Abstract
Background
Infections increase mortality and morbidity and often limit immunosuppressive treatment in rheumatoid arthritis patients.
Objective
To analyze the occurrence of serious infections and the associated factors in a cohort of rheumatoid arthritis patients under real-life conditions.
Methods
We analyzed data from the REAL, a prospective observational study, that evaluated Brazilian RA patients, with clinical and laboratory data collected over a year. Univariate and multivariate analyses were performed from the adjustment of the logistic regression model Generalized Estimating Equations (GEE), with the primary outcome being the occurrence of serious infection, defined as need for hospitalization or use of intravenous antibiotics for its treatment.
Results
841 patients were included with an average follow-up time of 11.2 months (SD 2.4). Eighty-nine serious infections occurred, corresponding to 13 infections per 100 patient-years. Pulmonary fibrosis, chronic kidney disease (CKD) and central nervous system disease increased the chances of serious infection by 3.2 times (95% CI: 1.5–6.9), 3.6 times (95% CI: 1.2–10.4) and 2.4 times (95% CI: 1.2–5.0), respectively. The use of corticosteroids in moderate doses increased the chances by 5.4 times (95% CI: 2.3–12.4), and for each increase of 1 unit in the health assessment questionnaire (HAQ), the chance increased 60% (95% CI: 20–120%).
Conclusion
The use of corticosteroids at moderate doses increased the risk of serious infection in RA patients. Reduced functionality assessed by the HAQ and comorbidities were other important factors associated with serious infection in this cohort.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease with an estimated global prevalence of 0.5–1%. In Brazil, this prevalence is close to the lower limit of this range [1, 2].
Studies carried out in different countries have shown an increased risk of infection among patients with RA. The estimated risk is twice as high in this population compared to subjects without RA, and the risk of serious infections (SI) requiring hospitalization is also higher [3, 4]. Infections increase mortality and morbidity and often limit immunosuppressive treatment [5,6,7]. The risk of infection in this population may be influenced by treatment, the characteristics of rheumatic disease and the clinical and social conditions of the patients [8,9,10,11,12,13,14].
Prospective national data in real-life studies assessing infections in patients with RA are still scarce. The most recent studies are limited to evaluate the risk associated with the use of biologic and small molecule drugs [15, 16]. The REAL cohort itself, a multicentric prospective study from which these data was drawn, has already been analyzed regarding the sociodemographic [17, 18] profile, cardiovascular comorbidities [19], treatment [20, 21] and disease activity [22] in RA patients, but this analysis has not included infections events.
Given the large therapeutic arsenal currently available for the treatment of RA, we believe that it is extremely important to understand the risk factors associated with serious adverse effects to safely choose the ideal treatment for each patient. Thus, this study aims to evaluate the incidence and factors related to the occurrence of SI, defined as the need for hospitalization or use of intravenous antibiotics for its treatment, among patients with RA under real-life conditions in Brazil.
Methods
Study design and setting
We analyzed data from the national multicenter prospective observational cohort, the REAL study [18], collected between August 2015 and May 2017. Eleven tertiary health care centers in Brazil based in four macroregions of the country participated in this cohort. Each center was expected to enroll 100 patients consecutively. All participating centers are part of the Unified Health System (Sistema Único de Saúde or SUS, in Portuguese), the Brazilian public health system, and have specialized rheumatology care. Three visits were conducted: the inclusion to the study, the second one (between five and seven months after the first visit), and the third one (between eleven and thirteen months after the inclusion visit).
Participants
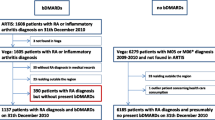
All participants were older than 18 years and met American College of Rheumatology (ACR) 1987 or American College of Rheumatology and European League Against Rheumatism (ACR/EULAR) 2010 classification criteria for RA and were previously followed up in rheumatology services for at least 6 months before inclusion. Initially, information from 1115 patients were collected. However, 274 patients were excluded due to lack of information regarding the infectious events in two or more visits during follow-up. Thus, 841 patients were included with an average follow-up time of 11.2 months (SD 2.4). All patients attended the first visit. After that, 150 patients attended only the 6-months visit and 145 patients attended only the 12-month visit, resulting in 696 and 691 patients at each visit, respectively (Fig. 1).
This study was approved by the local Research Ethics Committee (Comissão de Ética em Pesquisa—COEP)—Federal University of Minas Gerais. All the REAL study participating centers received approval from their Institutions’ Ethics Committees. Signed informed consent was obtained from every study participant.
Variables and data sources
All data were extracted from the database prepared and provided by the coordination of the REAL study. In da Rocha Castelar-Pinheiro et al. [18] it is possible to see the structure of the cohort and details about the data collected.
For statistical analysis, the following data were extracted: sex, age, socioeconomic status, lifestyle habits including smoking and alcoholism, presence of extra-articular manifestations (EAMs), presence of comorbidities, Charlson comorbidity index, history of previous infections, use of medications, time of disease duration, disease activity indices measured by the Disease Activity Score 28 ESR and CRP (DAS 28 ESR, DAS 28 CRP) and Clinical Disease Activity Index (CDAI), functionality index measured by the Health Assessment Questionnaire (HAQ), number of swollen and tender joints, serologic—rheumatoid factor and anti-cyclic citrullinated peptide antibody (anti-CCP)—and inflammatory tests—C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
Comorbidities were grouped into categories and patients could be included in any of them if they had at least one of the diagnoses. These groups were: cardiovascular disease, pulmonary disease, metabolic disease, chronic kidney disease, gastrointestinal tract disease, central nervous system disease, neoplastic diseases, osteoporosis, acquired immunodeficiency syndrome (AIDS) and obesity. Due to its clinical significance, the presence of pulmonary fibrosis as EAM was evaluated in the “extra-articular manifestation” group and as a separate variable.
The variables age, socioeconomic level, dose of corticosteroids and disease activity indices—DAS28 and CDAI—were evaluated in categories and as continuous variables. Disease duration, Charlson comorbidity index and Charlson comorbidity index adjusted for age, CRP and ESR levels, and HAQ were evaluated as continuous variables.
Serious infections were defined as the need for hospitalization or the use of intravenous antibiotics for the treatment of the infection. Information about infections were self-reported and based on medical reports.
Statistical methods
The results of the descriptive analysis were obtained using measures of central tendency (mean) and measures of dispersion (standard deviation) for quantitative variables. For qualitative variables, frequencies and percentages were obtained. These results were presented by visit for variables with potential for variation over time.
For univariate analysis, comparisons over time (defined as first, second and third visit) were performed from the adjustment of the logistic regression model Generalized Estimating Equations (GEE) [23]. Patients with missing information of any variable were excluded from the adjustments of the univariate models.
Variables with p values lower than 0.20 in the univariate analysis were indicated to compose the multivariate model of the initial regression unless they were collinear with another variable. In the case of collinearity, the most statistically significant and clinically appropriate variable was included in the multivariable analysis. Only characteristics with a p value equal to or less than 0.05 remained in the final adjustment. The associations found were quantified from the odds ratio (OR) of the model and its respective 95% confidence interval.
The groups of comorbidities with a significant p value (p < 0.20) were submitted to a further univariate analysis that comprised comorbidities of each group separately to assess the relation to SI. GEE logistic regression model adjustments were performed. We assessed the corticosteroid dose that was most associated with the occurrence of SI maximizing the sum of sensitivity and specificity for each equivalent dose of prednisolone.
Analyses were performed in R version 3.6.3, PASW Statistics 18, and MINITAB 17 software.
Results
Among the 841 patients eligible for the study, 757 (90.0%) were female, and 324 (38.5%) were 60 years of age or older. The mean age at the beginning of the study was 56 years (SD 11.4 years). On average, patients had been diagnosed with RA for 14 years (SD 9.5 years), showing the greater number of patients with established RA and a long disease duration in this cohort. Considering EAMs, 151 (18%) patients had at least one extra-articular manifestation, and 34 (4%) had pulmonary fibrosis. The sociodemographic characteristics of the study population and the presence of comorbidities and EAMs can be found in Table 1. Regarding treatment, on average of the three visits, 47.6% of patients used corticosteroids, and 92.6% used doses smaller than the equivalent of 10 mg of prednisolone per day. Approximately 90% of patients were using synthetic disease-modifying antirheumatic drugs (DMARDs) and half of them (56.7% on average of the three visits) were using it in monotherapy. Approximately 40% of patients were using biological or synthetic target specific DMARDs (Table 2). Most patients had moderate disease activity, and the mean HAQ over the period was 0.851 (Table 3).
Over the observation period, patients had 92 infection events and urinary tract was the most common site, followed by lower respiratory tract (Table 4). Regarding SI, 70 patients (8.32%) had 89 events, corresponding to 13 SI per 100 patient-years. Five of these patients had three SI events and nine had two. After six months, 45 (6.5%) patients presented with some SI, and in the last six months of follow-up, 30 (4.3%). No case of sepsis was documented.
After the univariate analysis (Table S1), we excluded from the subsequent analysis the presence of smoking or alcoholism, age, sex, gastrointestinal tract diseases, neoplasms, history of previous infection, obesity, and number of swollen joints for presenting p values ≥ 0.20. The final multivariate model (Table 5) showed that after six months a patient is 2.2 times (95% CI: 1.8–2.8) more likely to have a SI. The presence of pulmonary fibrosis, chronic kidney disease (CKD) and central nervous system (CNS) disease increased the chances of SI by 3.2 times (95% CI: 1.5–6.9), 3.6 times (95% CI: 1.2–10.4) and 2.4 times (95% CI: 1.2–5.0), respectively, with CNS disease including hemiplegia, Parkinson disease, dementia and psychiatric illness. The daily use of corticosteroids in doses equal to or higher than to 15 mg of prednisolone (or equivalent) increased the chances by 5.4 times (95% CI: 2.3–12.4), and for each increase of 1 unit in the HAQ, the chance of presenting SI increased by 60% (95% CI: 20–120%). The association between corticosteroid use and SI was dose-dependent until daily doses equal to or higher than 15 mg of prednisolone (or equivalent) in which the highest association was found.
Evaluating the groups of comorbidities, the presence of cardiovascular disease, pulmonary disease, metabolic disease, CKD and CNS disease were those that had a statistical association with SI in the initial univariate analysis. When performing further univariate analysis (Table S2), we observed that “other coronary diseases” presented a p value lower than 0.05, as well as chronic obstructive pulmonary disease (COPD), CKD classified as “moderate to severe” and the presence of “hemiplegia” and “psychiatric diseases”. Among the metabolic diseases, neither diabetes with nor without target organ damage alone had a relationship with the occurrence of infections.
Discussion
As RA itself is known to be related to an increased risk of infection [24], it is of great interest to know which other factors presented by the patients may lead to an additional risk. Some of these factors can be specific to each population, with different socioeconomic conditions, management protocols and access to treatment and there is little data available about the South American population.
We found an incidence of 13 infections per 100 patient-years. In 2002, Doran [3] saw an incidence of 9.57 SI per 100 patient-years, whereas the incidence of all infection events in this cohort was 19.64 per 100 patient-years. More recently, in 2019, a large national US cohort [24] showed a high frequency (19.6 per 100 patient-years) of SI when following patients for long periods (15 years). However, in 2020, Wang observed 10.8 SI per 100 patients-year after a year of segment in an Australian cohort [25].
Our study associated the occurrence of SI events with the presence of comorbidities – such as pulmonary fibrosis, CKD, central nervous system diseases –, the use of moderate doses of corticosteroids, and reduced functionality as assessed by the HAQ. These results were similar to those already described in other cohorts [8,9,10,11,12, 26] and some of these factors can be associated, such as higher corticosteroid doses and pulmonary fibrosis. In a recent Taiwan cohort [27], Ng KH et al showed a relationship between corticosteroid daily use and mortality due to infection and disease activity in patients with pulmonary fibrosis associated with RA.
In contrast, factors that were also related to the occurrence of infectious events in other cohorts, such as disease duration, disease activity assessed by CDAI, elevation of inflammatory reactants and diabetes [11, 12, 24, 28] did not achieve statistical significance to be included in our final model. This can be justified by the homogeneity of the cohort sample, in which patients with moderate disease activity and with a long disease duration (14 years on average) predominated. Furthermore, the association between SI and diabetes was not always achieved in RA cohorts [15, 25, 29]. BIOBADABRASIL, Brazilian registry for biologic drugs, didn’t found association between diabetes and SI in patients with rheumatic disease either [15].
Regarding the use of corticosteroids, much effort has been done to identify a dose limit considered safe for use in the treatment of RA. Previous studies were able to show that the use of corticosteroids, even at low doses, such as equal to or less than 5 mg of prednisolone (or equivalent) per day, was associated with a higher risk of infections. This risk increases with the dose escalation of the medication [8, 11]. In this cohort, the association between corticosteroid use and SI also occurred in a dose-dependent manner, with the highest association found with doses equal to or higher than 15 mg of prednisolone (or equivalent). According to the most recent recommendations of the EULAR and ACR, if steroids are necessary as initial or bridging therapy, they should be discontinued as soon as possible [30, 31].
We found no statistically significant association between the use of any type of DMARD and the occurrence of SI. This finding differs from what is described in the literature [13,14,15, 29]. In the South American registry for biologic monitoring [32], Ranza et al. found an adjusted hazard ratio (HR) of 2.03 [1.05–30.9] comparing biologic DMARDs and synthetic DMARDs, and Quartuccio [29] observed that this risk was higher in the beginning of any biologic drug.
Reduced functionality assessed by the HAQ was another important factor associated with SI in the REAL cohort. Similarly, Weaver et al found a 30% increase in the risk of SI for every 0.4-unit HAQ-DI increase [9]. This metric can be influenced by pain, swollen joints, damage, deformities but also by fatigue and depression [33]. Previous studies showed that early treatment, tight disease control and biologic use can reduce progression of disability on RA [34, 35].
When performing the additional univariate analysis, “other coronary disease” and COPD were associated with SI with an OR of 7.48 (95% CI: 2.45–22.81) and 3.92 (95% CI: 1.78–8.59) respectively. The association between coronary disease and infections complications has already been described in literature, wherein certain infections agents have been implicated in atherosclerotic disease [36, 37]. Furthermore, SI is associated with 10-fold risk of 30-day death after an acute myocardial infarction [38] However, no causal relationship between chronic coronary disease and SI has been found and further studies are needed for better understanding this data. In turn, COPD is a known risk factor for SI and the risk for chronic infections and multidrug-resistant organisms is also higher compared to the general population [39].
The main limitation from this study is about selection bias, since data were collected only in tertiary centers. In these hospitals, there is a greater number of patients with severe and refractory diseases, for whom the use of biological medications and small molecules are available according to the national treatment protocol [39]. Theoretically, these patients with severe disease and a high level of immunosuppression form the group with the greater risk of SI [7, 9].
Furthermore, information was not available on some variables that could potentially influence access to the health system and the risk of SI such as color and education level. However, social level, another potential influencer of access to the healthcare system, was included and did not reach statistical significance (p > 0.05).
Given the important frequency of respiratory infections in this cohort, the lack of information on vaccination coverage is another limitation. Data was collected before the COVID19 pandemic, but airway infections such influenza, pneumococcus and Hemophilus B are important causes of SI that are potentially preventable with vaccination. In a systematic review, Furer et al showed a higher incidence and prevalence of vaccine preventable infection in patients with autoimmune inflammatory rheumatic disease (AIRD) compared to general population [40]. Other cohorts have shown a suboptimal vaccine coverage through patients with AIRD [41], even in tertiary centers [42, 43].
In addition, as this is an observational study, information and confounding biases are possible. This may justify the absence of sepsis diagnoses among SI and the small number of patients with a history of SI (16 patients) before the start of the follow-up. Another study limitation was the number of patients who did not attended all the visits (35%), as expected in a real-life study, in which losses are usually higher.
Despite those limitations, this study, as derived from a real-life nationwide cohort (REAL study), included a large representative population sample. Data assessing SI in this population in Brazil are still scarce.
Conclusion
In conclusion, in the REAL cohort the administration of corticosteroids at moderate dosages increased the susceptibility to severe infections. Reduced functionality assessed by the HAQ and comorbidities were other important factors associated with SI in this cohort.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RA:
-
Rheumatoid arthritis
- SI:
-
Serious infection
- ACR:
-
American college of rheumatology
- EULAR:
-
European league against rheumatism
- EAMs:
-
Extra-articular manifestations
- DAS:
-
Disease activity score
- CDAI:
-
Clinical disease activity index
- HAQ:
-
Health assessment questionnaire
- anti-CCP:
-
Anti-cyclic citrullinated peptide antibody
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- AIDS:
-
Acquired immunodeficiency syndrome
- GEE:
-
Generalized estimating equations
- SD:
-
Standard deviation
- OR:
-
Odds ratio
- DMARDs:
-
Disease-modifying antirheumatic drugs
- CKD:
-
Chronic kidney disease
- CNS:
-
Central nervous system
References
Smolen JS, Aletaha D, Mcinnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055): ISSN 1474-547X. https://www.ncbi.nlm.nih.gov/pubmed/27156434.
Senna ER, et al. Prevalence of rheumatic diseases in Brazil: a study using the COPCORD approach. J Rheumatol. 2004;31(3):594–97. ISSN 0315-162X. https://www.ncbi.nlm.nih.gov/pubmed/14994410.
Doran MF, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287–93. ISSN 0004-3591. https://www.ncbi.nlm.nih.gov/pubmed/12355475.
Smitten AL, et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol. 2008;35(3):387–93. ISSN 0315-162X. https://www.ncbi.nlm.nih.gov/pubmed/18260176.
Haviv-Yadid Y, et al. Mortality of patients with rheumatoid arthritis requiring intensive care: a single-center retrospective study. Clin Rheumatol. 2019;38(11):3015–23. ISSN 1434-9949. https://www.ncbi.nlm.nih.gov/pubmed/31254235.
Ogdie A, et al. Cause-specific mortality in patients with psoriatic arthritis and rheumatoid arthritis. Rheumatology (Oxford). 2017;56(6):907–11. ISSN 1462-0332. https://www.ncbi.nlm.nih.gov/pubmed/28158384.
Subesinghe S, et al. Biologic prescribing decisions following serious infection: results from the British society for rheumatology biologics register-rheumatoid arthritis. Rheumatology (Oxford). 2018;57(12):2096–100. ISSN 1462-0332. https://www.ncbi.nlm.nih.gov/pubmed/29986108.
George MD, et al. Risk for serious infection with low-dose glucocorticoids in patients with rheumatoid arthritis: a cohort study. Ann Intern Med. 2020;173(11):870–78. ISSN 1539-3704. https://www.ncbi.nlm.nih.gov/pubmed/32956604.
Weaver A, et al. Rheumatoid arthritis disease activity and disability affect the risk of serious infection events in RADIUS 1. J Rheumatol. 2013;40(8):1275–81. ISSN 0315-162X. https://www.ncbi.nlm.nih.gov/pubmed/23772079.
Doran MF, et al. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2294–300. ISSN 0004-3591. https://www.ncbi.nlm.nih.gov/pubmed/12355476.
Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford). 2013;52(1):ISSN 1462-0332. https://www.ncbi.nlm.nih.gov/pubmed/23192911.
Chandrashekara S, et al. Influence of disease duration and socioeconomic factors on the prevalence of infection and hospitalization in rheumatoid arthritis: KRAC study. Int J Rheum Dis. 2019;22(7):1216–25. ISSN 1756-185X. https://www.ncbi.nlm.nih.gov/pubmed/30977300.
Ozen G, et al. Risk of serious infection in patients with rheumatoid arthritis treated with biologic versus nonbiologic disease-modifying antirheumatic drugs. ACR Open Rheumatol. 2019;1(7):424–32. ISSN 2578-5745. https://www.ncbi.nlm.nih.gov/pubmed/31777822.
Ibrahim A, et al. Risk of infection with methotrexate therapy in inflammatory diseases: a systematic review and meta-analysis. J Clin Med. 2018;81. ISSN 2077–0383 https://www.ncbi.nlm.nih.gov/pubmed/30583473.
Cecconi M, et al. Incidence of infectious adverse events in patients with rheumatoid arthritis and spondyloarthritis on biologic drugs-data from the Brazilian registry for biologics monitoring. J Clin Rheumatol. 2020;26(2):73–78. ISSN 1536-7355. https://www.ncbi.nlm.nih.gov/pubmed/32073519.
Lomonte ABV, et al. Tofacitinib, an oral Janus kinase inhibitor, in patients from Brazil with rheumatoid arthritis: pooled efficacy and safety analyses. Medicine (Baltimore). 2018;97(31):e11609. ISSN 1536-5964. https://www.ncbi.nlm.nih.gov/pubmed/30075534.
Sacilotto NC, et al. Real - rheumatoid arthritis in real life - study cohort: a sociodemographic profile of rheumatoid arthritis in Brazil. Adv Rheumatol. 2020;60(1):20. ISSN 2523-3106. https://www.ncbi.nlm.nih.gov/pubmed/32171331.
da Rocha Castelar-pinheiro G, et al. The REAL study: a nationwide prospective study of rheumatoid arthritis in Brazil. Adv Rheumatol. 2018;58(1):9. ISSN 2523-3106. https://www.ncbi.nlm.nih.gov/pubmed/30657089.
Vicente GNS, et al. Cardiovascular risk comorbidities in rheumatoid arthritis patients and the use of anti-rheumatic drugs: a cross-sectional real-life study. Adv Rheumatol. 2021;61(1):38. ISSN 2523-3106. https://www.ncbi.nlm.nih.gov/pubmed/34172097.
Gomides APM, et al. Causes of synthetic disease-modifying drug discontinuation in rheumatoid arthritis: data from a large real-life cohort. PLoS One. 2019;14(3):e0213219. ISSN 1932-6203. https://www.ncbi.nlm.nih.gov/pubmed/30822348.
Gomides APM, et al. High levels of polypharmacy in rheumatoid arthritis-A challenge not covered by current management recommendations: data from a large real-life study. J Pharm Pract. 2021;34(3):365–71. ISSN 1531-1937. https://www.ncbi.nlm.nih.gov/pubmed/31451091.
Guimarães MFBR, et al. Discordance between the patient’s and physician’s global assessment in rheumatoid arthritis: data from the REAL study-Brazil. PLoS One. 2020;15(3):e0230317. ISSN 1932-6203. https://www.ncbi.nlm.nih.gov/pubmed/32168350.
Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22.
Mehta B, et al. Serious infection risk in rheumatoid arthritis compared with non-inflammatory rheumatic and musculoskeletal diseases: a US national cohort study. RMD Open. 2019;5(1):e000935. ISSN 2056-5933. https://www.ncbi.nlm.nih.gov/pubmed/31245055.
Wang D, et al. Severe infections remain common in a real-world rheumatoid arthritis cohort: a simple clinical model to predict infection risk. Eur J Rheumatol. 2020;8(3):133–38. ISSN 2147-9720. https://www.ncbi.nlm.nih.gov/pubmed/33372891.
Pieringer H, et al. RABBIT risk score and ICU admission due to infection in patients with rheumatoid arthritis. Clin Rheumatol. 2017;36(11):2439–45. ISSN 1434-9949. https://www.ncbi.nlm.nih.gov/pubmed/28905133.
Ng KH, et al. Analysis of risk factors of mortality in rheumatoid arthritis patients with interstitial lung disease: a nationwide, population-based cohort study in Taiwan. RMD Open. 2022;8(2):e002343. ISSN. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9403156.
Accortt NA, et al. Impact of sustained remission on the risk of serious infection in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70(5):679–84. ISSN 2151-4658. https://www.ncbi.nlm.nih.gov/pubmed/28960869.
Quartuccio L, et al. Risk of serious infection among patients receiving biologics for chronic inflammatory diseases: usefulness of administrative data. J Adv Res. ISSN 2090–1232 2019;15:87–93. https://www.ncbi.nlm.nih.gov/pubmed/30581616.
Smolen JS, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99. ISSN 1468-2060. https://www.ncbi.nlm.nih.gov/pubmed/31969328.
Fraenkel L, et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73(7):1108–23. ISSN 2326-5205. https://www.ncbi.nlm.nih.gov/pubmed/34101376.
Ranza R, de la Vega MC, Laurindo IMM, et al. Changing rate of serious infections in biologic-exposed rheumatoid arthritis patients. Data from South American registries BIOBADABRASIL and BIOBADASAR. Clin Rheumatol. 2019;38:2129–39. https://doi.org/10.1007/s10067-019-04516-2.
Toussirot E. Predictive factors for disability as evaluated by the health assessment questionnaire in rheumatoid arthritis: a literature review. Inflamm Allergy Drug Targets. 2010;9(1):51–59. https://pubmed.ncbi.nlm.nih.gov/20230368.
Michaud K, et al. Treatment and nontreatment predictors of health assessment questionnaire disability progression in rheumatoid arthritis: a longitudinal study of 18,485 patients. Arthritis Care Res. 2011;63(3):366–72. https://doi.org/10.1002/acr.20405.
Monti S, Montecucco C, Bugatti S, et al. Rheumatoid arthritis treatment: the earlier the better to prevent joint damage. RMD Open. 2015;15(1):e000057. https://doi.org/10.1136/rmdopen-2015-000057.
Rezaee-Zavareh MS, et al. Infectious and coronary artery disease. ARYA Atheroscler. 2016;12(1). ISSN 1735-3955. Disponível em. https://www.ncbi.nlm.nih.gov/pubmed/27114736.
Sipilä PN, et al. Severe infection and risk of cardiovascular disease: a multicohort study. Circulation. 2023;147(21). ISSN 1524-4539. Disponível em. https://www.ncbi.nlm.nih.gov/pubmed/36971007.
de Oliveira PP, et al. Serious infections among unselected patients with ST-elevation myocardial infarction treated with contemporary primary percutaneous coronary intervention. Am Heart J. 2016;181. ISSN 1097-6744. Disponível em https://www.ncbi.nlm.nih.gov/pubmed/27823693.
Cavallazzi R, Ramirez J. Community-acquired pneumonia in chronic obstructive pulmonary disease. Curr Opin Infect Dis. 2020;33(2). ISSN 1473-6527. Disponível em https://www.ncbi.nlm.nih.gov/pubmed/32022741.
Saúde MD. Clinic protocol and therapeutic guidelines for rheumatoid arthritis and juvenile idiopathic arthritis. Joint Ordinance N 16, 2021. https://www.gov.br/saude/pt-br/assuntos/protocolos-clinicos-e-diretrizes-terapeuticaspcdt2021.
Furer V, et al. Incidence and prevalence of vaccine preventable infections in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD): a systemic literature review informing the 2019 update of the EULAR recommendations for vaccination in adult patients with AIIRD. RMD Open. 2019;5(2). ISSN 2056-5933. Disponível em https://www.ncbi.nlm.nih.gov/pubmed/31673420.
Qendro T, et al. Suboptimal immunization coverage among Canadian rheumatology patients in routine clinical care. J Rheumatol. 2020;47(5). ISSN 0315-162X. Disponível em https://www.ncbi.nlm.nih.gov/pubmed/31308211.
Jacques M, et al. Low influenza, pneumococcal and diphtheria-tetanus-poliomyelitis vaccine coverage in patients with primary Sjögren’s syndrome: a cross-sectional study. Vaccines. 2019;8(1). ISSN 2076-393X. Disponível em https://www.ncbi.nlm.nih.gov/pubmed/31877764.
Acknowledgments
We thank the Brazilian Society of Rheumatology and the Rheumatology team of the Clinical Hospital, Federal University of Minas Gerais, for support of this project.
Funding
This work was supported by the Brazilian Society of Rheumatology (BSR). For this project, BSR received specific grant support from the following companies: Bristol-Myers Squibb Farmacêutica Ltda; Eli Lilly do Brasil Ltda; Janssen-Cilag Farmacêuticos Ltda; Laboratórios Pfizer Ltda; Produtos Roche Químicos e Farmacêuticos S.A. and UCB Biopharma Ltda. The funding body or the companies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
A.L.B.A: medical student responsible for the study, participated in all planning, execution and preparation of the manuscript. M.F.B.R.G., M.R.C.P., L.M.H.M. and G.C.P.: advisor and supervisor, responsible for outlining the study, participated in the analysis, data interpretation and critical review of the content. L.M.H.M. and G.C.P.: participated in the process of execution and content review. M.F.B.R.G., M.R.C.P., L.R.P., A.P.M.G.R., K.R.B., P.L.J.R., R.D.N.G., G.R.W.C., S.C.R., C.V.B., A.P., L.M.H.M., G.C.P.: responsible for the ‘Rheumatoid Arthritis in Real Life’ database. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the National Commission of Ethics in Research (CONEP—Comissão Nacional de Ética em Pesquisa)—Ministry of Health. The coordinating center was the University of the State of Rio de Janeiro, and the approval number was 45781015.8.1001.5259. Each of the centers also obtained approval from the respective Institutional Review Boards. All patients signed the informed consent form.
Consent for publication
Not applicable.
Conflict of interest
Ana Luisa Bagno de Almeida 1 (MD)—none. Maria Fernanda B. Resende Guimarães 1 (MD, PhD)—personal fees and/or nonfinancial support from AbbVie, Bristol-Myers-Squibb, Janssen, Novartis, Pfizer, Roche and UCB. Maria Raquel da Costa Pinto 1 (MD, PhD)—none. Letícia Rocha Pereira 2 (MSc, PhD candidate)—none. Ana Paula Monteiro Gomides Reis 3—none (MD, PhD). Karina Rossi Bonfiglioli 4 (MD, PhD)—personal fees and/or nonfinancial support from Abbvie, Boheringer Ingelheim, Bristol-Myers-Squibb, Janssen, Novartis and Roche. Paulo Louzada-Junior 5 (MD, PhD)—none. Rina Dalva Neubarth Giorgi 6 (MD, MSc)—personal fees and/or nonfinancial support from AbbVie, Bristol-Myers-Squibb, Eli Lilly, Janssen and UCB. Gláucio Ricardo Werner de Castro 7 (MD, PhD)—none. Sebastião Cezar Radominski 8 (MD, PhD)—consulting fees, speaking fees and institutional support for clinical trials from AbbVie, Amgen, Bristol-Myers-Squibb, Lilly, Pfizer, and Roche. Claiton Viegas Brenol 9 (MD, PhD)—personal fees and/or nonfinancial support from AbbVie, Bristol-Myers-Squibb, Janssen, Novartis, Pfizer, Roche and UCB. Alisson Pugliesi 10 (MD)—none. Licia Maria Henrique da Mota 3 (MD, Ph. D)—personal or institutional support from AbbVie, Janssen, Pfizer and Roche; has delivered speeches at events related to this work and sponsored by AbbVie, Boehringer Ingelheim, GSK, Janssen, Libbs, Lilly, Novartis, Pfizer, Roche, Sandoz, and UCB. Geraldo da Rocha Castelar-Pinheiro 2 (MD, PhD, Full Professor)—none.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Almeida, A., Guimarães, M., da Costa Pinto, M. et al. Predictors of serious infections in rheumatoid arthritis—a prospective Brazilian cohort. Adv Rheumatol 64, 23 (2024). https://doi.org/10.1186/s42358-024-00363-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-024-00363-1