Abstract
Background
In patients with atrial fibrillation (AF), most biomarkers are still of limited use due to cost-effectiveness and complexity in clinical practice.
Hypotheses
Biomarkers from routine blood tests improve the current risk stratification in AF patients.
Methods
This prospective study enrolled 600 patients diagnosed with non-valvular AF, of whom 537 were analyzed. Platelet count; platelet distribution width (PDW); red cell distribution width (RDW); and creatinine, D-dimer, and troponin I levels were measured at enrollment.
Results
During the mean follow-up period (2.2 ± 0.6 years), 1.9% patients developed ischemic stroke. According to the optimal cutoff of each biomarker, the risk of ischemic stroke was higher in patients with RDW ≥ 13.5%, creatinine ≥ 1.11 mg/dL, or PDW ≥ 13.2% (significant biomarkers; P value: < 0.01, 0.04, or 0.07, respectively). These 3 significant biomarkers had higher information gain than clinical risk factors in predicting ischemic stroke. The cumulative incidence of ischemic stroke was 1.2%, 1.1%, 8.4%, and 40.0% in patients with 0, 1, 2, and 3 significant biomarkers, respectively (P-for-trend < 0.001). Patients with ≥ 2 significant biomarkers had a significantly higher risk of ischemic stroke than those with < 2 significant biomarkers (adjusted hazard ratio 11.5, 95% confidence interval 3.3–40.2, P < 0.001). The predictability for ischemic stroke was significantly improved when ≥ 2 significant biomarkers were added to the CHA2DS2–VASc score (area under the curve 0.790 vs. 0.620, P = 0.043).
Conclusion
Routine blood tests can provide better risk stratification of AF along with clinical risk factors.
Similar content being viewed by others
Introduction
In the prediction of thromboembolic risk in patients with non-valvular atrial fibrillation (AF), the CHA2DS2–VASc score (1 point for congestive heart failure, hypertension, age 65–74 years, diabetes mellitus, vascular disease, and female sex, and 2 points for age ≥ 75 years and prior stroke/transient ischemic attack/thromboembolic event) is the most widely used system in clinical practice. However, the CHA2DS2–VASc score has shown modest predictive ability [1], and thromboembolic events still occur in patients with a CHA2DS2–VASc score of 0 or 1 [2]. To refine the traditional risk stratification system, approaches based on several biomarkers have emerged for the assessment of thromboembolic risk [3, 4]. By employing several biomarkers, new scoring systems have recently been proposed to enhance the predictability of thromboembolic events and to provide guidance for anticoagulation therapy in patients with AF [5, 6]. However, in real-world clinical practice, the use of most newly introduced biomarkers is still limited owing to cost-effectiveness issues and complexity of utilization. Further, it is not easy to obtain those biomarkers only for the purpose of risk stratification of patients with non-valvular AF. Thus, the CHA2DS2–VASc score remains the most predominant tool for risk stratification in patients with AF despite its limitations. From this viewpoint, we hypothesized that a biomarker would be practically useful if it could be easily obtainable in clinical practice and if it could provide incremental prognostic value over the CHA2DS2–VASc score. In this regard, we designed a prospective cohort study to identify clinically relevant predictors among easily accessible biomarkers in clinical practice.
Methods
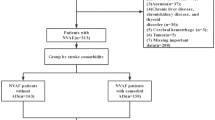
A total of 600 patients who visited the outpatient clinic of Seoul National University Hospital and were diagnosed with non-valvular AF between 2015 and 2017 were prospectively enrolled to estimate the efficacy of simple biomarkers. All patients with AF older than 19 years who agreed to be registered in the study were included. Informed consent was obtained from all patients before enrollment. The exclusion criteria were a history of stroke/transient ischemic attack/thromboembolic events and a prosthetic device exposed to the left heart, such as a left atrial appendage occluder, prosthetic mitral valve, prosthetic aortic valve, and atrial septal defect closing device. Among the initially enrolled 600 patients, 537 patients were included in the final analysis after excluding 10 patients with previous stroke/transient ischemic attack/thromboembolic events not recognized at the initial visit, 24 patients who refused blood sample collection after enrollment, 3 patients who experienced an ischemic stroke event after study enrollment before baseline blood sampling, and 26 patients who were lost to follow-up after the initial visit (Fig. 1). The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (No. 1410-062-619), and this study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients before enrollment.
We collected data on biomarkers from all patients within 6 months after enrollment. Platelet count, platelet distribution width (PDW), red cell distribution width (RDW), creatinine level, D-dimer level, and troponin I level were analyzed as markers of platelet reactivity, general patient performance, renal function, thromboembolism, and myocyte injury, respectively. These biomarkers were selected because they are easily obtainable as part of routine blood tests in common practice, and their efficacy in predicting thromboembolic events in patients with AF has been reported [5, 7,8,9,10,11]. To obtain biomarkers, a blood sample was acquired by a professional nurse through vein puncture and was collected in an anticoagulant (ethylenediaminetetraacetic acid [EDTA] or citrate) for CBC test. RDW and PDW were calculated by [standard deviation (SD) of mean corpuscular volume (MCV)/mean MCV] × 100 for red blood cell and platelet, respectively. Assays for serum creatinine were achieved at the Seoul National University Hospital laboratory. We finally selected the significant biomarkers in predicting the endpoint based on the Cox proportional hazard analysis.
The primary endpoint was thromboembolic events, defined as incident transient ischemic attack, ischemic stroke, or systemic arterial embolism, during the follow-up period. The follow-up period ended on December 31, 2018. The event duration was defined as the time from the collection of blood samples for evaluating each biomarker to the achievement of the primary endpoint.
Continuous variables are shown as mean ± standard deviation or median (interquartile range) based on the distribution of variables, and categorical variables are shown as number (percentage). Comparisons between 2 groups were performed using the Student’s t test or the Mann–Whitney U test for continuous variables, as appropriate. The optimal cutoff of each biomarker was assessed by maximizing the difference in log-rank statistics. Cox proportional hazard regression was performed to examine the risk of the endpoint according to the biomarkers, and biomarkers with P < 0.10 were considered significant biomarkers. The cumulative incidence according to the optimal cutoff of significant biomarkers was compared using Kaplan–Meier survival analysis and the log-rank test. Information gain was used to compare the relative importance of significant biomarkers with clinical risk factors. Variables with higher information gain were considered more important in the prediction of the endpoint. For sensitivity analysis, 10,000 bootstrapping was performed, and the mean value is presented with the 95% confidence interval. The discriminatory ability of the model was estimated using the area under the curve (AUC) in receiver operating characteristic analysis. The reclassification performance of the model with the CHA2DS2–VASc score and the selected biomarkers was compared with that of model with the CHA2DS2–VASc score alone, using the category-free net reclassification index (NRI) and the relative integrated discrimination improvement (IDI). P values < 0.05 were considered statistically significant. All analyses were performed using R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The baseline characteristics of the study population are shown in Table 1. The mean age was 67.7 ± 7.6 years, and the proportion of patients with a CHA2DS2–VASc score of ≥ 2 was 66.5%. The proportion of patients who received anticoagulation at enrollment was 71.1%. During the mean follow-up period of 2.2 ± 0.6 years, thromboembolic events occurred in 10 patients and all were ischemic stroke. Patients with incident ischemic stroke were older and had higher CHA2DS2–VASc scores than those without ischemic stroke. The value of each biomarker at baseline according to the incidence of ischemic stroke is shown in Additional file 1: Supplementary Table 1. According to the optimal cutoff of each biomarker based on maximal log-rank statistics, the risk of ischemic stroke was higher in patients with RDW ≥ 13.5%, creatinine ≥ 1.11 mg/dL, or PDW ≥ 13.2% (P < 0.01, P = 0.04, or P = 0.07, respectively; Table 2). The result was similar after adjustment for anticoagulant use and the CHA2DS2–VASc score (Table 2). Accordingly, we defined RDW, creatinine, and PDW as significant biomarkers.
The cumulative incidence of ischemic stroke was higher in patients with RDW ≥ 13.5%, creatinine ≥ 1.11 mg/dL, or PDW ≥ 13.2% than in the other patients (log-rank P < 0.001, P = 0.030, or P = 0.055, respectively; Additional file 2: Supplementary Figure 1). According to the information gain criterion, RDW ≥ 13.5% was the most important predictor of ischemic stroke, followed by creatinine ≥ 1.11 mg/dL and PDW ≥ 13.2%, and all these 3 significant biomarkers had higher information gain than clinical risk factors in the prediction of ischemic stroke (Fig. 2a). The cumulative incidence of ischemic stroke was 1.2%, 1.1%, 8.4%, and 40.0% in patients with 0, 1, 2, and 3 significant biomarkers, respectively (P-for-trend < 0.001, Fig. 2b). The risk of ischemic stroke also increased with an increase in the number of significant biomarkers (Additional file 1: Supplementary Table 2). Patients with ≥ 2 significant biomarkers had a significantly higher risk of ischemic stroke than those with < 2 significant biomarkers (unadjusted hazard ratio [HR] 12.1, 95% CI 3.5–41.9, P < 0.001). This result persisted after adjustment for anticoagulant use and the CHA2DS2–VASc score (adjusted HR 11.5, 95% CI 3.3–40.2, P < 0.001).
a Relative importance of significant biomarkers and clinical risk factors in the prediction of ischemic stroke. b Cumulative incidence of ischemic stroke according to the number of significant biomarkers. The significant biomarkers were RDW ≥ 13.5%, creatinine ≥ 1.11 mg/dL, and PDW ≥ 13.2%. PDW, platelet distribution width; RDW, red cell distribution width
Among 178 patients with a CHA2DS2–VASc score of ≤ 1, the rate of ischemic stroke was significantly higher in those with ≥ 2 significant biomarkers than in those with < 2 (11.1% vs. 0.0%, P = 0.040; Additional file 1: Supplementary Table 3). Among 355 patients with a CHA2DS2–VASc score of ≥ 2, the rate of ischemic stroke was still significantly higher in those with ≥ 2 significant biomarkers than in those with < 2 (11.8% vs. 1.6%, P = 0.002; Additional file 1: Supplementary Table 3). In the prediction of ischemic stroke, the predictive and discriminatory abilities were significantly improved when ≥ 2 significant biomarkers were added to the CHA2DS2–VASc score (AUC 0.790 vs. 0.620, P = 0.043; category-free NRI 0.855, P = 0.007; IDI 0.047, P = 0.015; Fig. 3).
Incremental prognostic value of ≥ 2 significant biomarkers over the CHA2DS2-VASc score. *category-free NRI. The significant biomarkers were RDW ≥ 13.5%, creatinine ≥ 1.11 mg/dL, and PDW ≥ 13.2%. IDI integrated discrimination improvement; NRI net reclassification index; PDW platelet distribution width; RDW red cell distribution width
Discussion
In the current prospective study of patients with non-valvular AF, we aimed to identify clinically relevant biomarkers that are easily accessible in clinical practice and to determine their prognostic value. The major findings were as follows: (1) RDW, creatinine, and PDW were significant biomarkers in predicting ischemic stroke during the prospective follow-up; (2) these 3 biomarkers showed higher importance than clinical risk factors in the prediction of ischemic stroke; (3) the risk of ischemic stroke was significantly higher in patients with ≥ 2 significant biomarkers than in those with < 2 significant biomarkers; (4) the addition of ≥ 2 significant biomarkers to the CHA2DS2–VASc score provided a significant improvement of the predictive ability for ischemic stroke.
New scoring systems based on biomarkers have been developed and validated. In the subanalysis of a cohort from the ENGAGE AF-TIMI 48 trial (Effective Anticoagulation with Factor Xa Next Generation in AF—Thrombolysis in Myocardial Infarction 48), incorporation of troponin I, N-terminal B-type natriuretic peptide, and D-dimer significantly enhanced the risk assessment for stroke, systemic embolic event, or death in patients with AF [5]. In another subanalysis from the ENGAGE AF-TIMI 48 trial, the thrombolysis in myocardial infarction-AF score was demonstrated to assist in the prediction of a composite clinical outcome [12]. Another novel scoring system—the ABC (age, biomarker, clinical history) risk score, including high-sensitivity troponin T and N-terminal B-type natriuretic peptide—was developed [6], and its performance was recently validated in an external cohort [13]. Although all these scoring systems have shown the possibility of improving risk stratification in patients with AF by employing biomarkers, their application remains limited. One of the reasons might be the requirement for an additional blood test, time, and cost in real-world clinical practice. In this regard, we focused on biomarkers that can be assessed as part of routine clinical tests without additional blood sampling or cost and sought to comprehensively evaluate their efficacy to improve the current risk stratification of patients with AF. As a result, we found that RDW, creatinine, and PDW were clinically relevant in thromboembolic risk prediction in patients with AF, and that the combination of these factors can provide better predictive ability in addition to clinical risk factors. These 3 biomarkers are robust in terms of generalizability because the values of PDW and RDW are usually available in most patients undergoing routine complete blood count tests, and creatinine is also a general marker usually measured in most patients.
Although the exact mechanism should be dissected in the future studies, The prognostic value of each biomarker in patients with AF has already been suggested. RDW has been shown to be a predictor of thromboembolic events or all-cause mortality in patients with AF [14, 15]. Consistent with previous studies, we proved the predictive value of RDW for ischemic stroke among patients with AF in a prospective manner. Interestingly, RDW was the most important biomarker in the current study. Several studies seem to support the importance of RDW in patients with AF. An elevated RDW was found to be significantly associated with carotid atherosclerosis [16, 17] and was independently correlated with the presence of left atrial/left atrial appendage thrombus [18]. One study suggested that RDW was significantly associated with poor anticoagulation control in patients with AF [19]. Biochemically, RDW has been considered a marker of inflammation, nutritional deficiency, and oxidative stress [20]. These studies implied that RDW represents not a single but several diverse mechanisms of ischemic stroke in patients with AF, which may partially explain the predictive value of RDW was higher than a single risk factor in the current study. The relationship between impaired renal function and increased thromboembolism risk has been well established in patients with AF [21, 22]. Its prognostic value for thromboembolic risk and cardiac mortality when added to the CHA2DS2–VASc score has been proposed [23]. We also demonstrated the efficacy of creatinine in predicting ischemic stroke and postulated that renal function can be considered a marker for enhancing the predictive ability of the CHA2DS2–VASc score. PDW, a marker reflective of platelet activity, was also a significant biomarker, as in a previous study [7]. One of the determinants of hypercoagulability is platelet hyperactivity, and it is associated with thrombogenesis in AF [24]. Combined together, the 3 significant biomarkers reflect systemic inflammatory status, renal function, and hypercoagulable state, and these attributes may complement the aspect of thromboembolic risk, which cannot be reflected by the CHA2DS2–VASc score alone.
The current study had several limitations. First, a potential bias might exist because the high-risk group based on the traditional scoring system, not on biomarkers, underwent intensive medical therapy, and this could affect the incidence rate of ischemic stroke. However, we measured the biomarkers at baseline regardless of therapy, and the biomarkers could still reflect the patients’ clinical status at enrollment. Second, the cutoff of each biomarker may not be generalizable to different study populations. Despite the limitations, this study still has strengths, as it was a prospective cohort study designed only for the evaluation of simple biomarkers. Third, the number of events was not enough to postulate the linear relationship between significant biomarkers and the incidence of ischemic stroke in the current study. Therefore, future studies are warranted to validate our findings.
Conclusion
In this study, RDW, creatinine, and PDW, which can be assessed as part of routine blood tests, were robust predictors of ischemic stroke in a prospective cohort of patients with AF. The combination of these 3 biomarkers provided incremental predictive value for ischemic stroke over the CHA2DS2–VASc score. Therefore, the clinical application of these biomarkers can provide better risk stratification of patients with AF in daily practice.
Availability of supporting data
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AF:
-
Atrial fibrillation
- IDI:
-
Integrated discrimination improvement
- NRI:
-
Net reclassification index
- PDW:
-
Platelet distribution width
- RDW:
-
Red cell distribution width
References
Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–72.
Kim TH, Yang PS, Uhm JS, et al. CHA2DS2–VASc score (congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female) for stroke in Asian patients with atrial fibrillation: a Korean nationwide sample cohort study. Stroke. 2017;48:1524–30.
Hijazi Z, Oldgren J, Siegbahn A, et al. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J. 2013;34:1475–80.
Hijazi Z, Oldgren J, Siegbahn A, et al. Application of biomarkers for risk stratification in patients with atrial fibrillation. Clin Chem. 2017;63:152–64.
Ruff CT, Giugliano RP, Braunwald E, et al. Cardiovascular biomarker score and clinical outcomes in patients with atrial fibrillation: a subanalysis of the ENGAGE AF-TIMI 48 randomized clinical trial. JAMA Cardiol. 2016;1:999–1006.
Hijazi Z, Lindbäck J, Alexander JH, et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J. 2016;37:1582–90.
Lyu QS, Liu B, Huang C, et al. The association between platelet distribution width and stroke in atrial fibrillation patients. Ann Clin Lab Sci. 2019;49:143–7.
Du J, Wang Q, He B, et al. Association of mean platelet volume and platelet count with the development and prognosis of ischemic and hemorrhagic stroke. Int J Lab Hematol. 2016;38:233–9.
Cha MJ, Lee HS, Kim HM, et al. Association between red cell distribution width and thromboembolic events in patients with atrial fibrillation. Eur J Intern Med. 2017;46:41–6.
Cha MJ, Cho Y, Oh IY, et al. Validation of conventional thromboembolic risk factors in a Korean atrial fibrillation population—suggestion for a novel scoring system, CHA2DS2-VAK. Circ J. 2018;82:2970–5.
Hijazi Z, Siegbahn A, Andersson U, et al. High-sensitivity troponin I for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOLE) trial. Circulation. 2014;129:625–34.
Fanola CL, Giugliano RP, Ruff CT, et al. A novel risk prediction score in atrial fibrillation for a net clinical outcome from the ENGAGE AF-TIMI 48 randomized clinical trial. Eur Heart J. 2017;38:888–96.
Berg DD, Ruff CT, Jarolim P, et al. Performance of the ABC scores for assessing the risk of stroke or systemic embolism and bleeding in patients with atrial fibrillation in ENGAGE AF-TIMI 48. Circulation. 2019;139:760–71.
Wan H, Yang Y, Zhu J, et al. The relationship between elevated red cell distribution width and long-term outcomes among patients with atrial fibrillation. Clin Biochem. 2015;48:762–7.
Saliba W, Barnett-Griness O, Elias M, et al. The association between red cell distribution width and stroke in patients with atrial fibrillation. Am J Med. 2015;128:192.e11–8.
Söderholm M, Borné Y, Hedblad B, et al. Red cell distribution width in relation to incidence of stroke and carotid atherosclerosis: a population-based cohort study. PLoS ONE. 2015;10:e0124957.
Lappegård J, Ellingsen TS, Vik A, et al. Red cell distribution width and carotid atherosclerosis progression. The Tromsø study. Thromb Haemost. 2015;113:649–54.
Kaya A, Tukkan C, Alper AT, et al. Increased levels of red cell distribution width is correlated with presence of left atrial stasis in patients with non-valvular atrial fibrillation. North Clin Istanb. 2017;4:66–72.
Lee KH, Cho JG, Park HW, et al. Role of red cell distribution width in the relationship between clinical outcomes and anticoagulation response in patients with atrial fibrillation. Chonnam Med J. 2018;54:113–20.
Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol. 2000;7:113–6.
Banerjee A, Fauchier L, Vourc’h P, et al. A prospective study of estimated glomerular filtration rate and outcomes in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Chest. 2014;145:1370–82.
Nakagawa K, Hirai T, Takashima S, et al. Chronic kidney disease and CHADS(2) score independently predict cardiovascular events and mortality in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2011;107:912–6.
Lin WY, Lin YJ, Chung FP, et al. Impact of renal dysfunction on clinical outcome in patients with low risk of atrial fibrillation. Circ J. 2014;78:853–8.
Makowski M, Smorag I, Makowska J, et al. Platelet reactivity and mean platelet volume as risk markers of thrombogenesis in atrial fibrillation. Int J Cardiol. 2017;235:1–5.
Acknowledgements
Not applicable.
Funding
This study was supported by Grant No. 25-2014-0150 from the Seoul National University Hospital Research Fund.
Author information
Authors and Affiliations
Contributions
Seokhun Yang and Myung-Jin Cha have made substantial contributions to the conception, design of the work, the acquisition, analysis, interpretation of data, and have drafted the work and revised it. Soongu Kwak, Soonil Kwon, Seoyoung Lee, Jiesuck Park, You-jung Choi, Inki Moon, Euijae Lee, So-Ryoung Lee, and Eue-Keun Choi have made substantial contributions to the conception, interpretation of data, and have reviewed the work and revised it. Seil Oh has1 made substantial contributions to the conception, design of the work, the acquisition, analysis, interpretation of data, and has drafted the work and revised it. All the authors have approved the submitted version.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (No. 1410-062-619).
Informed consent
Informed consent was obtained from all patients before enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 2.
Cumulative incidence of ischemic stroke according to (a) RDW ≥ 13.5%, (b) creatinine ≥ 1.11 mg/dL, and (c) PDW ≥ 13.2%. PDW, platelet distribution width; RDW, red cell distribution width.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, S., Cha, MJ., Kwak, S. et al. Prognostic value of routine blood tests along with clinical risk factors in predicting ischemic stroke in non-valvular atrial fibrillation: a prospective cohort study. Int J Arrhythm 21, 10 (2020). https://doi.org/10.1186/s42444-020-00018-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42444-020-00018-4