Abstract
Background
Ankylosing Spondylitis (AS) patients face several challenges due to the nature of the disease and its physical and psychological complications. Sleep disorders are among the most important concerns. Sleep disorders can aggravate the signs and symptoms of the disease and ultimately reduce the quality of patients’ lives. This study uses a systematic review and meta-analysis to pool the reported prevalence of sleep disorders among AS patients.
Methods
To find related studies, the WoS, PubMed, ScienceDirect, Scopus, Embase, and Google Scholar databases were systematically searched without a lower time limit. Heterogeneity among the identified studies was checked using the I2 index, and the Begg and Mazumdar correlation test examined the existence of published bias. Comprehensive Meta-Analysis (v.2) software was adopted to analyze the data.
Results
In the review of 18 studies with a sample size of 5,840, the overall pooled prevalence of sleep disorders among AS patients based on the random effects method was found to be 53% (95% CI: 44.9–61). The highest and lowest prevalence was in Egypt at 90% and Australia at 19.2%, respectively. Our meta-regression results show that with the increase in ‘sample size’ and ‘year of publication’, the overall prevalence of sleep disorders in patients with AS decreases (p < 0.05).
Conclusion
The results of the present study indicate a high and significant prevalence of sleep disorders among AS patients. Thus, health policymakers and healthcare providers must focus on timely diagnosis and effective educational and therapeutic interventions for the prevention and proper treatment of sleep disorders in this population of patients.
Similar content being viewed by others
Background
Ankylosing spondylitis (AS) is a chronic progressive spinal inflammatory arthritis and belongs to a group of arthritis called spondyloarthritis. Clinical manifestations usually emerge in the third decade of life. AS characteristically affects the axial skeleton, sacroiliac joints, entheses, and extra-skeletal sites such as the bowel, skin, and eye, and rarely the lungs and heart can be affected [1,2,3,4].
Inflammation in AS can cause bone erosion, new bone formation, and excessive bone remodeling and slowly fills the interarticular spaces and eventually fuses the joints in the spine, which triggers severe pain and reduces spinal mobility and stiffness [1,2,3,4,5,6]. This bone destruction and misplaced growth lead to major limitations in spine mobility, physical activity, and impaired functional ability. [5, 6]. In addition, these patients are at a greater risk of suffering from depression and anxiety [7, 8]. Such burdens negatively affect the quality of life of the patients [9].
Sleep quality is one of the important factors affecting quality of life [10]. Sleep disorders are one of the public health problems, so that most people have experienced sleep disorders in their life [11,12,13]. The findings from previous research indicate that patients with AS suffer from more sleep disorders than healthy individuals [14,15,16]. Patients with AS are affected by various sleep disorders, including sleep onset and continuation disorders, restless leg syndrome, and obstructive sleep apnea (OSA) [10, 14, 16,17,18]. Nevertheless, previous research findings present different sleep disorder prevalences among AS patients. For instance, in the studies conducted in Egypt, China, and Australia, the prevalence of sleep disorders was reported as 90%, 31%, and 19.2%, respectively [10, 16, 19].
Sleep disorders in AS patients can be influenced by the experience of axial pain and inflammatory back pain after midnight [20, 21], severeness of the disease [10], and fatigue and functional limitations experienced by the patients [6, 20]. In addition, under the influence of symptoms and complications of the disease, these patients experience anxiety and depression, which can affect sleep quality [16, 20, 22,23,24].
Sufficient and high-quality sleep regulates several internal bodily processes, such as metabolism, tissue restoration and recovery, immune-inflammatory response, and synaptic homeostasis, and improves cognitive function, memory, and mood [25, 26]. Accordingly, sleep disorders are related to increasing the risk of impaired cognitive functions, signs of depression, sleepiness during the day, and thus increasing the probability of accidents, falls and related deaths, fatigue, and reducing the physical capacity of a person in performing daily activities [12, 26]. Similarly, and even more sternly, sleep disorders affect the quality of life of AS patients [6]. The findings of existing research highlight the role of sleep disorder in the occurrence of depression and anxiety [16, 20, 22] and fatigue [20, 27] and that there is a significant positive relationship between sleep disorder and AS [6, 28].
Considering the negative effects of sleep disorders on an AS patient’s life, there is a need for policymakers and health professionals to be trained on the impacts of the disease. It is also imperative to allocate the necessary resources for pertinent therapeutic interventions. Considering dissimilar reported results of existing studies on the relationship between AS and sleep disorders, this study aims to obtain the pooled prevalence of sleep disorders among patients with AS.
Methods
This study is a systematic review and meta-analysis, which was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [29].
In order to find related studies, the WoS, PubMed, ScienceDirect, Embase, Scopus, and Google repositories and databases were searched using keywords of sleep, “sleep quality”, “sleep disturbance”, “sleep wake disorder”, “sleep disorder”, “sleep initiation and maintenance disorders”, sleeplessness, insomnia, “ankylosing spondylitis”, “ankylosing Apondyloarthritis”, “ankylosing spondylarthritis”, “spondylitis ankylopoetica”, “B disease”, “Marie Strumpell disease”, “spondylarthritis ankylopoetica”, and “Bechterew disease”. The initial search was conducted on 25th November 2022, and searches were updated on 23rd December 2022.
Additionally, lists of references within the identified articles were manually searched to ensure the comprehensiveness of the search and to find further relevant studies. No restrictions were placed on the year of publication of the articles. Study information was transferred into the EndNote (x8) reference management software.
Inclusion and exclusion criteria
Studies that fulfilled the inclusion criteria were retained in the study selection process. The inclusion criteria entailed cross-sectional, cohort, and case-control studies that reported the prevalence of sleep disorders among AS patients and had sufficiently reported sufficient related information. Exclusion criteria were case reports, case series, intervention studies, review studies, studies not published in English, and those whose full text was unavailable.
The International Classification of Sleep Disorders-Third Edition (ICSD3) is a reliable and authoritative source for diagnosing sleep disorders. According to this text, sleep disorders are divided into seven main categories that are insomnia disorders, sleep-related breathing disorders, central disorders of hypersomnolence (hypersomnia), circadian rhythm sleep-wake disorders, sleep-related movement disorders, parasomnias, and other types of sleep disorders [30,31,32,33]. This study did not focus on the prevalence of insomnia disorders, sleep-related breathing disorders such as obstructive sleep apnea, hypersomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders such as restless leg syndrome and parasomnia. Therefore, our analysis did not include several studies that only reported the prevalence of obstructive sleep apnea and restless leg syndrome.
Study selection
Following the initial search, duplicated studies in different repositories were removed, and only a copy was retained. Subsequently, the titles and abstracts of articles were reviewed per the inclusion and exclusion criteria, and irrelevant articles were removed. In the next step, the full texts of the remaining studies were evaluated based on the inclusion and exclusion criteria, and at this step, further irrelevant studies were omitted. To avoid bias, two researchers conducted all the steps of reviewing sources and data extraction independently, and the reasons for omitting articles were noted. In cases where there was a difference of opinion between two researchers, the review of the article was finalized by a third person to reach a consensus.
Quality evaluation
The Newcastle-Ottawa Scale is a quality assessment tool for observational studies that the Cochrane Collaboration recommends. The NOS assigns up to a maximum of nine points for the least risk of bias in three domains: selection of study groups, comparability of groups, and ascertainment of exposure and outcomes for case-control and cohort studies. Eventually, articles were classified as high quality (scoring ≥ 5 points) or low quality (scoring < 5 points).
Data extraction
Data extraction was conducted by two researchers using a separate pre-prepared checklist with the following fields: First author’s name, year of publication, place of study, type of study, AS diagnostic criteria, tools used to investigate sleep disorder, age of patients, sample size, and prevalence of sleep disorder.
Statistical analysis
The heterogeneity among the studies was assessed using the I2 test. The Begg and Mazumdar correlation test at a significance level of 0.1 was used to examine publication bias, and the corresponding funnel plot was drawn. Data analysis was conducted within the Comprehensive Meta-Analysis (v.2) software.
Results
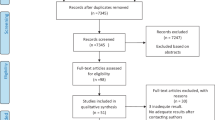
After systematically searching the research repositories and databases, 1,177 studies were identified and transferred into the EndNote reference management software. After removing 366 duplicate studies, the titles and abstracts of the remaining 811 articles were examined based on inclusion and exclusion criteria. Following this examination, 174 studies were retained for secondary evaluation. At this stage, the full texts of the 174 studies were examined following the inclusion and exclusion criteria, and a further 150 articles were excluded. After evaluating the quality of the remaining 24 studies, six further articles were omitted as they were deemed low quality. Finally, 18 studies were included in the meta-analysis (Fig. 1). The information from 18 studies is outlined in Table 1 [6, 10, 14, 16, 19, 20, 22, 27, 28, 34,35,36,37,38,39,40,41,42].
In the review of 18 studies with a sample size of 5,840, the I2 test illustrated high heterogeneity (I2: 96.05), and thus random effects method was adopted to conduct the analysis. Our meta-analysis shows that the pooled prevalence of sleep disorders among AS patients is 53% (95% CI: 44.9–61) (Fig. 2). The included studies were published between 1999 and 2022. The highest reported prevalence, with a rate of 90%, belonged to the study of Abdulaziez and Asaad, which was conducted in Egypt [19], and the lowest prevalence, with a rate of 19.2%, was reported in the work of Tymms et al. in Australia [10].
The Begg and Mazumdar correlation test was conducted at the significance level of 0.1, which indicated the absence of publication bias among the studies (p: 0.288) (Fig. 3).
We also conducted a meta-regression analysis to examine the effect of other factors that may be affecting the heterogeneity of the studies. Our meta-regression analysis showed that with the increase in ‘sample size’, the prevalence of sleep disorders in AS patients decreased (p < 0.05) (Fig. 4). Moreover, with the increase in ‘year of publication’, the prevalence of sleep disorders among AS patients decreased (p < 0.05) (Fig. 5).
Discussion
Ankylosing Spondylitis is a chronic inflammatory disease that has a negative effect on the quality of life [21]. The findings of previous studies indicate the existence of disorders in the quality, duration, and efficiency of sleep, difficulties in falling asleep, the presence of restless leg syndrome, and obstructive sleep apnea among AS patients [37, 38, 46,47,48,49].
This systematic review and meta-analysis were conducted to pool the prevalence of sleep disorders in patients with AS. As a result, 18 studies with a sample size of 5,840 people were evaluated, and their reported results were analyzed. Accordingly, the pooled prevalence of sleep disorders among AS patients is 53%. Moreover, considering our meta-regression analyses, it was found that with the increase in ‘sample size’ and ‘year of publication’, the prevalence of sleep disorders significantly decreased.
In a systematic review by Leverment et al., poor sleep was reported in 35–90% of patients with axial spondyloarthritis [50]. Jiang et al. reported the prevalence of sleep disorders among AS patients as 31% [16]. In 4 other studies conducted in China, the prevalence of sleep disorders was reported from 35.4% in the study of Li et al. up to 67.6% in Nie et al. analysis. [20, 36, 38, 40]. The lowest prevalence (19.2%) among the retained studies was related to the work of Tymms et al. in Australia [10]. The differences in reported results of existing literature can be due to the variations in the tools adopted for assessing sleep disorders, alternative approaches to defining the cut-off point of the tools used, and the working methods within the studies.
As highlighted earlier, the findings show that the prevalence of sleep disorders decreases with the increase of ‘sample size’. Among the included studies, the highest prevalence belonged to the two studies of Abdulaziez and Asaad and Yüksel et al., with sample sizes of 20 people, 90% and 80%, respectively [19, 42].
In the study of Aydin et al., the scores of 5 out of the seven subscales of PSQI, i.e., subjective sleep quality, sleep duration, habitual sleep efficiency, sleep disturbances, and daytime dysfunctions in patients, were significantly higher than in healthy individuals. However, the scores of the other two subscales, sleep latency and the use of sleeping pills, were insignificant between the two groups. In fact, in the study, the main concern was related to the continuation of sleep and not the sleep onset; this may be because the inflammatory back pain intensifies in the second half of the sleep duration [46]. Inflammatory back pain and axial pain are among the main causes of sleep disorders in patients with AS [21]. In the study of Da Costa et al., 88% of patients with spondyloarthropathy had difficulty staying asleep [51].
In a report by Li et al., AS patients had higher scores than the control group in the subscales of subjective sleep quality, sleep latency, sleep efficiency, sleep disorders, and medication use. However, the difference between the two groups was insignificant regarding sleep duration and disruption in daily functioning [53]. Compared to that study, the current research has selected more databases for search and covers a longer time range (until 2022), and accordingly, the sensitivity of the present study is higher. Also, the analysis of factors causing heterogeneity based on meta-regression is considered so that an analysis can be performed on the high heterogeneity of the study.
Abdulaziez and Asaas conducted a study using polysomnography (PSG) to objectively evaluate sleep quality in patients with AS. They reported that compared to the healthy individuals, patients had a lighter sleep with an increase in Non-rapid eye movement (NREM) stage I and II, which means a reduction in deep sleep. In addition, slow-wave sleep was reduced among the patients, indicating a reduction in deep sleep [38, 43,44,45].
The pro-inflammatory cytokine, tumor necrosis factor-alpha (TNF-α), is one of the important cytokines in the inflammation process in patients. Several TNF-α inhibitors have been developed to reduce spinal pain and inflammation in this disease [54,55,56]. The findings of the study of Karatas et al. demonstrate that in patients with severe AS activity who underwent anti-TNF therapy, compared to patients who were in remission and were not treated with anti-TNF-α drugs three months after the treatment, the PSQI scores decreased significantly, which means that their sleep quality improved meaningfully compared to the healthy group. However, PSG-related parameters such as NREM stage I and II after three months of treatment did not show a difference between the two groups [37]. In the study of In et al., After evaluating the sleep quality of the patients using PSG, the total sleep time and its efficiency in the anti-TNF group was significantly higher than the group receiving NSAIDS, stage I was considerably shorter, and the rapid eye movement (NRM) stage was markedly longer than the NSAIDs group [57]. Therefore, according to the literature, the type of treatment can affect the quality of sleep and the prevalence of sleep disorders.
Disease activity can be another factor in reports on the severity of sleep disorders. In this regard, the study of Tymms et al. showed that patients with a more severe AS experience the symptoms of insomnia seven times more [10]. The findings of other studies also indicate the negative impact of more severe disease activity on the sleep quality of AS patients [6, 14, 18, 21, 37]. More disease activity causes increased structural damage and disruption of spine mobility, which can cause difficulty in changing position while sleeping and thus disturb the patients’ sleep [18, 40]. Also, the findings indicate a significant positive correlation between sleep disorder scores and metrics (measurements related to the pelvis and spine) measured by Bath Ankylosing Spondylitis Metrology Index (BASMI) [6, 19, 28]. However, reported results in an article by Li et al. found no significant relationship between metrological indicators and sleep quality [38].
Night pain is one of the prominent features of AS disease, which can affect the quality of sleep among patients [16]. The findings of existing literature indicate a significant relationship between pain experience and sleep quality disruption [18, 20, 22, 37, 38]. In a study by Nie et al., nighttime back pain was one of the key factors resulting in sleep disturbance. Experiencing pain before and during the night causes problems in falling asleep and reduces the duration and quality of sleep [20].
AS also significantly impacts a patient’s psychological state [23]. In the study by Zhang et al., the prevalence of depression among AS patients was reported about 35% [23]. Furthermore, the findings of Jiang et al. and Nie et al. showed that 31.6% and 48% of AS patients experience anxiety, respectively [16, 20]. Additionally, the prevalence of anxiety disorders in patients suffering from insomnia and patients without insomnia was reported as 76.1% and 33.3%, respectively [22].
The findings of previous studies indicate the existence of a significant relationship between sleep disorder and variables such as older age [19, 20, 38], experiencing more fatigue [19, 20, 27, 34, 46], delay in diagnosing the disease [20], longer duration of the disease [18,19,20], greater severity and duration of morning stiffness [18, 38, 46], lower quality of life [18, 19, 22, 28, 37, 61], higher CRP values [6, 18, 38, 46], presence of extraspinal manifestations of the disease [20], presence of functional limitations [6, 19,20,21,22, 37, 38, 46], lower level of education [38] and female gender [6] among AS patients. In the study of Wadeley et al., it was found that women with axial spondylarthritis experience sleep disorders three times more than men [52]. Further, in the study of Hultgren et al., prevalence of sleep disorder in women and men with AS was reported as 81% and 50%, respectively [35].
Regarding the relationship between educational level and sleep quality, the results of the study by Li et al. showed that there is a significant negative relationship between the overall score of sleep quality and years of education, which means that the higher a patient’s education, the less likely she/he is to experience a sleep disorder [38]. Also, in the study of Jiang et al., higher education patients experienced less anxiety and depression [16]. Therefore, according to pertinent literature, higher education seems to enable a patient to gain sufficient knowledge and awareness about the disease and its associated considerations. This also can help the patient to be able to control the disease and its symptoms. Nonetheless, in the findings of the study of Nie et al. no relationship between sleep disorder and the level of education of a patient was found [20].
The findings of the study of Günaydin et al. showed that the experience of fatigue in AS patients is influenced by sleep disorder [27]. Moreover, as sleep disturbance can increase the experience of pain, it seems that sleep disturbance lowers the threshold of tolerance and strengthens the pain signals, thus resulting in the person focusing more on the feeling of pain [38]. In the study of Purabdollah et al., it was reported that a significant relationship was found between sleep disorders and pain [58,59,60,61,62]. This study states that the severity of pain and sleep problems can predict inflammatory markers that can be clues to the severity of the disease. Therefore, relieving pain and improving sleep can reduce the severity of the disease [63].
This study found a relatively high pooled prevalence of sleep disorders among AS patients. Also, it is known that sleep disorders affect various aspects of patients’ lives. Therefore, regular follow-ups and therapeutic interventions, including non-pharmacological treatments, as well as effective and quality education offered by healthcare providers, are vital to improving the patients with AS physical and mental health. Moreover, pertinent interventions and policies will be instrumental in treating and preventing sleep disorders among patients.
Limitations
One of the limitations of the study is that we included a number of researchers that had not used specific sleep disorder questionnaires to report the prevalence of sleep disorders. Additionally, two used PSQI cut-off points were used for measuring sleep disorders within the included studies. Moreover, the included studies used different sleep disorder questionnaires to report the prevalence. The report of sleep disorder was based on self-report questionnaires that provide the possibility of subjective evaluation of sleep quality. The mentioned cases may have affected the accurate reporting of the prevalence of sleep disorders in AS patients. Another limitation of the current research was that many studies could not be included due to either lack of access to their full text or their omissions after the quality evaluation stage.
Conclusion
The findings of the present study indicate the high prevalence of sleep disorders among AS patients, which means that policymakers and service providers in the field of healthcare, including nurses, should pay more attention to this population of patients and devise necessary interventions for timely diagnosis and treatment. Effective and quality educational and therapeutic interventions for patients and their families require regular follow-up. Additionally, more support for AS patients would be necessary to prevent and appropriately treat sleep disorders and associated complications.
Data Availability
Datasets are available through the corresponding author upon reasonable request.
Abbreviations
- AS:
-
Ankylosing Spondylitis
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- ICSD3:
-
The International Classification of Sleep Disorders-Third Edition
- NOS:
-
The Newcastle-Ottawa Scale
- SQI:
-
Pittsburg Sleep Quality Index
- USI:
-
Uppsala Sleep Inventory
- ISI:
-
Insomnia Severity Index
- ICD:
-
International Classification of Diseases
References
Hwang MC, Ridley L, Reveille JD. Ankylosing spondylitis risk factors: a systematic literature review. Clin Rheumatol. 2021;40(8):3079–93.
Mauro D, Thomas R, Guggino G, Lories R, Brown MA, Ciccia F. Ankylosing spondylitis: an autoimmune or autoinflammatory disease? Nat Rev Rheumatol. 2021;17(7):387–404.
Tam C, Gu LS, Yu J. Pathogenesis of ankylosing spondylitis. Nat Rev Rheumatol. 2010;6:399–405. https://doi.org/10.1038/nrrheum.2010.79.
Braga M, Lara-Armi FF, Neves JSF, Rocha-Loures MA, Terron-Monich MS, Bahls-Pinto LD, et al. Influence of IL10 (rs1800896) polymorphism and TNF-α, IL-10, IL-17A, and IL-17F serum levels in Ankylosing Spondylitis. Front Immunol. 2021;12:653611.
He Q, Luo J, Chen J, Yang J, Yao C, Xu C, et al. The validity and reliability of quality of life questionnaires in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review and meta-analysis. Health Qual Life Outcomes. 2022;20(1):116.
Chen CH, Chen HA, Liao HT, Chen CH. Association of sleep disturbance with calcitonin, disease severity and health index among patients with ankylosing spondylitis. Medicine. 2021;100:32.
Nam B, Koo BS, Nam SW, Shin JH, Song Y, Cho SK, et al. Gender differences in factors associated with low quality of life and depression in korean patients with ankylosing spondylitis. Qual life research: Int J Qual life aspects Treat care rehabilitation. 2021;30(8):2299–310.
Park JY, Howren AM, Zusman EZ, Esdaile JM, De Vera MA. The incidence of depression and anxiety in patients with ankylosing spondylitis: a systematic review and meta-analysis. BMC Rheumatol. 2020;4:12.
Song Y, Chen H. Predictors of Health-Related Quality of Life in patients with Ankylosing Spondylitis in Southwest China. Patient Prefer Adherence. 2021;15:1887–94.
Tymms K, Butcher BE, Sletten TL, Smith T, O’Sullivan C, Littlejohn G, et al. Prevalence of sleep disturbance and the association between poor disease control in people with ankylosing spondylitis within the australian clinical setting (ASLEEP study): a real-world observational study using the OPAL dataset. Clin Rheumatol. 2022;41(4):1105–14.
Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132(3):292–9.
Sindi S, Pérez LM, Vetrano DL, Triolo F, Kåreholt I, Sjöberg L, et al. Sleep disturbances and the speed of multimorbidity development in old age: results from a longitudinal population-based study. BMC Med. 2020;18(1):382.
Zhao M, Tuo H, Wang S, Zhao L. The Effects of Dietary Nutrition on Sleep and Sleep Disorders. Mediat Inflamm. 2020;2020:3142874.
Demirci S, Demirci K, Dogru A, Inal EE, Koyuncuoglu HR, Sahin M. Restless legs syndrome is associated with poor sleep quality and quality of life in patients with ankylosing spondylitis: a questionnaire-based study. Acta Neurol Belgica. 2016;116(3):329–36.
Durmus D, Sarisoy G, Alayli G, Kesmen H, Cetin E, Bilgici A, et al. Psychiatric symptoms in ankylosing spondylitis: their relationship with disease activity, functional capacity, pain and fatigue. Compr Psychiatr. 2015;62:170–7.
Jiang Y, Yang M, Lv Q, Qi J, Lin Z, Liao Z, et al. Prevalence of psychological disorders, sleep disturbance and stressful life events and their relationships with disease parameters in chinese patients with ankylosing spondylitis. Clin Rheumatol. 2018;37(2):407–14.
Tekatas A, Pamuk ON. Increased frequency of restless leg syndrome in patients with ankylosing spondylitis. Int J Rheum Dis. 2015;18(1):58–62.
Maatallah K, Makhlouf Y, Ferjani H, Cherif I, Ben Nessib D, Triki W et al. Factors associated with the inflammatory process in pain in ankylosing spondylitis. Pan Afr Med J. 2022;41.
Abdulaziez O, Asaad T. Sleep problems in ankylosing spondylitis: polysomnographic pattern and disease related variables. Egypt Rheumatologist. 2012;34(2):59–65.
Nie A, Wang C, Song Y, Xie X, Yang H, Chen H. Prevalence and factors associated with disturbed sleep in outpatients with ankylosing spondylitis. Clin Rheumatol. 2018;37(8):2161–8.
Urkmez B, Keskin Y. Relationship between sleep quality and physical activity level in patients with ankylosing spondylitis. Mod Rheumatol. 2020;30(6):1053–9.
Hakkou J, Rostom S, Mengat M, Aissaoui N, Bahiri R, Hajjaj-Hassouni N. Sleep disturbance in moroccan patients with ankylosing spondylitis: prevalence and relationships with disease-specific variables, psychological status and quality of life. Rheumatol Int. 2013;33(2):285–90.
Zhang L, Wu Y, Liu S, Zhu W. Prevalence of Depression in Ankylosing Spondylitis: a systematic review and Meta-analysis. Psychiatry Invest. 2019;16(8):565–74.
Webers C, Vanhoof L, Leue C, Boonen A, Köhler S. Depression in ankylosing spondylitis and the role of disease-related and contextual factors: a cross-sectional study. Arthritis Res therapy. 2019;21(1):215.
Fox JL, Scanlan AT, Stanton R, Sargent C. Insufficient sleep in young athletes? Causes, Consequences, and potential treatments. Sports Med (Auckland NZ). 2020;50(3):461–70.
Chennaoui M, Léger D, Gomez-Merino D. Sleep and the GH/IGF-1 axis: consequences and countermeasures of sleep loss/disorders. Sleep Med Rev. 2020;49:101223.
Günaydin R, Göksel Karatepe A, Ceşmeli N, Kaya T. Fatigue in patients with ankylosing spondylitis: relationships with disease-specific variables, depression, and sleep disturbance. Clin Rheumatol. 2009;28(9):1045–51.
Batmaz I, Sariyildiz MA, Dilek B, Bez Y, Karakoc M, Cevik R. Sleep quality and associated factors in ankylosing spondylitis: relationship with disease parameters, psychological status and quality of life. Rheumatol Int. 2013;33(4):1039–45.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Kansagra S. Sleep Disorders in adolescents. Pediatrics. 2020;145(Suppl 2):204–S209.
Sateia MJ. International classification of Sleep Disorders-Third Edition. Chest. 2014;146(5):1387–94.
Benca RM, Teodorescu M. Sleep physiology and disorders in aging and dementia. Handb Clin Neurol. 2019;167:477–93.
Halioua B, Chelli C, Misery L, Taieb J, Taieb C. Sleep Disorders and Psoriasis: an update. Acta Derm Venereol. 2022;102:adv00699.
Aissaoui N, Rostom S, Hakkou J, Berrada Ghziouel K, Bahiri R, Abouqal R, et al. Fatigue in patients with ankylosing spondylitis: prevalence and relationships with disease-specific variables, psychological status, and sleep disturbance. Rheumatol Int. 2012;32(7):2117–24.
Hultgren S, Broman JE, Gudbjornsson B, Hetta J, Lindqvist U. Sleep disturbances in outpatients with ankylosing spondylitis - a questionnaire study with gender implications. Scand J Rheumatol. 2000;29(6):365–9.
Jiang Y, Yang M, Wu H, Song H, Zhan F, Liu S, et al. The relationship between disease activity measured by the BASDAI and psychological status, stressful life events, and sleep quality in ankylosing spondylitis. Clin Rheumatol. 2015;34(3):503–10.
Karatas G, Bal A, Yuceege M, Firat H, Gurcay E, Ardic S, et al. Evaluation of sleep quality in patients with ankylosing spondylitis and efficacy of anti-TNF- therapy on sleep problems: a polisomnographic study. Int J Rheum Dis. 2018;21(6):1263–9.
Li Y, Zhang S, Zhu J, Du X, Huang F. Sleep disturbances are associated with increased pain, disease activity, depression, and anxiety in ankylosing spondylitis: a case-control study. Arthritis Res Ther. 2012;14(5):R215.
Song BW, Jeong HJ, Kim BY, Cho YW, Son CN, Kim SS, et al. Bath Ankylosing Spondylitis Disease Activity Index is Associated with the quality of Sleep in Ankylosing Spondylitis Patients. J Rheumatic Dis. 2021;28(3):143–9.
Song Y, Wang C, Chen H. Functional limitation and associated factors in outpatients with ankylosing spondylitis in Southwest China. Clin Rheumatol. 2017;36(4):871–7.
Ward MM. Health-related quality of life in ankylosing spondylitis: a survey of 175 patients. Arthritis Care Res. 1999;12(4):247–55.
Yüksel GA, Kurtuluş D, Tireli H. Sleep quality and restless legs syndrome in patients with ankylosing spondylitis. Med J Haydarpaşa Numune Train Res Hosp. 2019;59(1):78–83.
Chu P, Ju YS, Hinze AM, Kim AHJ. Measures of Sleep in Rheumatologic Diseases: Sleep Quality patient-reported outcomes in Rheumatologic Diseases. Arthritis Care Res. 2020;72(Suppl 10):410–30.
Sochal M, Małecka-Panas E, Gabryelska A, Talar-Wojnarowska R, Szmyd B, Krzywdzińska M et al. Determinants of Sleep Quality in Inflammatory Bowel Diseases. J Clin Med. 2020;9(9).
Zhang J, Zhou Y, Ma Z. Multi-target mechanism of Tripteryguim wilfordii Hook for treatment of ankylosing spondylitis based on network pharmacology and molecular docking. Ann Med. 2021;53(1):1090–8.
Aydin E, Bayraktar K, Turan Y, Kurt Omurlu I, Tastaban E, Sendur OF. Sleep quality in patients with ankylosing spondylitis. Annals of the Rheumatic Disease. 2013;71.
Solak O, Fidan F, Dundar U, Turel A, Aycicek A, Kavuncu V, et al. The prevalence of obstructive sleep apnoea syndrome in ankylosing spondylitis patients. Rheumatology. 2009;48(4):433–5.
Tsao CH, Huang JY, Huang HH, Hung YM, Wei JC, Hung YT. Ankylosing spondylitis is Associated with Risk of New-Onset Obstructive Sleep Apnea: a Nationwide Population-Based Cohort Study. Front Med (Lausanne). 2019;6:285.
Walsh JA, Song X, Kim G, Park Y. Evaluation of the comorbidity burden in patients with ankylosing spondylitis using a large US administrative claims data set. Clin Rheumatol. 2018;37(7):1869–78.
Leverment S, Clarke E, Wadeley A, Sengupta R. Prevalence and factors associated with disturbed sleep in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review. Rheumatol Int. 2017;37(2):257–71.
da Costa D, Zummer M, Fitzcharles MA. Determinants of sleep problems in patients with spondyloarthropathy. Musculoskelet Care. 2009;7(3):143–61.
Wadeley A, Clarke E, Leverment S, Sengupta R. Sleep in ankylosing spondylitis and non-radiographic axial spondyloarthritis: associations with disease activity, gender and mood. Clin Rheumatol. 2018;37(4):1045–52.
Li Z, Fu T, Wang Y, Dong C, Shao X, Li L, et al. Sleep disturbances in ankylosing spondylitis: a systematic review and meta-analysis. Psychol health Med. 2019;24(8):911–24.
Hu N, Chen X, Wang S, Yuan G, Wang Q, Shu H, et al. The association of polymorphisms in TNF and ankylosing spondylitis in common population: a meta-analysis. European spine journal: official publication of the european spine Society, the european spinal deformity Society, and the european section of the cervical. Spine Res Soc. 2021;30(6):1402–10.
Rockstrom MD, Chen L, Taishi P, Nguyen JT, Gibbons CM, Veasey SC, et al. Tumor necrosis factor alpha in sleep regulation. Sleep Med Rev. 2018;40:69–78.
Coates LC, Marzo-Ortega H, Bennett AN, Emery P. Anti-TNF therapy in ankylosing spondylitis: insights for the clinician. Ther Adv Musculoskelet Dis. 2010;2:37–43. https://doi.org/10.1177/1759720X09359728.
In E, Turgut T, Gulkesen A, Yolbas S, Akgol G, Koca SS. Sleep quality is related to Disease activity in patients with Ankylosing Spondylitis: a Polysomnographic Study. Jcr-Journal of Clinical Rheumatology. 2016;22(5):248–52.
Smith KJ, Au B, Ollis L, Schmitz N. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: a systematic review and meta-analysis. Exp Gerontol. 2018;102:109–32.
Pitharouli MC, Hagenaars SP, Glanville KP, Coleman JRI, Hotopf M, Lewis CM, et al. Elevated C-Reactive protein in patients with Depression, Independent of Genetic, Health, and psychosocial factors: results from the UK Biobank. Am J Psychiatry. 2021;178(6):522–9.
Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696–709.
Duruoz MT, Ulutatar F, Ozturk EC, Unal-Ulutatar C, Sanal Toprak C, Kayhan O. Assessment of the validity and reliability of the Jenkins Sleep Scale in ankylosing spondylitis. Int J Rheum Dis. 2019;22(2):275–9.
Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. 2019;23(4):2324–32.
Purabdollah M, lakdizaji S, Rahmani A. Relationship between Sleep, Pain and inflammatory markers in patients with rheumatoid arthritis. J Caring Sci. 2017;6(3):249–55.
Acknowledgements
By the Student Research Committee of Kermanshah University of Medical Sciences.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NS, and RH contributed to the design; MM statistical analysis participated in most of the study steps. MM and RH and NS prepared the manuscript. AH and SHSH and HK and MM assisted in designing the study and helped in the interpretation of the study. All authors have read and approved the content of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salari, N., Sadeghi, N., Hosseinian-Far, A. et al. Prevalence of sleep disturbance in patients with ankylosing spondylitis: a systematic review and meta-analysis. Adv Rheumatol 63, 33 (2023). https://doi.org/10.1186/s42358-023-00315-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-023-00315-1