Abstract
Background
Microparticles (MPs) are membrane-derived vesicles released from cells undergoing activation or apoptosis with diverse proinflammatory and prothrombotic activities, that have been implicated in the pathogenesis of systemic sclerosis (SSc). We aimed to evaluate the plasma levels of platelet-derived microparticles (PMPs), endothelial cell-derived microparticles (EMPs), and monocyte-derived microparticles (MMPs) in SSc patients, and the association between MPs and the clinical features of SSc.
Methods
In this cross-sectional study, 70 patients with SSc and 35 age- and sex-matched healthy controls were evaluated. Clinical and nailfold capillaroscopy (NFC) data were obtained from all patients. Plasma levels of PMPs (CD42+/31+), EMPs (CD105+), and MMPs (CD14+) were quantified by flow cytometry.
Results
Patients were mainly females (90%), with a mean age of 48.9 years old. PMP, EMP, and MMP levels were significantly increased in SSc patients compared to controls (79.2% ± 17.3% vs. 71.0% ± 19.8%, p = 0.033; 43.5% ± 8.7% vs. 37.8% ± 10.4%, p = 0.004; and 3.5% ± 1.3% vs. 1.1% ± 0.5%, p < 0.0001, respectively). PMP levels were significantly higher in patients with positive anti-topoisomerase-I antibodies (p = 0.030) and in patients with a disease duration > 3 years (p = 0.038). EMP levels were lower in patients with a higher modified Rodnan skin score (p = 0.015), and in those with an avascular score > 1.5 in NFC (p = 0.042).
Conclusion
The increased levels of PMPs, EMPs and MMPs in scleroderma patients might indicate a possible role for these agents in the pathogenesis of this challenging disease.
Similar content being viewed by others
Background
Systemic sclerosis (SSc) is a chronic autoimmune rheumatic disease (ARD), characterized by the pathogenic triad of microvascular damage, innate and adaptive immune dysregulation, and progressive tissue fibrosis of multiple organs [1]. SSc is a rare multifaceted disease, with high morbidity and mortality rates. Microvascular injury, including endothelial activation and progressive narrowing of the blood vessel, are early events in SSc and drive inflammation and fibrosis in a self-amplifying process [2, 3]. Platelet activation has also been considered an important player in SSc vascular and organ damage [4, 5].
Microparticles (MPs) are membrane-derived vesicles released from cells undergoing activation or apoptosis that contain a wide array of surface, cytoplasmic, and nuclear molecules [6], thus reflecting their cellular origin. MPs display a wide range of important biological properties, with diverse proinflammatory and prothrombotic activities, mediating cell–cell communication [6,7,8], vascular reactivity and angiogenesis [9]. In the circulation, MPs mostly originate from platelets, endothelial cells and leukocytes [10]. Due to the accumulating evidence of the role of MPs as conveyors of cell information, especially acting in the mechanisms of inflammation, thrombosis, and angiogenesis [6, 9], they have been implicated in the pathogenesis of SSc and other ARDs, including systemic lupus erythematosus, antiphospholipid syndrome, and rheumatoid arthritis [6, 11, 12].
Previous studies have reported plasma levels of MPs in SSc and their association with SSc clinical features [12,13,14,15,16,17,18], but with divergent results. Nevertheless, most studies have shown increased concentrations of MPs, particularly those derived from endothelial cells (EMPs) and platelets (PMPs), in patients with SSc compared to healthy controls [12, 13, 15, 16]. Therefore, these molecules are likely to contribute to the disease immunopathogenesis and may have antifibrotic and/or profibrotic behavior in SSc [9]. Few studies have evaluated monocyte-derived microparticles (MMPs) in SSc [13, 14]. Increased levels of MMPs likely reflect monocyte activation during inflammation and the innate immune response in ARD [6] and might couple inflammation and coagulation, considering that monocytes can display tissue factor and release IL-1β [11, 19]. In SSc, monocytes have higher migratory, chemotactic, and adhesive properties and contribute to fibrogenesis, producing profibrotic mediators, such as TGF-β and collagen type I [9, 20].
In this study, we aimed to evaluate the plasma levels of PMPs, EMPs, and MMPs in SSc patients compared to healthy controls, and to evaluate the association of MPs with SSc clinical features and microvascular involvement.
Materials and methods
Study design and patient selection
We conducted a cross-sectional study in which 70 SSc patients regularly followed in the Scleroderma Outpatient Clinic from the Federal University of São Paulo’s Medical School Hospital were consecutively recruited. All patients were over 18 years old, fulfilled the 2013 ACR/EULAR classification criteria for SSc [21] and were on stable pharmacological treatment for the last 3 months prior to recruitment. Patients were excluded if they had diagnosis of any other ARD, coronary diseases, cerebrovascular diseases, severe peripheral arterial conditions, active infections (including tuberculosis and hepatitis) or neoplastic conditions. Smokers and pregnant and lactating women were also excluded, as were patients who used anticoagulants or antiplatelet agents. Additionally, 35 sex- and age-matched healthy controls were also recruited for comparison analysis.
This study was conducted in accordance with the Declaration of Helsinki and all participants agreed and signed the informed consent form. The study was approved by the Local Ethics Committee (#2.630.953).
Clinical assessment
Clinical and demographic characteristics from all patients were obtained from electronic medical records at the same time as blood sample collection and included age, sex, disease subtype, and disease duration (defined as the time between the first non-Raynaud symptom and baseline visit). We also collected data about the presence of anticentromere (ACA) and anti-topoisomerase I (anti-Scl-70) antibodies and the presence of internal organ manifestations based on complementary exams routinely performed in all patients. Interstitial lung disease (ILD) was defined as the presence of interstitial abnormalities in chest high resolution computerized tomography. Pulmonary arterial hypertension (PAH) was considered in patients with Group I PAH confirmed by right heart catheterization, according to previous well-established criteria [22]. Esophageal dysmotility was considered when confirmed in esophagogram or esophageal manometry exams. A history of scleroderma renal crisis (SRC) was considered if new onset of significant hypertension (> 150/85 mmHg) and decreased renal function (≥ 30% reduction in estimated glomerular function rate) was registered in electronic medical records, and/or when confirmed by renal biopsy [23]. Finally, cardiac involvement was considered by the presence of myocardiosclerosis as confirmed by cardiac magnetic resonance. For all data, only exams performed in the last 12 months of the evaluation were considered. The modified Rodnan skin score (mRSS) and the presence of active digital ulcers (DUs) were assessed on the same day as blood sample collection. The mRSS was assessed by clinical palpation performed by the same experienced rheumatologist (SMO) in all patients at the 17 body areas preconized, and was graded on a scale of 0–3, with a maximum total score of 51 [24].
Nailfold capillaroscopy (NFC) was performed in the same month as the blood sample collection, with a stereomicroscope under 10 to 40 × magnification (SZ6145TR, Olympus, Tokyo, Japan). The variables assessed were the number of capillary loops per millimeter, the number of enlarged capillaries, the number of giant capillaries, and the number of microhemorrhages. The avascular score was rated from 0 to 3, as previously described [25]. Data obtained from eight fingers, excluding the thumbs, were expressed as the mean values. The scleroderma pattern was reviewed and classified into early, active, or late, as previously standardized by Cutolo et al. [26].
Microparticle acquisition and analysis
For MP acquisition, we used the protocol previously described by Fonseca et al. [27]. Peripheral blood samples (four milliliters) from SSc patients and healthy controls were collected in citrate tubes and immediately centrifuged at 160 × g for 10 min at 20 °C to obtain platelet-rich plasma (PRP), which was then centrifuged at 1500 × g for six minutes at 20 °C to obtain platelet-poor plasma (PPP). Thereafter, 70 µl of PPP were stained with fluorescent cell-specific mouse anti-human IgG1 monoclonal antibodies for identification and detection of PMP CD42+/CD31+ (FITC-conjugated anti-CD42 and PE-conjugated anti-CD31), EMP CD105+ (APC-conjugated anti-CD105), and MMP CD14+ (FITC-conjugated CD14) (Becton Dickinson Biosciences, San Jose, CA, USA). Subsequently, the samples were incubated for 20 min in the dark at room temperature. IgG1 isotypes were used as controls. After incubation, 200 µl of isotonic solution (PBS) were added, and the samples were immediately read at high flux mode on a flow cytometer (FACSCalibur, Becton Dickinson, San Jose, CA, USA), with approximately 30,000 events acquired. The data obtained were then analyzed using CellQuestPro software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) (Fig. 1). Plasma levels of MPs are presented as the percentages of positive acquired events.
Statistical analysis
Statistical analysis was conducted using IBM Statistical Package for Social Sciences (SPSS) version 28.0 (Chicago, IL, USA) and GraphPad Prism version 9.2.0 (San Diego, CA, USA). Variables are described as the means ± standard deviation (SD), unless otherwise stated. Considering that microparticles presented a normal distribution, as evaluated by Shapiro–Wilk and Kolmogorov–Smirnov tests, the percentages of microparticles were compared by paired Student’s t test. Categorical variables were analyzed using the χ2 test. A significance level of less than 5% for alpha risk (p < 0.05) was assumed in all tests.
Results
The clinical and demographic features of the studied population are summarized in Table 1. The patients were mainly females (90%), with a mean age of 48.9 ± 13.4 years old and a mean disease duration of 6.4 ± 4.0 years. Most patients had limited cutaneous SSc (62.9%) and ILD (54.3%). Regarding vascular complications, 40% presented a late scleroderma pattern in NFC, 55.7% had a history of previous or active DU (21.4% with active DU at clinical assessment), and 5.7% of patients had PAH.
For all sources of MPs studied, patients with SSc presented significantly increased plasma levels of MPs when compared to healthy controls (mean ± SD): 79.2% ± 17.3% versus 71.0% ± 19.8% for PMPs (p = 0.033); 43.5% ± 8.7% versus 37.8% ± 10.4% for EMPs (p = 0.004); and 3.5% ± 1.3% versus 1.1% ± 0.5% for MMPs (p < 0.0001), respectively (Fig. 2).
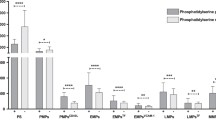
Associations found between MP levels and SSc clinical features are shown in Fig. 3. We observed significantly higher PMP plasma levels in SSc patients with positive anti-Scl-70 antibodies than in those with negative anti-Scl-70 antibodies (85.9% ± 9.5% vs. 77.7% ± 18.4%, p = 0.030), and in patients with a disease duration of more than 3 years than in those patients with a disease duration ≤ 3 years (82.4% ± 15.1% vs. 71.6% ± 20.0%, p = 0.038). In addition, we observed significantly lower EMP plasma levels in SSc patients with a mRSS > 15 compared to those with a mRSS ≤ 15 (40.0% ± 4.1% vs. 44.3% ± 9.3%, p = 0.015), as well as in patients with more severe capillary loss (avascular score > 1.5) in NFC compared to those with less severe capillary loss (avascular score ≤ 1.5) (39.8% ± 10.1% vs. 45.0% ± 8.3%, p = 0.042). No associations were observed between MMP plasma levels and SSc clinical features.
Associations found between microparticles (MP) and clinical features of systemic sclerosis (SSc). A Percentage of platelet-derived MP in SSc patients according to the presence of anti-Scl-70. B Percentage of platelet-derived MP in SSc patients according to disease duration. C Percentage of endothelial cell-derived MP in SSc patients according to modified Rodnan skin score (mRSS). D Percentage of endothelial cell-derived MP in SSc patients according to capillary loss on nailfold capillaroscopy (NFC)
There were no significant associations between plasma MP levels and disease subtype (limited or diffuse SSc), presence of active DU, or visceral manifestations (ILD, PAH, esophagopathy, cardiac involvement, and SRC). Moreover, the use of vasodilators and immunosuppressors did not affect the plasma levels of the different sources of MPs studied (data not shown).
Discussion
In the present study, we demonstrated increased levels of MPs derived from platelets, endothelial cells and monocytes in the blood of patients with SSc. In addition, a significant association with clinical features of SSc was found, including higher levels of PMPs in patients with positive anti-Scl-70 antibodies and longer disease duration, and lower EMPs levels in SSc patients with more severe cutaneous involvement, and more severe peripheral microangiopathy, as defined by NFC.
Our results are in accordance with previous studies, in which increased levels of different sources of MPs were found [12,13,14,15,16, 28]. As the elevated levels of various cytokines are a main feature of SSc, and platelets, endothelial cells and monocytes are activated in SSc [29, 30], the increased levels of MPs found in our study strengthen the hypothesis that MPs may be involved in SSc pathogenic mechanisms.
Our study is, thus far, the first to demonstrate increased plasma levels of PMPs in patients with SSc who were positive for anti-Scl-70 and had a longer disease duration (more than 3 years). The anti-Scl-70 autoantibody has a high specificity for the diagnosis of SSc [31, 32], and it is considered an important marker of disease progression, as it correlates with more severe phenotypes, such as diffuse cutaneous disease, pulmonary involvement, cardiac involvement and a higher risk of mortality [31,32,33]. Interestingly, in 2008, Nomura et al. [14] demonstrated an increase in serum levels of PMPs and MMPs in 42 patients with SSc compared to healthy controls, and this increase was significantly greater in patients with ILD. Recently, Leleu et al. [34] also found an increase in the serum level of PMPs in 96 patients with SSc compared to healthy controls, and this increase was more pronounced in patients with ILD and longer disease duration, similar to our study. Our findings, along with previous studies, therefore reinforce the possible role of these molecules as prognostic markers in SSc. It is important to highlight that, in both aforementioned studies, the prevalence of ILD was higher than in our sample (59.5% in the sample by Nomura et al. and 81% in the sample by Leleu et al.), which could explain the lack of association between ILD and MP levels in our study.
Endothelial cell-derived MPs have also been studied in SSc. Recently, Lammi et al. [35] demonstrated a significant increase in EMPs in 20 patients with SSc compared to healthy controls. Furthermore, our results are similar to those of Guiducci et al. [13], who showed a significant increase in PMPs, EMPs and MMPs in 37 patients with SSc compared to healthy controls, with lower levels of MPs in patients with an mRSS > 10. Significantly elevated levels of EMPs were also demonstrated in 47 scleroderma patients, with lower levels of EMPs in patients with higher mRSS [16]. This inverse correlation between EMP levels and the degree of skin thickening, measured by the mRSS, the best validated outcome measure for skin fibrosis in SSc [13], indicates that higher amounts of circulating MPs are linked to a lower degree of fibrosis in the dermis in patients with SSc [13, 35]. Considering that upregulation of matrix metalloproteinase expression by MPs can enhance the synthesis of fibroblasts involved in extracellular matrix (ECM) degradation and that MPs contain proteolytic enzymes involved in the degradation of ECM [9, 36], the potential antifibrotic properties of MPs deserve further investigation.
Interestingly, we demonstrated that EMP levels were lower in SSc patients with an avascular score higher than 1.5 in the NFC exam, indicating an association of lower EMPs with more severe peripheral vasculopathy. This is in accordance with the findings of Michalska-Jakubus et al. [16], where significantly lower levels of EMPs were correlated with late NVC patterns and higher NVC scores for capillary loss. NFC is a well-established method to evaluate morphological abnormalities in the microcirculation [37], allowing the observation of specific SSc capillaroscopic changes secondary to its microangiopathy. In patients with SSc, hemorrhages, enlarged capillaries, capillary loss and distortion of the capillary architecture are present in early stages of the disease. The late nailfold capillaroscopic pattern and more severe capillary loss are associated with severe internal organ involvement and mortality in SSc [37,38,39]. Thus, EMPs might be a useful biomarker of vascular damage in SSc. Further studies are necessary to evaluate the role of EMPs as a predictive marker of more severe vasculopathy in SSc. Nonetheless, we did not observe associations between MPs and vascular manifestations, such as DU and PAH. A lack of association between DU and MPs has already been shown [13, 16], but previous studies have shown higher levels of EMPs in SSc patients with PAH [35, 40], strengthening the potential role of EMPs as endothelial injury biomarkers. The different results observed in our report might have been due to the low prevalence of patients with PAH in our sample.
We also evaluated the level of MMPs in our patients. Remarkably, the greatest difference between patients and controls was found in this source of MPs. Monocytes play a pivotal role in SSc pathogenesis, as they present proinflammatory and profibrotic phenotypes and contribute to fibrogenesis owing to their high expression of TGF-β [20]. In addition, monocytes in SSc display low caveolin-1 function, collaborating with ILD, due to the increased monocyte maturation toward myofibroblasts and hyperaccumulation of fibrocytes in these patients, regardless of monocyte blood levels [14, 41].
The limitations of our study include its relatively small sample size and the use of flow cytometry. Although flow cytometry is the chosen method for evaluating MPs, the antibodies used across studies have varied, hindering direct comparisons. Since we did not use beads in the MPs acquisition, we were not able to express their quantification in absolute values. Furthermore, in vitro studies evaluating the behavior of MPs in SSc patients could confirm the hypothesis of the antifibrotic potential of these agents and explore their potential as new therapeutic targets, which are urgently needed. We were also not able to quantify ILD extension on chest HRCT, to better evaluate this abnormality in our patients.
Conclusion
In summary, we have demonstrated that the plasma levels of platelet-derived, endothelial cell-derived, and monocyte-derived microparticles are increased in patients with SSc compared to healthy subjects. The demonstrated association of MPs with clinical SSc features, such as the presence of anti-topoisomerase I, skin thickness and severe capillary loss on nailfold capillaroscopy, indicates that these biologic agents may somehow contribute to the pathogenic mechanisms of this defiant disease and deserves further investigation.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACA:
-
Anticentromere antibody
- ARD:
-
Autoimmune rheumatic disease
- DU:
-
Digital ulcers
- ECM:
-
Extracellular matrix
- EMPs:
-
Endothelial cell-derived microparticles
- ILD:
-
Interstitial lung disease
- MMPs:
-
Monocytes-derived microparticles
- MPs:
-
Microparticles
- mRSS:
-
Modified Rodnan skin score
- NFC:
-
Nailfold capillaroscopy
- NVC:
-
Nailfold videocapillaroscopy
- PAH:
-
Pulmonary arterial hypertension
- PMPs:
-
Platelets-derived microparticles
- SRC:
-
Scleroderma renal crisis
- SSc:
-
Systemic sclerosis
References
Allanore Y, Simms R, Distler O, et al. Systemic sclerosis. Nat Rev Dis Primers. 2015;1:15002. https://doi.org/10.1038/nrdp.2015.2.
Saketkoo LA, Frech T, Varjú C, et al. A comprehensive framework for navigating patient care in systemic sclerosis: a global response to the need for improving the practice of diagnostic and preventive strategies in SSc. Best Pract Res Clin Rheumatol. 2021;35(3):101707. https://doi.org/10.1016/j.berh.2021.101707.
Cutolo M, Soldano S, Smith V. Pathophysiology of systemic sclerosis: current understanding and new insights. Expert Rev Clin Immunol. 2019;15(7):753–64. https://doi.org/10.1080/1744666X.2019.1614915.
Ntelis K, Solomou EE, Sakkas L, Stamatis-Nick L, Daoussis D. The role of platelets in autoimmunity, vasculopathy, and fibrosis: Implications for systemic sclerosis. Semin Arthritis Rheum. 2017;47(3):409–17. https://doi.org/10.1016/j.semarthrit.2017.05.004.
Pauling JD, O’Donnell VB, Mchugh NJ. The contribution of platelets to the pathogenesis of Raynaud’s phenomenon and systemic sclerosis. Platelets. 2013;24(7):503–15. https://doi.org/10.3109/09537104.2012.719090.
Pisetsky DS, Ullal AJ, Gauley J, Ning TC. Microparticles as mediators and biomarkers of rheumatic disease. Rheumatology (Oxford). 2012;51(10):1737–46. https://doi.org/10.1093/rheumatology/kes028.
Ratajczak MZ, Ratajczak J. Extracellular microvesicles/exosomes: discovery, disbelief, acceptance, and the future? Leukemia. 2020;34(12):3126–35. https://doi.org/10.1038/s41375-020-01041-z.
Morel O, Morel N, Freyssinet JM, Toti F. Platelet microparticles and vascular cells interactions: a checkpoint between the haemostatic and thrombotic responses. Platelets. 2008;19(1):9–23. https://doi.org/10.1080/09537100701817232.
Čolić J, Matucci-Cerinic M, Guiducci S, Damjanov N. Microparticles in systemic sclerosis, targets or tools to control fibrosis: this is the question! J Scleroderma Relat Disord. 2020;5(1):6–20. https://doi.org/10.1177/2397198319857356.
Roos MA, Gennero L, Denysenko T, et al. Microparticles in physiological and in pathological conditions. Cell Biochem Funct. 2010;28(7):539–48. https://doi.org/10.1002/cbf.1695.
Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6(1):21–9. https://doi.org/10.1038/nrrheum.2009.229.
McCarthy EM, Moreno-Martinez D, Wilkinson FL, et al. Microparticle subpopulations are potential markers of disease progression and vascular dysfunction across a spectrum of connective tissue disease. BBA Clin. 2016;7:16–22. https://doi.org/10.1016/j.bbacli.2016.11.003.
Guiducci S, Distler JH, Jüngel A, et al. The relationship between plasma microparticles and disease manifestations in patients with systemic sclerosis. Arthritis Rheum. 2008;58(9):2845–53. https://doi.org/10.1002/art.23735.
Nomura S, Inami N, Ozaki Y, Kagawa H, Fukuhara S. Significance of microparticles in progressive systemic sclerosis with interstitial pneumonia. Platelets. 2008;19(3):192–8. https://doi.org/10.1080/09537100701882038.
Maugeri N, Franchini S, Campana L, et al. Circulating platelets as a source of the damage-associated molecular pattern HMGB1 in patients with systemic sclerosis. Autoimmunity. 2012;45(8):584–7. https://doi.org/10.3109/08916934.2012.719946.
Michalska-Jakubus M, Kowal-Bielecka O, Smith V, Cutolo M, Krasowska D. Plasma endothelial microparticles reflect the extent of capillaroscopic alterations and correlate with the severity of skin involvement in systemic sclerosis. Microvasc Res. 2017;110:24–31. https://doi.org/10.1016/j.mvr.2016.11.006.
Iversen LV, Østergaard O, Ullman S, et al. Circulating microparticles and plasma levels of soluble E- and P-selectins in patients with systemic sclerosis. Scand J Rheumatol. 2013;42(6):473–82. https://doi.org/10.3109/03009742.2013.796403.
Iversen LV, Ullman S, Østergaard O, et al. Cross-sectional study of soluble selectins, fractions of circulating microparticles and their relationship to lung and skin involvement in systemic sclerosis. BMC Musculoskelet Disord. 2015;16:191. https://doi.org/10.1186/s12891-015-0653-8.
Scanu A, Molnarfi N, Brandt KJ, Gruaz L, Dayer JM, Burger D. Stimulated T cells generate microparticles, which mimic cellular contact activation of human monocytes: differential regulation of pro- and anti-inflammatory cytokine production by high-density lipoproteins. J Leukoc Biol. 2008;83(4):921–7. https://doi.org/10.1189/jlb.0807551.
Kania G, Rudnik M, Distler O. Involvement of the myeloid cell compartment in fibrogenesis and systemic sclerosis. Nat Rev Rheumatol. 2019;15(5):288–302. https://doi.org/10.1038/s41584-019-0212-z.
van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747–55. https://doi.org/10.1136/annrheumdis-2013-204424.
Humbert M, Kovacs G, Hoeper MM, et al. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2022;2022:2200879. https://doi.org/10.1183/13993003.00879-2022.
Denton CP, Lapadula G, Mouthon L, Müller-Ladner U. Renal complications and scleroderma renal crisis. Rheumatology (Oxford). 2009;48(Suppl 3):iii32–5. https://doi.org/10.1093/rheumatology/ken483.
Clements P, Lachenbruch P, Siebold J, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22(7):1281–5.
Sekiyama JY, Camargo CZ, Eduardo L, Andrade C, Kayser C. Reliability of widefield nailfold capillaroscopy and video capillaroscopy in the assessment of patients with Raynaud’s phenomenon. Arthritis Care Res (Hoboken). 2013;65(11):1853–61. https://doi.org/10.1002/acr.22054.
Cutolo M, Sulli A, Pizzorni C, Accardo S. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol. 2000;27(1):155–60.
Fonseca F, Ballerini AP, Izar MC, et al. Advanced chronic kidney disease is associated with higher serum concentration of monocyte microparticles. Life Sci. 2020;260:118295. https://doi.org/10.1016/j.lfs.2020.118295.
Dunne JV, Bankole J, Keen KJ. Systematic review of the role of microparticles in systemic sclerosis. Curr Rheumatol Rev. 2013;9(4):279–300. https://doi.org/10.2174/1573397109666140103001139.
Denton CP, Black CM, Abraham DJ. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2(3):134–44. https://doi.org/10.1038/ncprheum0115.
Guilpain P, Noël D, Avouac J. Editorial: key players in systemic sclerosis: the immune system and beyond. Front Immunol. 2021;12:770419. https://doi.org/10.3389/fimmu.2021.770419.
Kayser C, Fritzler MJ. Autoantibodies in systemic sclerosis: unanswered questions. Front Immunol. 2015;6:167. https://doi.org/10.3389/fimmu.2015.00167.
Ho KT, Reveille JD. The clinical relevance of autoantibodies in scleroderma. Arthritis Res Ther. 2003;5(2):80–93. https://doi.org/10.1186/ar628.
Boonstra M, Bakker JA, Grummels A, et al. Association of anti-topoisomerase I antibodies of the IgM isotype with disease progression in anti-topoisomerase i-positive systemic sclerosis. Arthritis Rheumatol. 2020;72(11):1897–904. https://doi.org/10.1002/art.41403.
Leleu D, Levionnois E, Laurent P, Lazaro E, Richez C, Duffau P, et al. Elevated circulatory levels of microparticles are associated to lung fibrosis and vasculopathy during systemic sclerosis. Front Immunol. 2020;11:532177.
Lammi MR, Saketkoo LA, Okpechi SC, et al. Microparticles in systemic sclerosis: potential pro-inflammatory mediators and pulmonary hypertension biomarkers. Respirology. 2019;24(7):675–83. https://doi.org/10.1111/resp.13500.
Distler JH, Jüngel A, Huber LC, et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci USA. 2005;102(8):2892–7. https://doi.org/10.1073/pnas.0409781102.
Lambova SN, Müller-Ladner U. Nailfold capillaroscopy in systemic sclerosis – state of the art: the evolving knowledge about capillaroscopic abnormalities in systemic sclerosis. J Scleroderma Relat Disord. 2019;4(3):200–11. https://doi.org/10.1177/2397198319833486.
Markusse IM, Meijs J, de Boer B, et al. Predicting cardiopulmonary involvement in patients with systemic sclerosis: complementary value of nailfold videocapillaroscopy patterns and disease-specific autoantibodies. Rheumatology (Oxford). 2017;56(7):1081–8. https://doi.org/10.1093/rheumatology/kew402.
Kayser C, Sekiyama JY, Próspero LC, Camargo CZ, Andrade LE. Nailfold capillaroscopy abnormalities as predictors of mortality in patients with systemic sclerosis. Clin Exp Rheumatol. 2013;31(2 Suppl 76):103–8.
Tura-Ceide O, Blanco I, Garcia-Lucio J, et al. Circulating cell biomarkers in pulmonary arterial hypertension: relationship with clinical heterogeneity and therapeutic response. Cells. 2021;10(7):1688. https://doi.org/10.3390/cells10071688.
Tourkina E, Bonner M, Oates J, et al. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1 scaffolding domain peptide. Fibrogenesis Tissue Repair. 2011;4(1):15. https://doi.org/10.1186/1755-1536-4-15.
Acknowledgements
The authors would like to thank all participants of the study.
Funding
This work was supported by the São Paulo Research Foundation (FAPESP Grant Number 2018/15216-2) and the Brazilian Rheumatology Society (FAPE-SBR), Brazil.
Author information
Authors and Affiliations
Contributions
SMO, CNF, MCOI, and CK contributed to the conception and design of the study. SMO, ILAT, and CNF performed the laboratory tests and contributed to acquisition, analysis and interpretation of the data. SMO and CK contributed to the analysis of the data and were the major contributor in writing the manuscript. All authors read critically and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and all participants agreed and signed the informed consent form. The study was approved by the Local Ethics Committee (#2.630.953).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Oliveira, S.M., de Azevedo Teixeira, I.L., França, C.N. et al. Microparticles: potential new contributors to the pathogenesis of systemic sclerosis?. Adv Rheumatol 63, 19 (2023). https://doi.org/10.1186/s42358-023-00299-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-023-00299-y