Abstract
Background
Several studies have compared the clinical features and outcomes of late- and early-onset systemic lupus erythematosus (SLE) patients. However, these previous studies were uncontrolled. The current study aimed to compare late- and early-onset SLE patients while controlling for sex and year at diagnosis (± 1 year).
Methods
The medical records of SLE patients in a lupus cohort from January 1994 to June 2020 were reviewed. Late-onset patients were identified as those with an age at diagnosis ≥ 50 years. The early-onset patients (age at diagnosis < 50 years) were matched by sex and year at diagnosis with the late-onset patients at a ratio of 2:1. Clinical manifestations, disease activity (mSLEDAI-2K), organ damage scores, treatment, and mortality were compared between the two groups.
Results
The study comprised 62 and 124 late- and early-onset patients, respectively, with a mean follow-up duration of 5 years. At disease onset, when comparing the early-onset patients with the late-onset patients, the latter group had a higher prevalence rate of serositis (37.0% vs. 14.5%, p < 0.001) and hemolytic anemia (50.0% vs. 33.9%, p = 0.034) but lower prevalence rate of malar rash (14.5% vs. 37.1%, p = 0.001), arthritis (41.9% vs. 62.1%, p = 0.009), leukopenia (32.3% vs. 50.0%, p = 0.022) and lymphopenia (50.0% vs. 66.1%, p = 0.034). The groups had similar SLE disease activity (7.41 vs. 7.50), but the late-onset group had higher organ damage scores (0.37 vs. 0.02, p < 0.001). The rates of treatment with corticosteroids, antimalarial drugs, or immunosuppressive drugs were not different. At their last visit, the late-onset patients still had the same pattern of clinically significant differences except for arthritis; additionally, the late-onset group had a lower rate of nephritis (53.2% vs. 74.2%, p = 0.008). They also had a lower level of disease activity (0.41 vs. 0.57, p = 0.006) and received fewer antimalarials (67.7% vs. 85.5%, p = 0.023) and immunosuppressive drugs (61.3% vs. 78.2%, p = 0.044), but they had higher organ damage scores (1.37 vs. 0.47, p < 0.001) and higher mortality rates/100-person year (3.2 vs. 1.1, p = 0.015). After adjusting for disease duration and baseline clinical variables, the late-onset patients only had lower rate of nephritis (p = 0.002), but still received fewer immunosuppressive drugs (p = 0.005) and had a higher mortality rate (p = 0.037).
Conclusions
In this sex- and year at diagnosis-matched controlled study, after adjusting for disease duration and baseline clinical variables, the late-onset SLE patients had less renal involvement and received less aggressive treatment, but had a higher mortality rate than the early-onset patients.
Similar content being viewed by others
Background
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disorder characterized by a wide range of clinical and laboratory manifestations. The clinical course is highly variable, characterized by remission and relapse. The disease onset usually occurs in adult females of reproductive age; however, it can occur in children and elderly individuals [1].
Several factors, including age, sex, ethnicity and socioeconomic status, have been shown to affect clinical manifestations and disease outcomes [2,3,4,5,6,7]. Studies have shown that late-onset SLE patients (usually defined as ≥ 50 years of age at diagnosis) tend to have more insidious onset and a milder clinical course but poorer outcomes and a higher mortality rate than early-onset SLE patients (age < 50 years at diagnosis) [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. Unfortunately, the majority of these previous studies compared the late- and early-onset SLE patients directly without matching based on sex or the year at diagnosis [9, 10, 12, 14,15,16,17,18,19,20,21, 23, 24]. Controlling for the year at diagnosis is also important in long-term clinical research, as it reflects the medical care provided during that period, which clearly affects the treatment outcomes.
This study aimed to compare clinical features, serologic abnormalities, disease activity and treatment outcomes between the late- and early-onset patients with SLE in Thailand after controlling for sex and year at diagnosis.
Methods
In this case‒controlled study, the medical records of SLE patients in the lupus cohort of the Division of Rheumatology, Faculty of Medicine, Chiang Mai University who were seen between January 1994 and June 2020 were reviewed. SLE was diagnosed according to the 1982 or the 1997 American College of Rheumatology (ACR) revised criteria for the classification of SLE [25, 26]. Patients in the cohort were usually followed up at 1- to 3-month intervals depending on disease activity or any other clinical features. Clinical manifestations and laboratory investigation, including complete blood counts, urine analysis (including 24-hour urine protein creatinine ratio [24 h UPCI] in cases with lupus nephritis), and renal and liver function were determined at every visit.
In this study, SLE patients were classified into late- and early-onset if SLE was diagnosed when the patients were ≥ 50 and < 50 years of age, respectively. Only those who were diagnosed with SLE at Chiang Mai University Hospital, those who were aged ≥ 18 years at diagnosis, and those who had been followed-up for ≥ 1 year were included in this study. The records of patients who had died within 1 year after entering the cohort were also included. Patients with drug-induced lupus, lupus-like syndrome associated with malignancies, and SLE patients with other connective tissue diseases or overlap syndrome or with missing clinical data of ≥ 25% were excluded. Late-onset patients were identified. The early-onset patients were sex- and year at diagnosis-matched with the late-onset patients (± 1 year) at a ratio of 2:1.
Demographic information was collected, including sex, age at diagnosis, year at diagnosis, underlying disease, all clinical manifestations, serology and laboratory findings, time to diagnosis (duration from first clinical manifestation to diagnosis), disease duration (duration from SLE diagnosis to June 2020 or last visit or death), treatment and outcomes. The other relevant clinical manifestations of SLE were also captured. SLE disease activity and organ damage were determined by the modified SLE Disease Activity Index 2000 (mSLEDAI-2K) [27] and the Systemic Lupus International Collaborating Clinics/ACR (SLICC/ACR) damage index (SDI) [28], respectively. The mortality and cause of death were also recorded. All clinical manifestations, laboratory findings, SLE disease activity and organ damage were obtained from reviews of available longitudinal data in medical records.
This study was performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. It was approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University, Thailand (No. 215/2020).
Statistical analysis
STATA 16.0 for Windows computer software (Stata Corporation, Texas, USA) was used for data processing and statistical analysis. Log or square root data transformation was used to improve the normality of the data distribution for statistical analysis. Continuous variables are presented as the mean and 95% confidence intervals (95% CI), and categorical variables are presented as percentages. Student’s t test or the Mann‒Whitney U test was used to compare continuous variables, and the chi-square test or Fisher’s exact test was used to compare categorical variables. To compare the outcome variables with controlled covariate factors, analysis of covariance (ANCOVA) and logistic regression were used for continuous and binary outcomes, respectively. Firth’s logistic regression was used for the binary outcomes of rare events. Cox proportional hazards regression was used to determine the survival difference between the two groups after adjusting for confounding effects of other variables. The sample size was calculated based on Appenzeller et al.’s study [8] with a type I error of 0.05, power of 0.8 for comparison, correlation of 0.7, and standard deviation of 0.5. The allocation ratio was 2:1. A sample size of at least 55 cases in the late-onset group and 110 cases in the early-onset group was required for the study. A p value of < 0.05 was considered statistically significant.
Results
The cohort comprised a total of 1,476 SLE patients (1,366 female and 110 male, ratio 12.4:1). Of these, 401 cases were excluded because the year at diagnosis could not be verified. Of the remaining 1,075 patients, 125 (11.63%) and 950 (88.37%) were late- and non-late-onset patients, respectively. Sixty-three patients with late-onset and 654 with early-onset were excluded because they were not diagnosed at Chiang Mai University Hospital or because they had missing data of ≥ 25%, follow-up of < 1-year, age at onset of < 18 years or overlapping syndrome. Thus, only 62 patients with late-onset and 296 with early-onset remained for matching (Supplementary Fig. 1). As 6 male early-onset patients could not be identified for matching the same year at diagnosis with the late-onset cases, 6 female early-onset patients with the same year at diagnosis were used instead of male patients.
Demographic characteristics
The demographic characteristics of the patients at the time of SLE diagnosis are shown in Table 1. The study comprised 62 late-onset (54 female, 87.1%) and 124 early-onset patients (114 female, 91.9%) with mean (95% CI) ages at diagnosis of 56.3 (55.1–57.5) and 30.9 (29.3–32.6) years, respectively. The late-onset patients had higher rates of underlying diseases, including hypertension (p < 0.001), diabetes mellitus (p = 0.012), dyslipidemia (p = 0.012), chronic kidney disease (p = 0.036) and cerebrovascular disease (p = 0.012). They also had shorter duration of follow-up (p = 0.019). Three of the early-onset patients had malignancies (one each of malignant thymoma, carcinoma of the cervix, and carcinoma of the breast).
Clinical and laboratory manifestations
The initial organ presentation between the late- and early-onset patients showed similar frequencies (Table 2). The articular, mucocutaneous and hematologic systems were the 3 most common organs involved. The duration from the first clinical presentation to diagnosis was also slightly shorter in the late-onset patients (2.4 months vs. 3.7 months, p = 0.169).
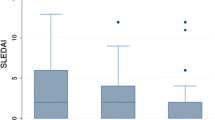
The hematologic, renal, articular, and mucocutaneous systems were the common organ involvements observed at SLE diagnosis in the late- and early-onset patients both at diagnosis and at the last visit (Table 3). At diagnosis, the late-onset patients had a higher frequency of serositis (p < 0.001) [particularly pleuritis (p = 0.005)] and hemolytic anemia (p = 0.034) but a lower frequency of malar rashes (p = 0.001), arthritis (p = 0.009), leukopenia (p = 0.022) and lymphopenia (p = 0.034) than the early-onset patients. The late-onset patients also tended to have a higher frequency of seizures but a lower frequency of oral ulcers. The mean (95% CI) number of ACR classification criteria and SLE disease activity (mSLEDAI-2K) were not different between the two groups. However, the late-onset patients had higher mean SDI scores (p < 0.001).
Comparisons between the late- and early-onset patients in clinical and laboratory manifestations at the last visit were adjusted by disease duration (Table 3). The late-onset patients still had a higher frequency of serositis (p = 0.011) [particularly pleuritis (p = 0.010)] but a lower frequency of malar rash (p = 0.001), oral ulcers (p = 0.024), renal involvement (p = 0.008), leukopenia (p = 0.025) and lymphopenia (p = 0.041) than the early-onset patients. They also tended to have a higher frequency of seizures but a lower frequency of arthritis. The late-onset patients had lower mean (95% CI) ACR classification criteria (p = 0.005) and mSLEDAI-2K scores (p = 0.006) but higher mean (95% CI) SDI scores (p < 0.001).
As the cumulative frequency of clinical and laboratory manifestations at the last visit was influenced by their frequency at diagnosis, comparisons of these variables between the two groups were also adjusted with the baseline clinical and laboratory values, in addition to disease duration, to set both groups with the same variable frequency at diagnosis (Table 3). It was interesting that many clinical and laboratory manifestations, showing significant differences between these two groups, lost their significance and left only renal involvement, anti-double stranded DNA antibody (anti-dsDNA) and mean number of the ACR classification criteria, which remained lower in the late-onset patients (p = 0.002, p = 0.049, and p = 0.008, respectively).
Fever, alopecia, fatigue, weight loss, arthralgia, Raynaud’s phenomenon, cutaneous vasculitis and lymphadenopathy were among the clinical manifestations of the non-ACR classification criteria commonly observed (≥ 10%) in both groups, both at diagnosis and at the last visit (Supplementary Table 1). At diagnosis, the late-onset patients had a higher frequency of weight loss (p = 0.001), arthralgia (p = 0.011) and protein-losing enteropathy (p = 0.036). At the last visit, when adjusted for disease duration, the late-onset patients had a higher frequency of weight loss (p = 0.009) and arthralgia (p = 0.016). In addition, they also had a higher frequency of cardiomyopathy (p = 0.046), lupus endocarditis (p = 0.049) and interstitial lung disease (p = 0.020) but a lower frequency of cutaneous vasculitis (p = 0.026) than the early-onset patients. Similarly, when adjusted by the baseline value of the clinical variables, in addition to disease duration, only cutaneous vasculitis remained lower in the late-onset patients (p = 0.025).
Treatment and outcomes
At diagnosis, although there was no difference between the late- and early-onset patients in the treatment received with corticosteroids, intravenous pulse methylprednisolone (IVMP), antimalarials, or immunosuppressive drugs (Table 4), the late-onset SLE patients received a lower rate of antimalarials (53.2% vs. 67.7%, p = 0.053) but had a slightly higher rate of immunosuppressive drugs (35.5% vs. 25.8%, p = 0.170). Among the immunosuppressive drugs, only mycophenolate mofetil (MMF) was more commonly used in late-onset patients (p = 0.028). One late-onset patient required hemodialysis due to acute renal failure from lupus nephritis.
At the last visit, the use of corticosteroids was not different between the two groups. However, the late-onset patients received fewer antimalarial (p = 0.023) and immunosuppressive drugs (p = 0.044) than the early-onset patients. Specifically, the late-onset patients received less azathioprine (p = 0.001), cyclophosphamide (p = 0.028), calcineurin inhibitors (p = 0.039) and MMF (p = 0.026). Three patients in the early-onset group and one in the late-onset group required hemodialysis due to acute renal failure from lupus nephritis during their follow-up period. When the medication used at diagnosis was adjusted in addition to disease duration, the frequency of antimalarial used between the two groups was no longer significantly different, but the differences in the use of immunosuppressive drugs between the two groups remained (Table 4).
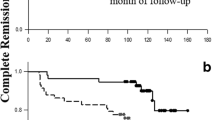
During the follow-up period, 11 (17.7%) late-onset and 10 (8.1%) early-onset patients died (p = 0.049). The incidence rate (IR)/100-person year of death was higher in the late-onset patients than in the early-onset patients (3.2 vs. 1.1, p = 0.015), even after adjusting for the presence of comorbidities at diagnosis (9.1 vs. 3.2, p = 0.037). Two patients with early-onset SLE died at home without clearly identifiable causes. Infection was the major cause of death in both the late- and early-onset SLE patients (Table 5).
Discussion
The prevalence of late-onset patients in this study (10.63%) was comparable to the prevalence rates observed in many previous reports (2–17%) [8,9,10, 12,13,14,15,16,17,18,19,20,21, 23, 29, 30]. In addition, a very high prevalence (39%) was reported by Alonse et al. from Spain [31]. The lower female-to-male ratio of 5.9:1 in the late-onset patients, compared to the ratio of 13.8:1 in the early-onset patients in this study, was comparable to many previous reports (2.6–9.7:1 and 5.7–18.1:1, respectively) [9,10,11,12,13, 15, 16, 19, 21, 23, 24, 29,30,31]. Interestingly, reports from China by Choi et al. [14] and Egypt by Mehat et al. [20] found a higher female to male ratio in the late-onset group when compared with early-onset patients. The lower female-to-male ratio in the late-onset patients might be due partly to a lack of estrogenic hormones during the menopausal period, as estrogen can trigger the development of SLE in susceptible individuals [32].
Despite many studies reporting on the difference in clinical manifestations between the late- and early-onset SLE patients, information on the initial clinical presentation between the two groups was limited. This study found that mucocutaneous, articular and hematologic involvements were the common presenting symptoms, which was similar to a report from China by Boddaert et al., who found that polyarthritis, serositis and malar rashes were among the common manifestations in their late-onset patients [19]. However, it was difficult to conclude that mucocutaneous and articular manifestations were more common initial presentations in the late-onset patients than in the early-onset patients, as they were common clinical manifestations in both age groups.
The duration from first clinical symptoms to SLE diagnosis generally took longer in the late-onset patients than in the early-onset patients [10, 12, 17, 21, 33]. The diagnostic delay in late-onset SLE patients might be related partly to SLE being uncommon at ages ≥ 50 years. The clinical presentation (e.g., hematologic or cardiopulmonary involvement) might not be seen typically in early-onset patients, thus causing the physician to search for other more common diseases, particularly malignancies, that occur in elderly individuals. In this study, no difference between the two groups was found in the duration from first clinical symptoms to diagnosis, which was similar to a report by Alonso et al. [31].
The differences in clinical and laboratory manifestations, disease activity, treatment and outcomes between the late- and early-onset SLE patients have been determined by several groups. Unfortunately, a majority of these studies were not controlled but instead compared the clinical manifestations directly between late- and early-onset patients and mainly compared the cumulative clinical manifestations, which showed differences in the results. Of those studies in which information on clinical manifestations at diagnosis was available, the late-onset patients had less mucocutaneous [24, 29, 30, 33], vasculitis [30], renal [12, 29, 30], and central nervous system involvement [30], less hypocomplementemia [9, 24, 29, 30], less positive anti-dsDNA [24, 29], and lower mean number of ACR classification criteria [9] and SLEDAI-2K scores [29] than the early-onset patients.
Regarding cumulative manifestations at the last visit, the late-onset SLE patients had lower rates of mucocutaneous [10, 12, 17, 19, 21, 23, 33, 34], renal [10, 12, 16, 17, 19, 23, 33, 34], arthritic [17, 19], neurological [12, 17, 34] and hematologic involvement, and particularly less leukopenia and thrombocytopenia [10, 17, 34]. They also had less hypocomplementemia [9, 12, 16, 17, 19, 31], less positive anti-dsDNA [17, 31], less positive anti-Smith antibody (anti-Sm) [12, 17, 31], and a lower mean number of ACR classification criteria [9, 12, 19, 21] and SLEDAI-2K scores [9, 17]. In contrast, the late-onset patients had more Sicca or Sjogren’s symptoms [10, 16, 31] and cardiopulmonary involvement [10, 21] than the early-onset patients.
Among the few controlled studies (Supplementary Table 2), Bertoli et al. [13] used data from the LUMINA (Lupus in minorities: nature versus nurture) cohort and matched the early-onset patients by sex and disease duration (randomly selected). They found that the late-onset patients had more comorbidities (hypertension, hypothyroidism, osteoporosis and arterial thrombosis) and higher cardiopulmonary, neurological and renal involvement than the early-onset patients. Disease activity at the onset and during the course of the disease was lower in the late-onset patients. Another controlled study in Brazil by Applezeller et al. [8] matched sex, ethnicity, disease duration and SDI scores at diagnosis and found that the late-onset patients had a lower prevalence of malar rash and articular involvement but a higher prevalence of hematologic involvement and anti-dsDNA antibodies. The late-onset group had significantly more comorbidities (hypertension). The level of SLE disease activity was also lower in late-onset patients. A controlled study from Canada by Aljohani et al. [11] matched early-onset patients by sex and date of first clinic visit within 5 years and found that the late-onset patients had less renal and neurological involvement, less abnormal immunologic abnormalities, and lower mean number of ACR classification criteria and SLEDAI-2K scores but higher comorbidities, both at diagnosis and during the last visit, than the early-onset patients.
The results from this study were also similar to those of previous controlled studies in that the late-onset SLE patients were found to have lower levels of mucocutaneous, articular and hematologic involvement (leukopenia and thrombocytopenia) than early-onset patients, except for hemolytic anemia, which was more common at diagnosis. Mucocutaneous, renal and hematologic involvement (leukopenia and lymphopenia) were also less frequent at the last visit (Supplementary Table 2). However, after adjusting for disease duration and the baseline frequency of each clinical and laboratory manifestation, only renal involvement was more prevalence in the early- rather than the late-onset patients at the last visit. This finding indicates that renal involvement occurred more frequently in the early- rather than late-onset patients during the follow-up period. This could be reflected by the higher mean number of ACR classification criteria in the early-onset patients.
The treatment received and outcomes of treatment among previous reports also showed different results. Several studies, including this one, found that the late-onset patients received less cumulative treatment with antimalarials and immunosuppressive drugs than the early-onset patients, which might be explained partly by less severe disease (particularly less renal, central nervous system and hematologic involvement) among the late-onset patients [8, 10,11,12, 16, 17, 19, 23, 33]. Such treatment, which was no different in the study by Mak et al. [21] and Sohn et al. [9], might be explained by the organ involvement being similar in both groups, which allowed these authors to conclude that the late-onset SLE might not be as mild as initially believed. However, this could not explain why the use of corticosteroids, antimalarials and immunosuppressive drugs was not different in the study by Bertoli et al. [13], where patients with early-onset disease had more cardiopulmonary and neurological involvement but less renal involvement. Another possibility might be treating physicians trying to avoid using corticosteroids and immunosuppressive drugs in late-onset or elderly patients to avoid potential complications from these medications. Also, the lower rate of antimalarials used in the late-onset patients might be because the treating physicians were afraid of the possible previously degenerative retinal alteration and higher risk of antimalarial retinopathy in this group of patients. Unfortunately, there were no data to support this.
The results from the aforementioned studies, including those in controlled studies and this study, indicated that although there were some differences in the clinical manifestations at diagnosis and during the follow-up visits between the late- and early-onset SLE patients, the former group seemed to have milder disease when determined by less internal organ involvement, lower mean number of ACR classification criteria and less SLE disease activity, as well as less treatment received, particularly with the use of corticosteroids and immunosuppressive drugs.
The higher level of damage (SDI scores) observed in the late-onset group than in the early-onset SLE patients in this study was also similar to that previously reported [8, 11, 13, 17] but was inconsistent to the results reported by Lanani et al. [12] and Sohn et al. [9]. The higher level of damage observed in the late-onset patients could not be explained solely by the treatment received, as these patients usually received fewer corticosteroids, antimalarials and immunosuppressive drugs, which are medications that can cause damage according to the SDI items. However, this difference could be related to underlying comorbidities that could contribute to damage. This was confirmed by the current study, as although the SDI was significantly higher in the late-onset group, the difference disappeared after adjusting for the baseline comorbidity condition.
The higher mortality rate observed in the late-onset patients in this study was not unexpected and was consistent with many previous reports [9, 10, 13, 16, 19, 23, 31]. Both SLE-related and non-SLE-related causes (particularly infection) were among the common causes of death in SLE patients in both groups. This study found that infection was the major cause of death in both the late- and early-onset patients. Cancer, cardiovascular diseases, and infections were among the major causes of death in the late-onset patients in other studies [8, 13, 16, 19, 31]. A study by Budhoo et al. [34] found no difference in mortality rate among the late- and early-onset patients; however, the median duration of their 4-year study might not be long enough to identify the difference in mortality between the two groups. It is interesting that although the study by Sohn et al. [9] reported a higher mortality rate in the late-onset patients, it was not different from that in the general population. Furthermore, SLE-related death was the major cause of death in the late-onset SLE patients. Interestingly, a meta-analysis found that the mortality rate in SLE patients had decreased globally during the past 50 years [35]. This improvement was more likely a result of more advanced diagnosis, better medical care, more aggressive treatment to control the disease, use of less toxic therapies, and early awareness of SLE among physicians and patients. Unfortunately, despite improvement in the mortality rate, renal involvement, cardiovascular disease and infections were among the major factors that contributed to death [36].
This study had some limitations. A large number of patients were excluded, particularly early-onset patients; therefore, the remaining patients may not represent the entire group in the determination of outcome measures. Those exclusions were made to ensure that the late- and early-onset patients were matched properly. However, the remaining number of patients in this study was sufficient for statistical power, and it was comparable to or even larger than some previous controlled studies [8, 11, 13]. The available data were collected from the cohort; thus, some clinical parameters and laboratory findings might be missing or not recorded. However, medical records with ≥ 25% missing data were excluded, which could strengthen the data in this study. Immunological laboratory investigations, including anti-Sm, anti-ribonucleoprotein, anti-cardiolipin, anti-Ro antigen, anti-La antigen, and anti-β2 glycoprotein-1 antibodies, were not routinely available at this institution, and they were costly. This could lead to some missing diagnosis when these antibodies were required to support it. However, due to the low prevalence of these antibodies, when compared to anti-nuclear antibodies (ANA) and anti-dsDNA, missing or errors in the diagnosis would be minimal. Sex was not completely matched between the late- and early-onset patients, but the non-matched cases accounted for less than 5% of the sample, which should not make a significant difference in statistical analysis. Additionally, this study used the mSLEDAI-2K instead of the original SLEDAI-2K instrument in the assessment of SLE disease activity. However, the mSLEDAI-2K has been shown to have a very strong correlation with the original SLEDAI-2K [27]. Therefore, the use of mSLEDAI-2K in the assessment of SLE disease activity between the late- and early-onset patients would not lead to significant differences between the two groups; however, the results of the current study could not be compared with studies using the original SLEDAI-2K instrument. In addition, the duration of follow-up was significantly different between the late- and early-onset patients, but the outcome in this study was adjusted for disease duration, which should improve the results of the statistical analysis. Last, this study included only Thai patients. Therefore, we cannot extrapolate the results to other ethnic groups.
Despite several limitations, this study had some strengths. It included cases that were diagnosed with SLE only at this institution. This ensured that the diagnosis was correct, and the data were collected properly from the beginning of the disease. The early-onset patients were matched by sex and year at diagnosis with the late-onset patients to ensure that both groups received similar medical treatment by treating physicians. As the period of the study progressed, with better understanding of management and care of the SLE patients, as well as advanced medical support, better outcomes resulted for the patients [35]. Thus, properly matched sex and year at diagnosis in this study avoided selection and treatment bias that could directly affect the outcomes, which could be the main strength of this study.
Conclusions
This sex- and year at diagnosis-matched controlled study found that late-onset SLE patients had some clinical differences from early-onset patients. The late-onset patients tended to have less SLE disease activity and a lower level of disease severity based on organ involvement and treatment received. The significant differences in many clinical parameters observed at diagnosis and during follow-up diminished after correction for the frequency of organ involvement at the baseline visit, except for renal involvement; this result indicated that only renal involvement occurred more frequently in the early-onset patients during the follow-up period. The higher damage score observed in the late-onset patients was due mainly to the higher prevalence of comorbidities at disease onset. The mortality rate in the late-onset patients was higher than that in the early-onset patients. Infection was the major cause of death in both groups.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 24 hr UPCI:
-
24-hour urine protein creatinine ratio
- ACR:
-
American College of Rheumatology
- ANA:
-
Anti-nuclear antibody
- anti-dsDNA:
-
Anti-double stranded DNA antibody
- anti-Sm:
-
Anti-Smith antibody
- IVMP:
-
Intravenous pulse methylprednisolone
- MMF:
-
Mycophenolate mofetil
- mSLEDAI-2K:
-
Modified Systemic Lupus Erythematosus Disease Activity Index 2000
- SDI:
-
Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index
- SLE:
-
Systemic lupus erythematosus
- SLEDAI-2K:
-
Systemic Lupus Erythematosus Disease Activity Index 2000
- SLICC:
-
Systemic Lupus International Collaborating Clinic
References
Dall’Era M, Wofsy D. Clinical features of systemic lupus erythematosus. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O’Dell JR, editors. Kelley & Furestein’s Textbook of Rheuamtology. Volume 2. Philadelphia: Elsevier; 2017. pp. 1345–67.
Jakes RW, Bae SC, Louthrenoo W, Mok CC, Navarra SV, Kwon N. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res (Hoboken). 2012;64(2):159–68. https://doi.org/10.1002/acr.20683.
Bruce IN, O’Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the systemic Lupus International collaborating clinics (SLICC) inception cohort. Ann Rheum Dis. 2015;74(9):1706–13. https://doi.org/10.1136/annrheumdis-2013-205171.
Uribe AG, Alarcon GS. Ethnic disparities in patients with systemic lupus erythematosus. Curr Rheumatol Rep. 2003;5(5):364–9. https://doi.org/10.1007/s11926-003-0022-8.
Boodhoo KD, Liu S, Zuo X. Impact of sex disparities on the clinical manifestations in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Med (Baltim). 2016;95(29):e4272. https://doi.org/10.1097/MD.0000000000004272.
Mongkoltanatus J, Wangkaew S, Kasitanon N, Louthrenoo W. Clinical features of Thai male lupus: an age-matched controlled study. Rheumatol Int. 2008;28(4):339–44. https://doi.org/10.1007/s00296-007-0442-2.
Tan TC, Fang H, Magder LS, Petri MA. Differences between male and female systemic lupus erythematosus in a multiethnic population. J Rheumatol. 2012;39(4):759–69. https://doi.org/10.3899/jrheum.111061.
Appenzeller S, Pereira DA, Costallat LT. Greater accrual damage in late-onset systemic lupus erythematosus: a long-term follow-up study. Lupus. 2008;17(11):1023–8. https://doi.org/10.1177/0961203308089695.
Sohn IW, Joo YB, Won S, Bae SC. Late-onset systemic lupus erythematosus: is it “mild lupus”? Lupus. 2018;27(2):235–42. https://doi.org/10.1177/0961203317716789.
Catoggio LJ, Soriano ER, Imamura PM, Wojdyla D, Jacobelli S, Massardo L et al. Late-onset systemic lupus erythematosus in Latin Americans: a distinct subgroup? Lupus. 2015;24(8):788–795. https://doi.org/10.1177/0961203314563134
Aljohani R, Gladman DD, Su J, Urowitz MB. Disease evolution in late-onset and early-onset systemic lupus erythematosus. Lupus. 2017;26(11):1190–6. https://doi.org/10.1177/0961203317696593.
Lalani S, Pope J, de Leon F, Peschken C, Members of Ca NFoL. Clinical features and prognosis of late-onset systemic lupus erythematosus: results from the 1000 faces of lupus study. J Rheumatol. 2010;37(1):38–44. https://doi.org/10.3899/jrheum.080957.
Bertoli AM, Alarcon GS, Calvo-Alen J, Fernandez M, Vila LM, Reveille JD, et al. Systemic lupus erythematosus in a multiethnic US cohort. XXXIII. Clinical [corrected] features, course, and outcome in patients with late-onset disease. Arthritis Rheum. 2006;54(5):1580–7. https://doi.org/10.1002/art.21765.
Choi JH, Park DJ, Kang JH, Yim YR, Lee KE, Lee JW, et al. Comparison of clinical and serological differences among juvenile-, adult-, and late-onset systemic lupus erythematosus in korean patients. Lupus. 2015;24(12):1342–9. https://doi.org/10.1177/0961203315591024.
Feng JB, Ni JD, Yao X, Pan HF, Li XP, Xu JH, et al. Gender and age influence on clinical and laboratory features in chinese patients with systemic lupus erythematosus: 1,790 cases. Rheumatol Int. 2010;30(8):1017–23. https://doi.org/10.1007/s00296-009-1087-0.
Cartella S, Cavazzana I, Ceribelli A, Inverardi F, Tincani A, Franceschini F. Evaluation of mortality, disease activity, treatment, clinical and immunological features of adult and late onset systemic lupus erythematosus. Autoimmunity. 2013;46(6):363–8. https://doi.org/10.3109/08916934.2013.794793.
Riveros Frutos A, Holgado S, Sanvisens Berge A, Casas I, Olive A, Lopez-Longo FJ, et al. Late-onset versus early-onset systemic lupus: characteristics and outcome in a national multicentre register (RELESSER). Rheumatology (Oxford). 2021;60(4):1793–803. https://doi.org/10.1093/rheumatology/keaa477.
Sassi RH, Hendler JV, Piccoli GF, Gasparin AA, da Silva Chakr RM, Brenol JC, et al. Age of onset influences on clinical and laboratory profile of patients with systemic lupus erythematosus. Clin Rheumatol. 2017;36(1):89–95. https://doi.org/10.1007/s10067-016-3478-4.
Boddaert J, Huong DLT, Amoura Z, Wechsler B, Godeau P, Piette JC. Late-onset systemic lupus erythematosus: a personal series of 47 patients and pooled analysis of 714 cases in the literature. Med (Baltim). 2004;83(6):348–59. https://doi.org/10.1097/01.md.0000147737.57861.7c.
Medhat BM, Behiry ME, Sobhy N, Farag Y, Marzouk H, Mostafa N, et al. Late-onset systemic lupus erythematosus: characteristics and outcome in comparison to juvenile- and adult-onset patients-a multicenter retrospective cohort. Clin Rheumatol. 2020;39(2):435–42. https://doi.org/10.1007/s10067-019-04776-y.
Mak SK, Lam EK, Wong AK. Clinical profile of patients with late-onset SLE: not a benign subgroup. Lupus. 1998;7(1):23–8. https://doi.org/10.1191/096120398678919723.
Maddison P, Farewell V, Isenberg D, Aranow C, Bae SC, Barr S, et al. The rate and pattern of organ damage in late onset systemic lupus erythematosus. J Rheumatol. 2002;29(5):913–7.
Merola JF, Bermas B, Lu B, Karlson EW, Massarotti E, Schur PH, et al. Clinical manifestations and survival among adults with (SLE) according to age at diagnosis. Lupus. 2014;23(8):778–84. https://doi.org/10.1177/0961203314526291.
Prevete I, Iuliano A, Cauli A, Piga M, Iannone F, Coladonato L, et al. Similarities and differences between younger and older disease onset patients with newly diagnosed systemic lupus erythematosus. Clin Exp Rheumatol. 2022. https://doi.org/10.55563/clinexprheumatol/oo5ymg.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. https://doi.org/10.1002/art.1780400928.
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. https://doi.org/10.1002/art.1780251101.
Uribe AG, Vila LM, McGwin G Jr, Sanchez ML, Reveille JD, Alarcon GS. The systemic lupus activity Measure-revised, the mexican systemic Lupus Erythematosus Disease Activity Index (SLEDAI), and a modified SLEDAI-2K are adequate instruments to measure disease activity in systemic lupus erythematosus. J Rheumatol. 2004;31(10):1934–40.
Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the systemic Lupus International collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–9. https://doi.org/10.1002/art.1780390303.
Formiga F, Moga I, Pac M, Mitjavila F, Rivera A, Pujol R. Mild presentation of systemic lupus erythematosus in elderly patients assessed by SLEDAI. SLE Disease Activity Index Lupus. 1999;8(6):462–5. https://doi.org/10.1177/096120339900800609.
Jeleniewicz R, Suszek D, Majdan M. Clinical picture of late-onset systemic lupus erythematosus in a group of polish patients. Pol Arch Med Wewn. 2015;125(7–8):538–44. https://doi.org/10.20452/pamw.2963.
Alonso MD, Martinez-Vazquez F, de Teran TD, Miranda-Filloy JA, Dierssen T, Blanco R, et al. Late-onset systemic lupus erythematosus in Northwestern Spain: differences with early-onset systemic lupus erythematosus and literature review. Lupus. 2012;21(10):1135–48. https://doi.org/10.1177/0961203312450087.
McMurray RW, May W. Sex hormones and systemic lupus erythematosus: review and meta-analysis. Arthritis Rheum. 2003;48(8):2100–10. https://doi.org/10.1002/art.11105.
Ho CT, Mok CC, Lau CS, Wong RW. Late onset systemic lupus erythematosus in southern chinese. Ann Rheum Dis. 1998;57(7):437–40. https://doi.org/10.1136/ard.57.7.437.
Budhoo A, Mody GM, Dubula T, Patel N, Mody PG. Comparison of ethnicity, gender, age of onset and outcome in South Africans with systemic lupus erythematosus. Lupus. 2017;26(4):438–46. https://doi.org/10.1177/0961203316676380.
Mak A, Cheung MW, Chiew HJ, Liu Y, Ho RC. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum. 2012;41(6):830–9. https://doi.org/10.1016/j.semarthrit.2011.11.002.
Lee YH, Choi SJ, Ji JD, Song GG. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25(7):727–34. https://doi.org/10.1177/0961203315627202.
Acknowledgements
The authors thank Mrs. Waraporn Sukitawut for her secretarial assistance.
Funding
This study had no funding support.
Author information
Authors and Affiliations
Contributions
JM, AW, NK and WL were responsible for the conception and design of the study. JM and WL collected data. JM and AW analyzed the data. JM, AW, NK and WL interpreted the data. JW and WL prepared the manuscript. All authors provided a critical review of intellectual content and approved the final version to be submitted for publication. Dr. Louthrenoo had full access to all of the data in the study and takes responsibility for the integrity and accuracy of the data and analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University, Thailand (No. 215/2020) and was performed in accordance with the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. Written informed consent was waived due to medical records review.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mongkolchaiarunya, J., Wongthanee, A., Kasitanon, N. et al. Comparison of clinical features, disease activity, treatment and outcomes between late-onset and early-onset patients with systemic lupus erythematosus. A sex- and year at diagnosis-matched controlled study. Adv Rheumatol 63, 20 (2023). https://doi.org/10.1186/s42358-023-00297-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-023-00297-0