Abstract
Background
Osteoarthritis (OA) affects the entire joint, causing structural changes in articular cartilage, subchondral bone, ligaments, capsule, synovial membrane, and periarticular muscles that afflicts millions of people globally, leading to persistent pain and diminished quality of life. The intra-articular use of platelet-rich plasma (PRP) is gaining recognition as a secure therapeutic approach due to its potential regenerative capabilities. However, there is controversial clinical data regarding efficacy of PRP for OA treatment. In this context, gathering scientific evidence on the effects of PRP in treating OA in animal models could provide valuable insights into understanding its impact on aspects like cartilage health, synovial tissue integrity, and the inflammatory process in affected joints. Thus, the objective of this study was to assess the effects of PRP injections on inflammation and histopathological aspects of cartilage and synovium in animal models of OA through a comprehensive systematic review with meta-analysis.
Methods
A electronic search was conducted on Medline, Embase, Web of Science, The Cochrane Library, LILACS, and SciELO databases for relevant articles published until June 2022. A random-effects meta-analysis was employed to synthesize evidence on the histological characteristics of cartilage and synovium, as well as the inflammatory process. The GRADE approach was utilized to categorize the quality of evidence, and methodological quality was assessed using SYRCLE’s RoB tool.
Results
Twenty-one studies were included in the review, with twelve of them incorporated into the meta-analysis. PRP treatment demonstrated superior outcomes compared to the control group in terms of cartilage histology (very low quality; p = 0.0002), synovium histology (very low quality; p < 0.0001), and reductions in proinflammatory markers, including IL-1 (low quality; p = 0.002), IL-6 (very low quality; p < 0.00001), and TNF-α (very low; p < 0.00001). However, PRP treatment did not yield a significant impact on PDGF-A levels (very low quality; p = 0.81).
Conclusion
PRP appears capable of reducing proinflammatory markers (IL-1, IL-6, TNF-α) and mitigating cartilage and synovium damage in animals with OA. However, the levels of evidence of these findings are low to very low. Therefore, more rigorous studies with larger samples are needed to improve the quality of evidence.
PROSPERO registration
CRD42022250314
Similar content being viewed by others
Introduction
Osteoarthritis (OA) stands as one of the most widespread joint diseases [1], impacting an estimated 302 million individuals globally, including over 30 million in the United States alone [1, 2]. The well-being, both physical and mental, of those affected is significantly compromised due to pain, inflammation, and a decline in functionality [3, 4]. Beyond affecting public health systems, OA incurs socioeconomic costs through reduced work productivity and premature retirement [3, 5,6,7].
Treatment options for OA encompass surgical, non-pharmacological, and pharmacological approaches [8]. While surgical interventions are typically reserved for advanced stages when conservative treatments have proven ineffective [9], both non-pharmacological and pharmacological strategies are employed across mild and severe cases to alleviate pain, reduce joint stiffness, and preserve functionality [10]. Non-pharmacological methods include physical exercise, lifestyle adjustments, and self-management programs, which are strongly advocated for individuals with OA affecting the hand, hip, knee, or polyarthritis [2, 11,12,13,14,15]. Conversely, pharmacological approaches involve medications administered orally or via joint injection, with varying levels of recommendation [2, 15].
Intra-articular applications with platelet-rich plasma (PRP) have received attention in the treatment of OA [16, 17]. PRP is a blood product with a high platelet concentration obtained through the centrifugation of autologous venous blood [18, 19]. This enriched substance has a large diversity of growth factors (GFs) and other bioactive mediators [20, 21]. The regenerative potential of these substances is based on the functions of metabolic regulation, cell proliferation and extracellular matrix synthesis [22, 23]. With regard to cartilaginous tissue, a significant increase was found in the synthesis of extracellular matrix in chondrocytes treated with GFs [24]. Moreover, PRP proved to be efficient at reducing inflammatory markers and apoptosis in vivo [25, 26].
In spite of reported benefits, the Osteoarthritis Research Society International (OARSI) and the American College of Rheumatology (ACR) strongly advises against the intra-articular injection of PRP in individuals with OA. This caution is driven by the presence of low-quality evidence supporting its efficacy and the lack of standardization in its manufacturing process, encompassing variables such as the duration and speed of centrifugation, the use of anticoagulants and activators, and the concentration of platelets [2, 15, 27]. In this context, the compilation of scientific evidence regarding the effects of PRP in the treatment of OA in animal models could offer valuable insights into comprehending the impact of this therapeutic approach on various aspects such as cartilage health, synovial tissue integrity, and the inflammatory cascade within the affected joints. Therefore, the aim of systematic review with meta-analysis to investigate the effect of the intra-articular injection of PRP on the inflammatory process and histopathological characteristics of cartilage and synovium in animal models with OA.
Methods
Protocol and registration
The present review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [28] and the recommendations of the Cochrane Collaboration [29]. The quality of the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE approach) [30].
The following question was used to guide this study: “How does the intra-articular injection of PRP affect the inflammatory process and histopathological characteristics of cartilage and synovium in animals with induced lesions aimed at developing OA?” To ensure a comprehensive analysis as well as the transparency of the methods and results, the protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration code: #CRD42022250314). As this was a systematic review of preclinical trials, there was no need for approval from an ethics committee.
Eligibility criteria
Types of studies
Preclinical trials that evaluated the inflammatory process and histopathological characteristics following the intra-articular injection of PRP in animals with OA were included. Papers published in English, Portuguese and Spanish were considered.
Types of participants
Studies with any animal model that developed OA through surgical or pharmacological interference were included.
Types of comparators
Studies with comparison groups (control) treated either with a placebo or not submitted to any treatment were included.
Types of treatments
Studies that employed the intra-articular injection of PRP as the treatment were included. No restrictions were imposed regarding the dose, concentration, or production method of PRP.
Outcome measures
Studies reporting results related to changes (improvement, worsening or no change) in the inflammatory process (inflammatory markers) and/or histopathology (proliferation rate of chondrocytes and synoviocytes, synthesis of glycosaminoglycan (GAG), thickness of the cartilage and/or synovium) were included.
Exclusion criteria
Clinical trials, case studies, animal models with multiple diseases, in vitro or ex-vivo experiments and studies with control groups other than a placebo group or group without treatment were excluded.
Development and data synthesis
Databases and search strategies
An electronic search was performed of the Medline, Embase, Web of Science, The Cochrane Library, LILACS and SciELO databases for relevant articles published up to June 2022. The search terms were selected considering the controlled vocabulary of the Medical Subject Headings (MeSH) database and uncontrolled vocabulary. The search strategy involved terms related to the topic of interest. Thus, the following combination of search terms was employed (“Platelet-Rich Plasma” OR “Platelet Gel” OR “Autologous Platelet Concentrate” OR “Autologous Conditioned Plasma” OR ACP) AND (Osteoarthritis) AND (Animals OR “Models, Animal” OR “Animal Experimentation”) AND (Inflammation OR “Intercellular Signaling Peptides and Proteins” OR Cartilage OR “Synovial Membrane”). A manual search was conducted by screening the reference lists of the studies included to identify potentially relevant studies not retrieved during the electronic search.
Selection of studies
Two independent reviewers (C.C. and H.G.M.) selected titles and abstracts of publications encountered during the electronic search based on the inclusion criteria. Potentially relevant studies were preselected for full-text analysis. The entire selection process was conducted by consensus. When a consensus was not reached, a third reviewer (K.N.Z.P.R.) was consulted to make the final decision. The StArt (State of the Art through Systematic Review) reference management software was used during the selection of the studies [31]. The StArt software automatically detected duplicates.
Data extraction
After the selection of the studies, the reviewers (C.C. e H.G.M.) worked independently. A standard form adapted from the model proposed by the Cochrane Collaboration was used to extract data on the study design, characteristics of the animals, treatment and comparison groups and outcomes [29].
Appraisal of methodological quality
The methodological quality was assessed using the SYRCLE’s risk of bias (RoB) tool for animal studies [32], analyzing risks related to selection, performance, detection, attrition and other biases. Two reviewers (E.M.G. and H.G.M.) scored the items independently, with disagreements resolved by a third reviewer (C.C.).
Data synthesis and analysis
The quality of the scientific evidence was analyzed using the GRADE approach, which has the following domains: limitations (risk of bias), inconsistency, indirectness, imprecision and publication bias [30]. The item 1 (limitations) was classified as “serious” when less than 75% of the studies included in comparison group fulfilled less than three items of the SYRCLE’s RoB tool. Meta-analyses were conducted using the RevMan 5 software [Review Manager 5.4 (RevMan)] [33]. Effect sizes were calculated using standardized mean differences (SMD) and with 95% confidence intervals (CI). The random effects models were used to calculate the pooled mean effect size. The effect size was classified as small (< 0.20), moderate (0.21 to 0.79) or large (> 0.80). The I2 statistic was used to assess heterogeneity among studies by comparison groups of meta-analyses, with values of ≥ 25, 50 and 75% interpreted as representing low, moderate and high heterogeneity, respectively [34].
Results
Description of studies
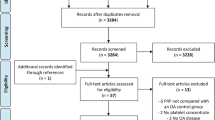
The electronic search of the databases led to the retrieval of 446 studies. After the selection process performed by consensus, 21 studies [16, 25, 26, 35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] were included in the present review, involving a total of 456 animal joints (243 in the PRP-treated group and 213 in the control group). Twelve studies were included on meta-analyses and the assessment of the quality of the evidence by GRADE approach [16, 25, 36, 37, 39, 41, 44,45,46, 48, 50, 52]. The details of the selection process and main reasons for exclusions of studies are presented in Fig. 1.
Characteristics of studies
The main characteristics of the 21 studies included are displayed in Table 1. The number of animals in the comparison groups ranged from 5 [43, 48, 50] and 16 [40, 49] and the average number of animals was 10. Different animal models were used in the studies included. Ten studies used a rodent model: three used Sprague-Dawley rats [16, 25, 43], four used albino rats [26, 46, 50, 51], two used Dunkin-Hartley guinea pigs [37, 48] and one used FVB/N mice [35]. Among those with a non-rodent model, seven studies used New Zealand rabbits [36, 38, 39, 42, 43, 49, 52], three used dogs [40, 45, 47] and one used Boer goats [41]. The vast majority of studies included (90%) used the knee as the target of the PRP treatment [16, 25, 35,36,37, 39,40,41,42,43,44,45,46,47,48, 50,51,52]. Two studies (9%) used the temporomandibular joint [38, 49] and one study (4,7%) used the ankle joint [26].
For the induction of OA, surgical procedures were performed in 12 of the 21 studies included [25, 35, 36, 38, 39, 41,42,43,44,45,46, 50]. Chemical methods were employed in five studies [16, 26, 49, 51, 52]. Four studies used animal models that developed OA naturally and therefore did not require any induction method [37, 40, 47, 48]. The different surgical procedures involved a meniscus transection [35, 46], sectioning of ligaments around the knee joint [43,44,45], a combination of both methods [25, 41], a bilateral destabilization of the temporomandibular joint [38] or the modified Hulth Protocol [36, 39, 42]. The chemical method most adopted was the injection of monosodium iodoacetate (MIA), which was used in three studies [16, 26, 49], followed by the injection of collagenase [52]. A formalin solution was used in one study [51].
Although the PRP preparation method is limited to the double-centrifugation method or kits developed by specialized companies, no standardization in the manufacturing steps of the blood product was found with regards to centrifugation time or speed, preparation temperature, the administration of activators or anticoagulants or platelet count (Table 2). The range of the platelet concentration reported in 13 studies was three to eight times higher than the normal concentration in blood [16, 35, 37, 38, 40,41,42,43,44,45, 47, 48, 52]. Divergences were also found regarding the dose of PRP applied, even in studies using the same species of animal. For instance, the dose in the experiments conducted in two studies [42, 44] had a difference of 2.5 mL, although both experimental units shared the same characteristics. Likewise, the dose administration regime diverged considerably. The number of applications ranged from one [16, 25, 47,48,49] to ten [39] and the frequency ranged from a single time [26, 36, 37, 39, 40, 42,43,44,45, 48, 49, 51] to up to three times per week [41, 46, 50].
The outcomes were evaluated using a variety of methods. Sixteen studies evaluated cartilage histology and/or synovium histology to investigate tissue regeneration [16, 25, 35, 37,38,39, 41,42,43, 45, 46, 48,49,50,51,52]. Thirteen studies investigated the inflammatory process by determining the expression of inflammatory markers or other molecules affected during joint inflammation, such as COL-2, MMP13, PDGF-A and VEGF [25, 26, 36, 39,40,41,42,43,44,45,46,47, 50]. The main classification systems used in the histological evaluations were the Modified Mankin Score [53] in nine studies [16, 25, 38, 41, 43, 45, 46, 48, 52], Pelletier [54] in four studies [37, 41, 42, 48] and OARSI [55] in three studies [35, 43, 46]. The most common immunodiagnostic method was ELISA test in eleven studies [25, 26, 36, 39,40,41,42,43,44, 46, 47], followed by immunohistochemical analysis in three studies [42, 45, 50].
Appraisal of methodological quality
The appraisal performed using the SYRCLE’s RoB tool revealed greater frequencies of high and uncertain risk, as illustrated in Fig. 2. The included studies met at least three items of SYRCLE’s RoB tool. Most of the included studies did not mentioned methods for blinding the investigators during the experiment or randomizing the selection of the animals to evaluate the outcomes. Moreover, a large part of the studies failed to report clearly how the allocation sequence of the animal models was generated, applied and concealed, how relevant characteristics were standardized for the treatment and control groups, how the randomization was performed in the lodging of the animals during the experiment or whether the study was apparently free of other problems that could result in biases. The summary of the methodological quality assessment of all studies is presented in Table 3.
Comparison of treatment with PRP versus control
Cartilage histology
The meta-analysis of eight studies [16, 25, 37, 41, 45, 46, 48, 52] indicated that treatment with PRP achieved superior results compared to the control for changes in cartilage, as the samples from these studies presented less cartilage damage after treatment (pooled sample of 119 animals [goat, dog, guinea pig, rabbit and rat]; very low quality of evidence [items met: inconsistency and publication bias]; SMD = −2.50 [large effect]; 95% CI: −3.83 to −1.18; p = 0.0002; I2 = 84% [high heterogeneity] and control groups received either placebo or no type of treatment) (Fig. 3A).
Synovium histology
The meta-analysis of two studies [37, 48] indicated that treatment with PRP achieved superior results compared to the control for changes in synovium, as the samples from these studies presented less synovitis (combined sample of 23 animals [guinea pigs]; very low quality of evidence [items met: inconsistency and indirectness]; SMD = −3.05 [large effect]; 95% CI = −4.43 to −0.77; p < 0.0001; I2 = 0% [small heterogeneity] and control groups received no type of treatment) (Fig. 3B).
Interleukin (IL)-1 levels
The meta-analysis of four studies [36, 39, 44, 45] indicated that treatment with PRP achieved superior results compared to the control regarding the concentration of IL-1, as the samples from these studies presented a lower concentration of this proinflammatory interleukin (combined sample of 83 animals [dog and rabbit]; low quality of evidence [items met: inconsistency, indirectness and publication bias]; SMD = −2.49 [large effect]; 95% CI = −4.03 to −0.94; p = 0.002; I2 = 83% [high heterogeneity] and control groups received no type of treatment) (Fig. 3C).
Interleukin-6 levels
The meta-analysis of three studies [25, 36, 39] indicated that treatment with PRP achieved superior results compared to the control regarding the concentration of IL-6, as the samples from these studies presented a lower concentration of this proinflammatory interleukin (combined sample of 71 animals [rabbit and rat]; very low quality of evidence [items met: inconsistency and publication bias]; SMD = −3.76 [large effect]; 95% CI = −5.37 to −2.14; p < 0.00001; I2 = 73% [moderate heterogeneity] and control groups received no type of treatment) (Fig. 3D).
Tumor necrosis factor alpha (TNF-α) levels
The meta-analysis of four studies [25, 36, 39, 45] indicated that treatment with PRP achieved superior results compared to the control for the concentration of TNF-α, as the samples from these studies presented a lower concentration of this pro-inflammatory cytokine (combined sample of 83 animals [dog, rabbit and rat]; very low quality of evidence [items met: inconsistency and publication bias]; SMD = −3.70 [large effect]; 95% CI = −5.19 to −2.22; p < 0.00001; I2 = 72% [moderate heterogeneity] and control groups received no type of treatment) (Fig. 3E).
Platelet-derived growth factor (PDGF)
The meta-analysis of two [46, 50] studies indicated that treatment with PRP did not obtain superior results compared to the control for the concentration of PDGF-A (combined sample of 35 animals [rat]; very low quality of evidence [items met: inconsistency and publication bias]; SMD = −1.51 [large effect]; 95% CI = −14.09 to 11.06; p = 0.81; I2 = 96% [high heterogeneity] and control groups received no type of treatment) (Fig. 3F).
Discussion
The aim of this systematic review with meta-analysis was to investigate the effect of the intra-articular injection of PRP on inflammatory process and histopathological characteristics of cartilage and synovium in animal models with OA. As the main and innovative result, the PRP treatment led to less cartilage damage and synovitis as well as reductions in the concentration of the proinflammatory markers IL-1, IL-6 and TNF-α in animal models. Based on the GRADE approach, the quality of the evidence assessed was low to very low. The effect size in the meta-analyses was large.
The level of evidence for both cartilage and synovium histology was very low with a large effect (p = 0.0002 [16, 25, 37, 41, 45, 46, 48, 52], and p < 0.0001 [37, 48], respectively). The Modified Mankin Score for cartilage and the Pelletier Score for synovium were employed, reporting lower means in comparison to the CG, indicating reduced cartilage damage and synovitis post-PRP treatment. For cartilage histology, PRP dosage ranged from 0.5 mL [16, 42] to 1 mL [45], and administration protocols included single [16] and weekly applications for three, four, and six weeks [37, 42, 45, 48]. Notably, shorter treatment protocols, such as a single PRP application, proved effective in minimizing cartilage damage caused by OA. For synovium histology, however, neither Chouhan et al. [48] nor Kanwat et al. [37] specified the dosage of PRP administrated, and both employed a single weekly application for three weeks. Although the meta-analysis exhibited low heterogeneity (0%), noteworthy is that both studies are from the same research group, contributing to the lowered evidence level (publication bias per the GRADE approach).
The evidence levels for IL-1, IL-6, and TNF-α concentrations after PRP treatment varied, with low evidence for IL-1 (p = 0.002) [36, 39, 44, 45] and very low evidence for IL-6 and TNF-α (p < 0.00001 [25, 36, 39], p < 0.00001 [25, 36, 39, 45], respectively). ELISA was the primary assessment method, consistently showing lower means post-PRP treatment, indicating reduced inflammatory processes. PRP dosage and treatment protocols varied across studies. For IL-1, PRP dosage ranged from 0.5 mL [36, 39] to 3 mL [44], and treatment protocols varied from weekly applications spanning three [44] to ten weeks [39]. In the case of IL-6, PRP dosage ranged from 0.5 mL [36, 39] to 1 mL [25], and treatment protocols ranged from a single injection [25] to five [36] or ten [39] weekly applications. Similarly, for TNF-α, PRP dosage ranged from 0.5 mL [36, 39] to 1 mL [25, 45], and treatment protocols ranged from a single injection [25] to four [45], five [36], or ten [39] weekly applications. Shorter treatment protocols, whether spanning three weeks for IL-1, or a single injection for IL-6 and TNF-α, demonstrated effectiveness in reducing concentrations across these inflammatory markers.
Concerning PDGF-A levels however, the findings indicate very low-quality evidence with a large effect (p = 0.81) [46, 50] suggesting that PRP is not superior to sham. Gamal et al. [50] observed a lower mean in the PRP-treated groups, whereas Almasry et al. [46] reported a higher mean, indicating elevated PDGF-A levels following PRP treatment. The administered PRP doses were 0.2 and 0.085 mL, respectively, with both studies employing a consistent application period of once per week for three weeks.
While this review enhances our understanding of the histological effects on cartilage and synovium following PRP injection, it is crucial to interpret these findings with caution. The majority of the evidence assessed through the GRADE approach was rated as very low, emphasizing the need for careful consideration and acknowledgment of potential limitations. It is also important to highlight that the experimental heterogeneity among the included studies was one of the main reasons for lowering the quality of the evidence. Furthermore, high heterogeneity was found for the histology of cartilage and synovium, as well as inflammatory markers such as IL-1, IL-6, and TNF-α. This high heterogeneity may be due to the different species of animals, the different PRP preparation methods, and the intervention protocols used by the studies (experimental heterogeneity). Even though most meta-analyses show high heterogeneity, our results are in agreement with the literature, given that about 25% of the meta-analyses developed present I2 values above 50% [34].
Meta-analyses of randomized clinical trials (RCTs) have suggested that PRP treatments improve pain and function in knee and hip OA patients [56, 57]. However, recent RCTs [58, 59] contradict these findings, showing no benefit of PRP over placebo. The meta-analyses, conducted prior to these RCTs, may have been compromised not only by comparisons with no first line treatments for knee OA like hyaluronic acid and corticosteroids but also by the inclusion of studies with low levels of evidence and methodological rigor. This could have significantly affected the evaluation of PRP efficacy. While our study observed reductions in proinflammatory markers following PRP treatment, the clinical significance, particularly regarding long-term joint health, remains uncertain given the conflicting evidence on PRP’s efficacy for pain and function improvement.
The appraisal of the methodological quality of the studies was performed using the SYRCLE’s’ RoB tool to determine the risk of bias and the GRADE approach was used to determine the quality of the evidence. Among the ten items on the SYRCLE scale, the criteria with the greatest risk of bias were item 5 (blinding of the investigators) and item 6 (randomization of animal selection for the evaluation of the outcomes). None of the studies included fulfilled the item 6. Regarding item 1, sixteen studies reported randomization during the generation of the allocation sequence, but none described the method used. Although the majority of studies reported apparent similarities in the baseline characteristics of the animal models, such as weight, age, and sex, only five studies [26, 37, 39, 48, 49] provided details on tests performed to determine statistical differences in these characteristics within the sample.
Regarding the GRADE analysis, the indirectness and imprecision were the main items responsible for lowering the quality of the evidence. There was a variety of tests used to assess outcomes, and different species of animals were used. This reason negatively affected item 3 (indirectness) of the GRADE approach. The sample size was low in all studies included. To fulfill item 4 (imprecision) from the GRADE approach, the sample size from all the included studies must reach over 200 animals by comparison groups. Therefore, more studies are needed.
The Modified Mankin Score was used in seven studies [16, 25, 37, 41, 45, 48, 52] and only one of these did not find a significant difference after the treatment [52], with a small effect size encountered. The six studies [16, 25, 37, 41, 45, 48] found significant differences and a large effect size. Thus, if Hermeto et al. [52] had increased the sample size, it is possible that the authors would have found a significant difference with large effect size.
This is the first study to synthesize evidence regarding the effects of PRP on the inflammatory process as well as histological characteristics of the cartilage and synovium in animals with OA. The type of treatment selected (PRP) proved to be promising for animals with OA and needs to be investigated better. Another strong point was the methodology adopted, which followed the PRISMA guidelines and recommendations of the Cochrane Collaboration and involved the GRADE approach. These points allowed a qualitative and quantitative analysis of the results. However, we must recognize divergence with regards to the sample (we had to pool the animals for the assessment of the quality of evidence through GRADE approach), the OA induction method, the different PRP preparation protocols and doses, the period and frequency of the applications and the measurement tools employed for the evaluation of the outcomes. We hope that our study would be of some help in the design of high-quality preclinical studies in the future.
Conclusions
Treatment with PRP seems to be capable of lowering concentrations of proinflammatory markers such as IL-1, IL-6 and TNF-α (very low level of evidence with a large effect) and cartilage and synovium damage (low level of evidence with a large effect) in animals with OA. Further studies with greater methodological rigor and larger samples are needed to improve the quality of evidence.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- GAG:
-
Glycosaminoglycan
- GFs:
-
Growth factors
- GRADE:
-
Grading of recommendations, assessment, development and evaluations
- IL:
-
Interleukin
- MeSH:
-
Medical subject headings
- OA:
-
Osteoarthritis
- PDGF:
-
Platelet-derived growth factor
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- PROSPERO:
-
International prospective register of systematic reviews
- PRP:
-
Platelet-rich plasma
- RoB:
-
Risk of bias
- SMD:
-
Standardized mean differences
- StArt:
-
State of the art through systematic review
- TNF-α:
-
Tumor necrosis factor alpha
References
Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey. Arthritis Care Res. 2016;68:574–80. https://doi.org/10.1002/acr.22721.
Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2020;72:149–62.
Kingsbury SR, Gross HJ, Isherwood G, Conaghan PG. Osteoarthritis in Europe: impact on health status, work productivity and use of pharmacotherapies in five European countries. Rheumatol (United Kingdom). 2014;53:937–47. https://doi.org/10.1093/rheumatology/ket463.
Veronese N, Stubbs B, Solmi M, Smith TO, Noale M, Cooper C, et al. Association between lower limb osteoarthritis and incidence of depressive symptoms: data from the osteoarthritis initiative. Age Ageing. 2017;46:470–76.
Hermans J, Koopmanschap MA, Bierma-Zeinstra SMA, Van Linge JH, Verhaar JAN, Reijman M, et al. Productivity costs and medical costs among working patients with knee osteoarthritis. Arthritis Care Res. 2012;64:853–61.
Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–41. https://doi.org/10.1038/nrrheum.2014.44.
Sharif B, Garner R, Hennessy D, Sanmartin C, Flanagan WM, Marshall DA. Productivity costs of work loss associated with osteoarthritis in Canada from 2010 to 2031. Osteoarthr Cartil. 2017;25:249–58. https://doi.org/10.1016/j.joca.2016.09.011.
Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr Cartil. 2010;18:476–99.
Ghouri A, Conaghan PG. Prospects for therapies in osteoarthritis. Calcif Tissue Int. 2020. https://doi.org/10.1007/s00223-020-00672-9.
Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104:293–311. https://doi.org/10.1016/j.mcna.2019.10.007.
Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31:596–611.
Fernandes L, Hagen KB, Bijlsma JWJ, Andreassen O, Christensen P, Conaghan PG, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72:1125–35.
Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465–74.
Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part three: aerobic exercise programs *. Clin Rehabil. 2017;31:612–24.
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27:1578–89.
Asjid R, Faisal T, Qamar K, Malik S, Umbreen F, Fatima M. Effect of platelet-rich plasma on mankin scoring in chemically-induced animal model of osteoarthritis. J Coll Physicians Surg Pakistan. 2019;29:1067–71. https://doi.org/10.29271/jcpsp.2019.11.1067.
Shen L, Yuan T, Chen S, Xie X, Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:1–12. https://doi.org/10.1186/s13018-017-0521-3.
Fang D, Jin P, Huang Q, Yang Y, Zhao J, Zheng L. Platelet-rich plasma promotes the regeneration of cartilage engineered by mesenchymal stem cells and collagen hydrogel via the TGF-β/SMAD signaling pathway. J Cell Physiol. 2019;234:15627–37. https://doi.org/10.1002/jcp.28211.
Lang S, Loibl M, Herrmann M. Platelet-rich plasma in tissue engineering: hype and hope. Eur Surg Res. 2018;59:265–75. https://doi.org/10.1159/000492415.
Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–34. https://doi.org/10.1016/j.tibtech.2006.02.010.
Irmak G, Gümüşderelioğlu M. Photo-activated platelet-rich plasma (PRP)-based patient-specific bio-ink for cartilage tissue engineering. Biomed Mater. 2020;15:065010. https://doi.org/10.1088/1748-605X/ab9e46.
Chen FM, Zhang M, Wu ZF. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–308. https://doi.org/10.1016/j.biomaterials.2010.04.053.
Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone biology and clinical applications. J Bone Jt Surg Ser A. 2002;84:1032–44. https://doi.org/10.2106/00004623-200206000-00022.
Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJMA, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthr Cartil. 2006;14:1272–80.
Ahmad MR, Badar W, Ullah Khan MA, Mahmood A, Latif N, Iqbal T, et al. Combination of preconditioned adipose-derived mesenchymal stem cells and platelet-rich plasma improves the repair of osteoarthritis in rat. Regener Med. 2020;15:2285–95.
Ragab GH, Halfaya FM, Ahmed OM, Abou El-Kheir W, Mahdi EA, Ali TM, et al. Platelet-rich plasma ameliorates monosodium iodoacetate-induced ankle osteoarthritis in the rat model via suppression of inflammation and oxidative stress. Evid Based Complement Altern Med. 2021;2021:1–13.
Kushida S, Kakudo N, Morimoto N, Hara T, Ogawa T, Mitsui T, et al. Platelet and growth factor concentrations in activated platelet-rich plasma: a comparison of seven commercial separation systems. J Artif Organs. 2014;17:186–92.
Page MJ, McKenzie J, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Aki EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Nihon Bika Gakkai Kaishi (Japanese J Rhinol). 2020;59:S59–S61.
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.1. In: The Cochrane Collaboration. 2020.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–26.
Fabbri S, Silva C, Hernandes E, Octaviano F, Di Thommazo A, Belgamo A Improvements in the StArt tool to better support the systematic review process. Proceedings of the 20th international conference on evaluation and assessment in software engineering. New York, NY, USA: ACM; 2016. pp. 1–5. https://doi.org/10.1145/2915970.2916013
Hooijmans CR, Rovers MM, De Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:1–9. https://doi.org/10.1186/1471-2288-14-43.
The Nordic Cochrane Centre TCCV 5.3.C. Revman 5 software. 2014.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. https://doi.org/10.1136/bmj.327.7414.557.
Jayaram P, Liu C, Dawson B, Ketkar S, Patel SJ, Lee BH, et al. Leukocyte-dependent effects of platelet-rich plasma on cartilage loss and thermal hyperalgesia in a mouse model of post-traumatic osteoarthritis. Osteoarthr Cartil. 2020;28:1385–93.
Ji HD, Huo XY, Zhang HQ, Wang YS, Shi X, Huo L. Platelet-rich plasma with sodium hyaluronate in repair of rabbit knee osteoarthritis. Chinese J Tissue Eng Res. 2015;19:6133–39. https://doi.org/10.3969/j.issn.2095-4344.2015.38.012.
Kanwat H, Mandeep Singh D, Devendra Kumar C, Alka B, Biman S, Aman H. The effect of intra-articular allogenic platelet rich plasma in Dunkin-Hartley guinea pig model of knee osteoarthritis. Muscle Ligaments Tendons J. 2019;07:426. https://doi.org/10.32098/mltj.03.2017.04.
Kütük N, Baş B, Soylu E, Gönen ZB, Yilmaz C, Balcioǧlu E, et al. Effect of platelet-rich plasma on fibrocartilage, cartilage, and bone repair in temporomandibular joint. J Oral Maxillofac Surg. 2014;72:277–84.
Lu W, Zhang E, Liu D, Ruan B, Xie S. Effect of platelet-rich plasma on infection markers and jak/stat pathway in rabbit models of knee osteoarthritis. Int J Clin Exp Med. 2020;13:8562–69. https://www.embase.com/search/results?subaction=viewrecord%26id=L2005531767%26from=export.
Parlak K, Arican M. Effect of intra-articular administration of autologous PRP and activated PRP on inflammatory mediators in dogs with osteoarthritis. Vet Med (Praha). 2020;65:62–70. https://doi.org/10.17221/36/2019-VETMED.
Wang Z, Zhai C, Fei H, Hu J, Cui W, Wang Z, et al. Intraarticular injection autologous platelet-rich plasma and bone marrow concentrate in a goat osteoarthritis model. J Orthop Res. 2018;36:2140–46.
Wu J, Zhang Z, Qin X, Cai X, Feng H. Platelet-rich-plasma alleviates pathological symptoms in a rabbit model of osteoarthritis. Int J Clin Exp Med. 2016;9:21038–47. http://www.embase.com/search/results?subaction=viewrecord%26from=export%26id=L613467865%0Ahttp://wt3cf4et2l.search.serialssolutions.com?sid=EMBASE%26issn=19405901%26id=doi:%26atitle=Platelet-rich-plasma+alleviates+pathological+symptoms+in+a+rabbit+model+of+osteoart.
Xin F, Wang H, Yuan F, Ding Y. Platelet-rich plasma combined with alendronate reduces pain and inflammation in induced osteoarthritis in rats by inhibiting the nuclear factor-kappa B signaling pathway. Biomed Res Int. 2020;2020. https://doi.org/10.1155/2020/8070295.
Yin WJ, Xu HT, Sheng JG, An ZQ, Guo SC, Xie XT, et al. Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in treating rabbit knee osteoarthritis. Med Sci Monit. 2016;22:1280–90.
Yun S, Ku SK, Kwon YS. Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. J Orthop Surg Res. 2016;11:1–12. https://doi.org/10.1186/s13018-016-0342-9.
Almasry SM, Soliman HM, El-Tarhouny SA, Algaidi SA, Ragab EM. Platelet rich plasma enhances the immunohistochemical expression of platelet derived growth factor and vascular endothelial growth factor in the synovium of the meniscectomized rat models of osteoarthritis. Ann Anat. 2015;197:38–49. https://doi.org/10.1016/j.aanat.2014.10.006.
Arican M, Şimşek A, Parlak K, Atli K, Sönmez G. Matrix metalloproteinases 2 and 9 activity after intra-articular injection of autologous platelet-rich plasma for the treatment of osteoarthritis in dogs. Acta Vet Brno. 2018;87:127–35. https://doi.org/10.2754/avb201887020127.
Chouhan DK, Dhillon MS, Patel S, Bansal T, Bhatia A, Kanwat H. Multiple platelet-rich plasma injections versus single platelet-rich plasma injection in early osteoarthritis of the knee: an experimental study in a guinea pig model of early knee osteoarthritis. Am J Sports Med. 2019;47:2300–07. https://doi.org/10.1177/0363546519856605.
Coskun U, Candirli C, Kerimoglu G, Taskesen F. Effect of platelet-rich plasma on temporomandibular joint cartilage wound healing: experimental study in rabbits. J Cranio-Maxillofacial Surg. 2019;47:357–64. https://doi.org/10.1016/j.jcms.2018.12.004.
Gamal N, Abou-Rabia NM, El Ebiary FH, Khalaf G, Raafat MH. The possible therapeutic role of platelet rich plasma on a model of osteoarthritis in male albino rat. Histological and immunohistochemical study. Egypt J Histol. 2019;42:554–66. https://doi.org/10.21608/ejh.2019.9750.1090.
Guner S, Buyukbebeci O. Analyzing the effects of platelet gel on knee osteoarthritis in the rat model. Clin Appl Thromb. 2013;19:494–98. https://doi.org/10.1177/1076029612452117.
Hermeto LC, DeRossi R, Oliveira RJ, Pesarini JR, Antoniolli-Silva ACMB, Jardim PHA, et al. Effects of intra-articular injection of mesenchymal stem cells associated with platelet-rich plasma in a rabbit model of osteoarthritis. Genet Mol Res. 2016;15:1–14.
van der Sluijs JA, Geesink RGT, van der Linden AJ, Bulstra SK, Kuyer R, Drukker J. The reliability of the mankin score for osteoarthritis. J Orthop Res. 1992;10:58–61. https://doi.org/10.1002/jor.1100100107.
Pelletier JP, Fernandes JC, Brunet J, Moldovan F, Schrier D, Flory C, et al. In vivo selective inhibition of mitogen-activated protein kinase kinase 1/2 in rabbit experimental osteoarthritis is associated with a reduction in the development of structural changes. Arthritis Rheum. 2003;48:1582–93.
Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell K, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil. 2006;14:13–29.
Nie LY, Zhao K, Ruan J, Xue J. Effectiveness of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled clinical trials. Orthop J Sport Med. 2021;9:1–11. https://doi.org/10.1177/2325967120973284.
Dong Y, Zhang B, Yang Q, Zhu J, Sun X. The effects of platelet-rich plasma injection in knee and hip osteoarthritis: a meta-analysis of randomized controlled trials. Clin Rheumatol. 2021;40:263–77. https://doi.org/10.1007/s10067-020-05185-2.
Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. 2021;326:2021–30.
Dório M, Pereira RMR, Luz AGB, Deveza LA, de Oliveira RM, Fuller R. Efficacy of platelet-rich plasma and plasma for symptomatic treatment of knee osteoarthritis: a double-blinded placebo-controlled randomized clinical trial. BMC Musculoskelet Disord. 2021;22:1–12. https://doi.org/10.1186/s12891-021-04706-7.
Acknowledgements
Not applicable.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Author information
Authors and Affiliations
Contributions
H.G.-M.: contributing to the conception and design; collecting data; interpreting data; drafting the article and revising it critically for important intellectual content; and approving the final version to be published; C.C.: contributing to the conception and design; collecting data; analyzing and interpreting data; drafting the article and revising it critically for important intellectual content; and approving the final version to be published; E.M.G.: contributing to the analyzing and interpreting data; drafting the article and revising it critically for important intellectual content; and approving the final version to be published; M.P.B.D.O.: contributing to the analyzing and interpreting data; drafting the article and revising it critically for important intellectual content; and approving the final version to be published; K.N.Z.P.R.: contributing to the conception and design; interpreting data; drafting the article or revising it critically for important intellectual content; and approving the final version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was not required for this study, as the data used comprises of peer-reviewed publications and information that could identify the subjects of the original studies was not included.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garcia-Motta, H., Carvalho, C., Guilherme, E. et al. Effects of intra-articular injection of platelet-rich plasma on the inflammatory process and histopathological characteristics of cartilage and synovium in animals with osteoarthritis: a systematic review with meta-analysis. Adv Rheumatol 64, 24 (2024). https://doi.org/10.1186/s42358-024-00364-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-024-00364-0