular contractions without VT. The origin of ventricular arrhythmia was localized in the RVOT in 24 patients (80%) and the LVOT in six patients (20%). Among patients with RVOT VA, the ventricular arrhythmia was eliminated by ablation in the septal aspect in 17 patients and the free wall in seven patients. For patients with LVOT VA, the successful site of ablation was the left coronary cusp in four patients and right coronary cusp in two patients. The mean fluoroscopy time was significantly longer among patients with LVOT VA compared to RVOT VA.
The V(RVOT)-V(HB) interval
The V(RVOT)-V(HB) intervals of patients with VA originating from the RVOT and LVOT are presented in Table 2 and Fig. 3a. Significant differences in mean and median values for the V(RVOT)-V(HB) interval were observed between two groups. The V(RVOT)-V(HB) interval was shorter in patients with LVOT VA, compared to that in patients with RVOT VA (mean 34.2 ± 12.6 ms vs. mean 56.3 ± 15.9 ms; P = 0.004). Receiver operating characteristic (ROC) analysis showed that the best discriminant value was ≤ 50 ms, with a sensitivity, specificity, positive predictive value, and negative predictive value of 100%, 62.5%, 40%, and 100%, respectively. The area under the curve (AUC) obtained by ROC analysis for V(RVOT)-V(HB) interval was 0.865 (Fig. 3b).
a Boxplots of the V(RVOT)-V(HB) intervals (interval from the RVOT electrogram to V signal in the His bundle electrogram) for idiopathic ventricular arrhythmia originating from the RVOT and LVOT. b ROC curve of the V(RVOT)-V(HB) interval. A V(RVOT)-V(HB) interval cutoff of ≤ 50 ms predicted an origin from the LVOT with 100% sensitivity and 62.5% specificity
QRS-V(HB) interval
QRS-V(HB) interval was also significantly different between patients with RVOT VA and LVOT VA (Table 2). The QRS-V(HB) interval was shorter in patients with LVOT VA, compared to that in patients with RVOT VA (mean 3.8 ± 13.0 ms vs. mean 20.6 ± 16.1 ms; P = 0.025). All patients with LVOT VA had a QRS-V(HB) interval of ≤ 15 ms (range − 18 to 15 ms), whereas 17 of 24 patients with RVOT VA had a QRS-V(HB) interval of 16 ms or more (Fig. 4a). Some overlap in QRS-V(HB) interval value between LVOT VA and RVOT VA was observed, and ROC analysis showed that the best discriminant value was ≤ 15 ms, with a sensitivity, specificity, positive predictive value, and negative predictive value of 100%, 70.8%, 45.2%, and 100%, respectively (AUC = 0.840) for the prediction of LVOT origin of VA (Fig. 4b).
a Boxplots of the QRS-V(HB) intervals (interval from the onset of the earliest QRS complex in 12-lead surface electrocardiogram to the V signal in the His bundle electrogram) for idiopathic ventricular arrhythmia originating from the RVOT and LVOT. b ROC curve of the QRS-V(HB) interval. A QRS-V(HB) interval cutoff of ≤ 15 ms predicted an origin from the LVOT with 100% sensitivity and 70.8% specificity
Discussion
In our retrospective analysis of a single-center registry, the interval from V(RVOT)-V(HB) and interval from QRS-V(HB) during VA were found to be useful in the localization of the origin of OT VAs. A V(RVOT)-V(HB) interval of ≤ 50 ms and QRS-V(HB) interval of ≤ 15 ms were the best cutoff values for the prediction of LVOT VA origin with high sensitivity and negative predictive value. Although there were substantial overlaps in these parameters between VAs of RVOT and LVOT origin, the median values were significantly different, and the current cutoff values were clinically practical to rule out an LVOT origin of VA.
Ventricular OTs have a complex three-dimensional anatomical structure, which complicates the recognition of VAs originating from these regions. Knowledge of the anatomy is crucial to understanding the electrophysiological features during the mapping of OT VAs. The His bundle is consistently located in the membranous portion of the interventricular septum, which lies between the juncture of the right and noncoronary cusps on the left side and the tricuspid annulus on the right side [18, 19]. Only the proximal portion of the RVOT merges with the tricuspid annulus and inflow portion of the RV close to the membranous septum, and the subpulmonary infundibulum of the RVOT overlies the anterior wall of the aortic sinuses. Consequently, the plane of the pulmonary valve and RVOT is located more superiorly than that of the aortic valve and LVOT, and the RVOT region is more distant from the His bundle compared with the LVOT in general. We measured the intervals of electrical activation time between references of the QRS complex or RVOT signal and the local V signal at the His bundle region; distinct anatomical distances from the RVOT or LVOT to His bundle region are reflected in a different range of parameters of OT VAs originating from each side.
The 12-lead surface ECG during VA provides comprehensive cues to regionalizing the origin of OT VA and assists in planning a focused EPS and ablation procedure. However, the precise differentiation of the VA focus is often not possible by examining the surface QRS morphology alone, primarily because VAs originating from separate locations of the OT can produce overlapping surface ECG morphologies. In addition, factors such as rotation of the cardiac axis [15], body habitus of the patients [14], and deviation of electrode placements [16] can alter the spatial relationship of the heart and surface electrodes and impair the consistency of surface ECG results, which limit the accuracy of algorithms predicting the origin of OT VAs based on these signals. Furthermore, as the surface ECG-based algorithms become more able to localize VAs with high accuracy and detail, multiple parameters are required, which may increase the chance of measurement error and increase intra- and inter-observer variability [17]. A number of surface ECG-based algorithms have been introduced and validated to distinguish the origin of OT VA [9,10,11,12,13], but each method has limitations. No single solution for every circumstance has been elucidated.
Although the noninvasive pre-procedural prediction of OT VA origin with surface ECG is helpful for planning ablation, differentiation of VA origin usually requires detailed mapping of both OTs. In this regard, parameters obtained during the EPS would supplement the initial localization of OT VA and contribute to an effective ablation procedure. The limited diagnostic accuracy of ECG-based algorithms has been concerning, especially in the case of OT VAs with precordial transition at lead V3 [12, 20]. In our cohort, six patients presented with left bundle branch block and inferior axis QRS morphology with precordial transition at lead V3 (RVOT VA, 5; LVOT VA, 1). Of these patients, both a V(RVOT)-V(HB) interval of ≤ 50 ms and QRS-V(HB) interval of ≤ 15 ms correctly classified the origin of VA with 100% accuracy.
The His bundle region and RVOT are routinely assessed with catheters during EPS for OT VAs, and hence, V(RVOT)-V(HB) and QRS-V(HB) target intervals could readily be measured without the additional introduction of catheters. Current data showed that V(RVOT)-V(HB) interval and QRS-V(HB) interval could reliably discriminate VAs of LVOT origin from those of RVOT origin. In particular, our cutoff values effectively discriminated an LVOT origin with a high negative predictive value. Based on these parameters, operators could avoid the unnecessary risk of LVOT exploration and focus on detailed mapping in the RVOT region with confidence.
Study limitations
The results of the present study should be interpreted in the context of several limitations. First, we performed a retrospective analysis with pre-acquired electrogram in established OT VA cases to construct the proposed criteria. A relatively small number of OT VA cases are included, especially in the LVOT VA group, which included only six cases. A prospective analysis with adequate sample size should be performed to validate and generalize our results. Second, the V(RVOT)-V(HB) interval and the QRS-V(HB) interval showed high sensitivity, but only moderate specificity, in discrimination of an LVOT origin of VA. Considerable RVOT VA cases were over the proposed cutoff points regarding these parameters, and the practical utility of our criteria is currently limited to excluding an LVOT origin of VA with high negative predictive values. Although our criteria do not contribute to identification of an LVOT origin of VA, unnecessary invasive procedures requiring access to left-sided cardiac chambers could possibly be avoided, as these procedures are accompanied by the risk of stroke or inadvertent coronary artery damage. Focused mapping of the RVOT can also be accomplished with our criteria.
Conclusions
The current study presents novel parameters based on intracardiac His bundle electrogram, which can differentiate the origin of OT VAs with acceptable diagnostic accuracy. The V(RVOT)-V(HB) and QRS-V(HB) intervals have practical utility in assisting the early recognition and exclusion of an LVOT origin of VAs by simple methods with routine catheter placement during EPS.
Availability of data and materials
The data used in the current study are available from the corresponding author on reasonable request.
References
Froment R, Gallavardin L, Cahen P. Paroxysmal ventricular tachycardia; a clinical classification. Br Heart J. 1953;15(2):172–8.
Buxton AE, Waxman HL, Marchlinski FE, Simson MB, Cassidy D, Josephson ME. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation. 1983;68(5):917–27.
Brooks R, Burgess JH. Idiopathic ventricular tachycardia. A review. Medicine. 1988;67(5):271–94.
Lerman BB, Stein KM, Markowitz SM. Mechanisms of idiopathic left ventricular tachycardia. J Cardiovasc Electrophysiol. 1997;8(5):571–83.
Prystowsky EN, Padanilam BJ, Joshi S, Fogel RI. Ventricular arrhythmias in the absence of structural heart disease. J Am Coll Cardiol. 2012;59(20):1733–44.
Coggins DL, Lee RJ, Sweeney J, Chein WW, Van Hare G, Epstein L, et al. Radiofrequency catheter ablation as a cure for idiopathic tachycardia of both left and right ventricular origin. J Am Coll Cardiol. 1994;23(6):1333–41.
Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della-Bella P, Dickfeld T, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm. 2014;11(10):e166–96.
Asirvatham SJ. Correlative anatomy for the invasive electrophysiologist: outflow tract and supravalvar arrhythmia. J Cardiovasc Electrophysiol. 2009;20(8):955–68.
Kamakura S, Shimizu W, Matsuo K, Taguchi A, Suyama K, Kurita T, et al. Localization of optimal ablation site of idiopathic ventricular tachycardia from right and left ventricular outflow tract by body surface ECG. Circulation. 1998;98(15):1525–33.
Ito S, Tada H, Naito S, Kurosaki K, Ueda M, Hoshizaki H, et al. Development and validation of an ECG algorithm for identifying the optimal ablation site for idiopathic ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol. 2003;14(12):1280–6.
Yang Y, Saenz LC, Varosy PD, Badhwar N, Tan JH, Kilicaslan F, et al. Using the initial vector from surface electrocardiogram to distinguish the site of outflow tract tachycardia. Pacing Clin Electrophysiol (PACE). 2007;30(7):891–8.
Betensky BP, Park RE, Marchlinski FE, Hutchinson MD, Garcia FC, Dixit S, et al. The V(2) transition ratio: a new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. J Am Coll Cardiol. 2011;57(22):2255–62.
Yoshida N, Yamada T, McElderry HT, Inden Y, Shimano M, Murohara T, et al. A novel electrocardiographic criterion for differentiating a left from right ventricular outflow tract tachycardia origin: the V2S/V3R index. J Cardiovasc Electrophysiol. 2014;25(7):747–53.
Fraley MA, Birchem JA, Senkottaiyan N, Alpert MA. Obesity and the electrocardiogram. Obesity Rev. 2005;6(4):275–81.
Yoshida N, Inden Y, Uchikawa T, Kamiya H, Kitamura K, Shimano M, et al. Novel transitional zone index allows more accurate differentiation between idiopathic right ventricular outflow tract and aortic sinus cusp ventricular arrhythmias. Heart Rhythm. 2011;8(3):349–56.
Anter E, Frankel DS, Marchlinski FE, Dixit S. Effect of electrocardiographic lead placement on localization of outflow tract tachycardias. Heart Rhythm. 2012;9(5):697–703.
Jamil-Copley S, Bokan R, Kojodjojo P, Qureshi N, Koa-Wing M, Hayat S, et al. Noninvasive electrocardiographic mapping to guide ablation of outflow tract ventricular arrhythmias. Heart Rhythm. 2014;11(4):587–94.
Sehar N, Mears J, Bisco S, Patel S, Lachman N, Asirvatham SJ. Anatomic guidance for ablation: atrial flutter, fibrillation, and outflow tract ventricular tachycardia. Indian Pacing Electrophysiol J. 2010;10(8):339–56.
Tabatabaei N, Asirvatham SJ. Supravalvular arrhythmia: identifying and ablating the substrate. Circ Arrhythmia Electrophysiol. 2009;2(3):316–26.
Tanner H, Hindricks G, Schirdewahn P, Kobza R, Dorszewski A, Piorkowski C, et al. Outflow tract tachycardia with R/S transition in lead V3: six different anatomic approaches for successful ablation. J Am Coll Cardiol. 2005;45(3):418–23.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
W-S Choe has made substantial contributions to the conception, design of the work, the acquisition, analysis, and interpretation of data and has drafted the work. S-RL, M-JC, and E-KC have made substantial contributions to the data collection, conception, and interpretation of data. SO has made substantial contributions to the data collection, conception, design of the work, the acquisition, analysis, and interpretation of data and has drafted the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (no. 2006-219-1138). Informed consent was waived because of the retrospective nature of the study and the analysis used anonymous clinical data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choe, WS., Lee, SR., Cha, MJ. et al. Differentiation of the right versus left outflow tract ventricular arrhythmias using local activation time at the His bundle electrogram. Int J Arrhythm 21, 15 (2020). https://doi.org/10.1186/s42444-020-00023-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42444-020-00023-7