Abstract
Objective
To describe parametric changes observed using scanning electron microscopy (SEM) in very early stages in posttraumatic osteoarthritis (OA) models in mice.
Methods
Mice (5/group) had their knees subjected to anterior cruciate ligament transection (ACLT), ACLT plus meniscectomy (MNCT) or sham surgery, sacrificed after 3, 7 or 14 days, had the articular cartilage evaluated under optical microscopy using Osteoarthritis Research Society International (OARSI) parameters as well as cartilage thickness, roughness, and a damage index using SEM.
Results
Alterations of the cartilage under optical microscopy were not significantly relevant among groups. SEM analysis revealed reduction of femoral and tibial cartilage thickness in ACLT and MNCT groups at 7 and 14 days, with increased cartilage roughness in MNCT group as early as 3 days postsurgery, being sustained up to 14 days. Articular damage index was significantly higher at 14 days post surgery in ACLT and MNCT vs control groups.
Conclusion
This is the first demonstration of very early quantitative changes in the cartilage of mice subjected to posttraumatic experimental OA using SEM, revealing increased roughness and thickness as early as 3 days post surgery. These changes may be used as early surrogates for later joint damage in experimental OA.
Similar content being viewed by others
Background
Osteoarthritis (OA) is the most common chronic arthritis representing a major cause of years of life lost with disability. Although the hands seem to be the most commonly affected joint, the knee is most frequent clinically involved joint [1, 2]. OA has a complex pathogenesis that involves genetic, inflammatory, and weight-bearing mechanisms that were previously thought to affect mainly chondrocytes and the cartilage extracellular matrix [3]. However, current knowledge suggests that OA develops as a relentless inflammation in the joint involving cells in the synovium and the adjacent subchondral bone as well as the menisci and surrounding muscles and ligaments leading to joint failure [1, 4].
Detection of early signs of damage to the joint is a major unmet need to understand the pathophysiology of OA [5]. Criticism has been raised regarding the use of animal OA models given that not uncommonly data generated either using such models or in vitro studies are not reproduced in humans. However, exceptions do apply, particularly with posttraumatic OA models in rodents, which also allow determining the time of initiation of the insult. Indeed, histopathological changes that reproduce those seen in humans are used to quantitate joint damage in animal OA models as well. Notwithstanding, genetically modified animals are an interesting tool to dissect the implication of specific mediators involved [6, 7]. Ethic issues apply both to human and animal studies. Therefore, strategies to reduce the number of animals to a minimum as well as shortening of the period of observation are strongly recommended, as defined in the ARRIVE guidelines [8]. Establishing early biomarkers may thus help define surrogates for late joint damage. Our aim was to describe parametric changes observed using scanning electron microscopy (SEM) in very early stages in posttraumatic OA models in mice. Our data show that ultrastructural changes happen as early as 3 days following surgery, which do also allow quantitation of disease severity.
Material and methods
Animals
Seventy-two Swiss mice (25–30 g) provided by the Animal Care Unit of the Federal University of Ceará (UFC—Fortaleza, Brazil), were randomly housed, 6 per group, in appropriate plastic cages at 23 ± 2 °C with a 12-h light–dark cycle (light from 06:00 AM to 6:00 PM) with access to water and food ad libitum.
Ethical approval
The study was conducted in accordance with the guidelines from the Brazilian Society of Laboratory Animal Science (SBCAL) and the protocol was approved by the Institutional Animal Care and Use Committee at the Faculdade de Medicina of the Universidade Federal Ceará in the city of Sobral, Ceará, Brazil (registration number 113/07). All experimental procedures complied with recommendations in ARRIVE guidelines [8].
Posttraumatic OA models
Mice were anesthetized (i.m.) with ketamine (50 mg/kg) and xylazine (10 mg/kg), as described previously [9]. Briefly, after local. aseptic surgery, a parapatellar incision was made, followed by lateral displacement of the patella, to provide access to the joint space and transection of both the medial collateral and anterior cruciate ligaments (ACLT group). Another group of mice was subjected to ACLT followed by transection of the medial meniscus (MNCT group). We have previously shown that animals subjected to MNCT display a more severe joint damage as compared to animals subjected solely to ACLT [10]. A control group was subjected to opening of the joint followed by a sham procedure. Surgical wounds were sutured by planes using vycril™ (6-0) and nylon (4-0) threads. Animals had the right distal femoral and proximal tibial extremities excised 3, 7 or 14 days post surgery and had their entire knee joints dissected of soft tissue and fixated in 10% v/v formaldehyde solution followed by decalcification with 5% v/v formic acid (in 10% v/v formaldehyde solution). The lateral and medial compartments of each sample were separated, embedded in paraffin, and serially sectioned at 5 μm in the sagittal plane of the articular surface, from the inner to the outer limits. Five sections per compartment (50 μm apart) were stained with hematoxylin–eosin or safranin. Semi-quantitative histopathology grading under optical microscopy (Olympus CX31, Biolab Equipamentos Brasil, São Paulo, SP, Brazil) was performed by two independent experienced observers (VCCG, ACRML) blinded to group allocation according to the Osteoarthritis Research Society International (OARSI) histopathology grading and staging system [11]. The maximal possible final score was 24. Results are expressed as the median (interquartile range—IQR) values for each treatment group.
Scanning electron microscopy (SEM)
The knee extremities were fixed in Karnovisky for at least 6 h in Cacodylate buffer. Specimens were trimmed with a diamond blade cutter, in a sagital plane, to obtain cartilage fragments that were dried in a desiccator for 24 h, followed by coating with a 5 nm layer of gold in a Quorum Metallizer QT150ES (Quorum Technologies, Laughton, England). The specimens were viewed and photographed in a high-resolution SEM inspect50 (FEI, Hillsboro, Oregon, USA) SEM. Quantitation was performed with ImageJ® software (version 1.51j—at https://imagej.nih.gov/ij/), using Surface 3D plot software that enables interactive representation of the entire surface in gray scale, with the possibility of adjustment for various angles, perspective, lightening and smoothing views. We defined 3 parameters to assess joint damage, namely cartilage thickness, roughness and damage, as follows: (a) Cartilage thickness was measured at nine randomly selected points, collecting 3 measurements in 3 different zones of each sample, in a total of 9 measures for each sample, using 80× magnification; (b) Cartilage roughness was measured as Ra (roughness profile in μm) means obtained with an ImageJ plugin Roughness calculation with 3 different measures at randomly selected areas of each sample, using a 14,000× magnification. Briefly, Ra values are the arithmetic average of the roughness profile obtained with the roughness plugin software that calculates surface roughness statistics based on a topographical image [12]; (c) Cartilage damage was measured using previously described parameters, as follows: grade 0—normal cartilage; grade I—initial exposure of collagenous fibres with partial loss of ground substance; grade II—exposure of collagenous fibres with marked loss of ground substance with partially detached fibres and lamellae showing prominence in the joint space; grade III—grade II lesions with fissure formation of the articular cartilage; grade IV—islands with exposure of the bony substance with total loss of cartilage [13].
Statistical analysis
Data were described as mean ± SEM for each parameter and normality was assessed using the Shapiro–Wilk normality test. Differences between means were compared using unpaired Student’s “t” test or one-way ANOVA followed by Tukey's test, as appropriate. Medians were compared using Kruskal–Wallis. P < 0.05 was considered significant. Analyzes were performed using IBM SPSS Statistics for Windows, version 20.0, Software Armonk, NY or GraphPad Prism 6, San Diego, CA, USA.
Results
Histopathological analysis of synovia and articular cartilage
The analysis using optical microscopy reproduced previous data published by our group [10], showing a mild synovitis in mice subjected to either ACLT or MNCT as compared to sham group. As expected, there was no difference regarding cartilage and/or subchondral bone changes across all groups, meaning no consistent structural damage under histopathology detected at any of the time points evaluated (Table 1).
Cartilage thickness
There was a significant reduction in cartilage thickness in both the femur and tibia of mice of either ACLT or MNCT groups sacrificed after 7 or 14 days, as compared to sham (Fig. 1a, b). Reduction of cartilage thickness was sustained until 14 days andappeared more pronounced in the tibial plateau of animals subjected to the MNCT model sacrificed after 14 days (P < 0.05), as compared to animals of the ACLT group, at the same time (Fig. 1b).
Assessment of femoral (a) and tibial (b) cartilage thickness. Mice were subjected to a sham procedure or transection of the medial collateral and anterior cruciate ligaments (ACLT) or ACLT followed by transection of the medial meniscus (MNCT). Groups were sacrificed 3, 7 or 14 days post surgery. Data represent mean ± sem of cartilage thickness (µm) using scanning electron microscope (SEM) images (9/group), compared using Student’s “t” test
Femoral cartilage roughness
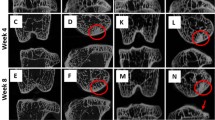
Figure 2 illustrates measurements of femoral cartilage roughness across various groups with a significant and remarkable increase in the MNCT group starting as early as 3 days post surgery, as compared to animals subjected to ACLT and sham groups, at the same time point (Fig. 2a). The increase in cartilage roughness is significantly sustained at 7 and 14 days post surgery in the MNCT group, as compared to both sham and ACLT groups. Representative illustrations of SEM imaging of the femoral cartilage are shown in Fig. 2b–d and h–j with their corresponding surface plot profiles for analysis of thickness shown in Fig. 2e–g, k–m.
Assessment of roughness of femoral cartilage. Mice were subjected to a sham procedure or transection of the medial collateral and anterior cruciate ligaments (ACLT) or ACLT followed by transection of the medial meniscus (MNCT). Groups were sacrificed 7 or 14 days post surgery. a Data represent mean ± sem of cartilage roughness (µm) using scanning electron microscope (SEM) images (9/group), compared using Student’s “t” test. Representative SEM imaging and roughness plots of the femoral cartilage of animals of the sham [b, e and h, k], ACLT [c, f and i, l], and MNCT [d, g and j, m] groups sacrificed at 7 or 14 days post surgery, respectively
Tibial cartilage roughness
Similar to what was shown in the femoral cartilage, Fig. 3a shows a significant increase in roughness ot the tibia cartilage as early as 3 days following MNCT. That increased roughness in the tibia cartilage is sustained in mice of the MNCT group sacrificed 7 or 14 days post surgery. Also, tibial roughness of the ACLT group sacrified at 14 days is also significantly increased, as compared to sham (Fig. 3a). Representative illustrations of SEM imaging of the tibial cartilage are shown in Fig. 3b–d and h–j and their corresponding surface plot profiles in Fig. 3e–g and k–m.
Assessment of roughness of tibial cartilage. Mice were subjected to a sham procedure or transection of the medial collateral and anterior cruciate ligaments (ACLT) or ACLT followed by transection of the medial meniscus (MNCT). Groups were sacrificed 7 or 14 days post surgery. a Data represent mean ± sem of cartilage roughness (µm) using scanning electron microscope (SEM) images (9/group), compared using Student’s “t” test. Representative SEM imaging and roughness plots of the tibial cartilage of animals of the sham [b, h and e, q], ACLT [c, i and f, l], and MNCT [d, j and g, m] groups sacrificed at 7 or 14 days post surgery, respectively
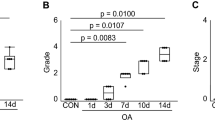
Cartilage damage
Evaluation of the cartilage damage index (see above) revealed no significant changes in both femoral and tibial extremities of all groups measured both at 3 and 7 days post surgery. Samples from the sham group showed a smooth cartilage surface, rendering a grade 0 for this group. Exposure of collagenous fibers with marked loss of ground substance, partly detached fibers and prominent lamellae into the joint space were observed in mice of both ACLT and MNCT groups sacrificed 14 days postsurgery, being significantly different from sham (Table 1).
Discussion
In the present study, we used SEM imaging to detect very early changes in the surface of the cartilage of the knee of mice subjected to posttraumatic experimental OA. Alterations were quantitative, reproducible, starting as early as 3 days and sustained until 14 days post surgery. In addition to be clearly different from control joints, quantitative evaluation of SEM data allowed discrimination between animals subjected to the less severe ACLT model from those subjected to the more severe MNCT model that includes damage to the medial meniscus (MNCT) [10].
Animal models remain a major tool to study the pathogenesis and evaluation of novel interventions to treat OA. Despite some drawbacks, alterations observed in OA models reproduce changes found in human OA, particularly following trauma [7]. In the present study, evaluation using optical histopathology showed a mild synovitis as early as 3 days following surgery that was similar in ACLT and MNCT models. We believe surgical trauma is very likely responsible for this early and mild synovitis. However, as expected, the histopathological analysis did not reveal relevant cartilage changes until 14 days post surgery. We have previously shown that mice subjected to ACLT have milder nociception and less joint damage as compared to animals subjected to the MNCT model [10]. It was also shown that mice subjected to the meniscus destabilization model of knee OA exhibit alterations of the expression of genes involved in matrix remodeling as early as 2 weeks following surgery [14]. Our data show that cartilage morphology evaluated using SEM imaging not only discriminate OA and control groups at a very early stage, but also allows evaluating disease severity. Thus, it might well be that SEM imaging data can be used as a surrogate for late damage at least in these OA models (Table 2).
Cartilage roughness, the earliest modification detected in our study, has already been used as a parameter of joint damage both in experimental and human OA [15, 16]. Accordingly, rats subjected to the ACLT model developed alterations in thickness, thinning and roughness of the cartilage overlying the femoral condyles and the tibial plateau, measured with contrast-enhanced micro-computed tomography (µCT). In that study, roughness variation was determined against a defined cartilage thickness whereas we compared roughness in a damaged cartilage to the image of a normal, smooth cartilage surface [16]. In humans, nuclear magnetic resonance (NMR) imaging showed a higher index of surface roughness of the femoral condyles and tibial plateau that was associated to increased severity grade assessed using plain radiography. Additionally, cartilage roughness distinguished patients with mild and severe disease [15]. We also constructed 2D models in order to obtain quantitative and reproducible data for validation as well as to facilitate visualization. We are not aware of previous studies using SEM to quantitate very early cartilage damage in OA models. Cartilage and subchondral bone changes were shown using equilibrium partitioning of an ionic contrast agent µCT occurring 2 weeks after induction of an OA model in rodents [17]. Increase in cartilage roughness was shown in rats subjected to transection of the medial meniscus using µCT as early as 1 week following surgery that was sustained and increased at 3 weeks. Those changes were associated with cartilage fibrillation seen at histopathology [5]. It was also previously shown that rats subjected to a meniscus destabilization OA model display alterations of the cartilage of the medial tibia coupled to disruption and decreased density of collagen fibers. That was linked to areas of subchondral bone perforation seen at various methods including SEM evaluated 4 weeks following surgery [18].
Histopathology is a traditional tool to study OA, providing consistent, reproducible, semiquantitative data [19]. However, techniques with higher resolution may provide reproducible, fast, consistently reliable data that also allow quantitative analysis in earlier time points. Electron microscopy offers the possibility of evaluating components of the extracellular matrix as well as intracellular structures such as alterations of the collagen network demonstrated in damaged (OA) human cartilage [20]. Structural damage follows biochemical changes happening in cells of the cartilage as well as in the extracellular matrix. Indeed, we have recently shown that glycosaminoglycans extracted from OA cartilage have increase in the molar mass with a decrease in sulfate content and the correspondent zeta potential, as compared to non-OA samples [21]. Those changes mirror data with SEM imaging showing morphological changes of proteoglycans and alterations of collagen fibers [20].
There was a decrease in cartilage thickness as early as 7 days following induction of the OA model, which was sustained up to one week later. Actually, cartilage thickness in the tibial plateau was further reduced at 14 days in animals subjected to the MNCT, as compared to those subjected to the milder ACLT model. Accordingly, early degenerative changes associated to alterations of components of the extracellular cartilage matrix were shown using NMR imaging in both rabbit and equine OA models [22, 23]. Also, chondrocyte death was demonstrated as early as one week following initiation of the ACLT model in rats [12].
Definition of early biomarkers is an unmet need in human OA. Significant increased cartilage roughness occurred as early as 3 days in both the femoral and tibial extremities indicating that changes occur randomly and simultaneously in the whole joint. We believe our data add to the concept that OA is the result of a joint failure [24], in this case following a trauma insult.
Our study has some limitations, which include the fact that electron microscopy includes a destructive protocol that precludes further analysis of the same material. Additionally, artifacts may be generated due to preparation requirements including fixation, coating, and dehydrating steps [20]. Although well standardized, the dehydration process may provoke artifacts due to shrinking of the sample, producing undulations, fissures, and humps [25]. Measurements made in randomly selected areas, using the arithmetic means, intended to overcome focal sampling issues. High resolution images do not allow an overview of the damage to the whole joint, which is also a limitation. However, electron microscopy may document alterations in cells of the cartilage and subchondral bone as well as in extracellular matrix components, such as proteoglycans and collagen [20]. In this regard, high resolution imaging serves as a complementary tool, rather than a substitute for other methods aiming to obtain structural data [26].
Conclusion
Our present data show that ultrastructural changes occur in the cartilage as early as 3 days following induction of experimental OA. High resolution SEM imaging provides quantitative, reproducible data to measure changes in experimental OA being able to discriminate severity of joint damage. Analysis of the cartilage with SEM may well turn out to be a surrogate for later cartilage damage in posttraumatic OA.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- ACLT:
-
Anterior cruciate ligament transection
- MNCT:
-
Meniscal transection
- NMR:
-
Nuclear magnetic resonance
- OA:
-
Osteoarthritis
- OARSI:
-
Osteoarthritis Research Society International
- SEM:
-
Scanning electron microscopy
References
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–59. https://doi.org/10.1016/S0140-6736(19)30417-9.
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2017;392:1789–58. https://doi.org/10.1016/S0140-6736(18)32279-7.
van den Bosch MHJ. Osteoarthritis year in review 2020: biology. Osteoarthr Cartil. 2021;29:143–50. https://doi.org/10.1016/j.joca.2020.10.006.
Berenbaum F, Walker C. Osteoarthritis and inflammation: a serious disease with overlapping phenotypic patterns. Postgrad Med. 2020;132:377–84. https://doi.org/10.1080/00325481.2020.1730669.
Reece DS, Thote T, Lin ASP, et al. Contrast enhanced μCT imaging of early articular changes in a pre-clinical model of osteoarthritis. Osteoarthr Cartil. 2018;26:118–27. https://doi.org/10.1016/j.joca.2017.10.017.
Thysen S, Luyten FP, Lories RJ. Targets, models and challenges in osteoarthritis research. Dis Model Mech. 2015;8:17–30. https://doi.org/10.1242/dmm.016881.
Lim NH, Wen C, Vincent TL. Molecular and structural imaging in surgically induced murine osteoarthritis. Osteoarthr Cartil. 2020;28:874–84. https://doi.org/10.1016/j.joca.2020.03.016.
Nunes RM, Girão VCC, Castro RR, et al. Increase in molar mass distinguishes chondroitin sulfate from osteoarthritis and normal extracellular cartilage matrix. Connect Tissue Res. 2020;7:1–8. https://doi.org/10.1080/03008207.2020.1825700.
de Melo Leite AC, Teotonio MA, Girão VC, et al. Meniscal transection rather than excision increases pain behavior and structural damage in experimental osteoarthritis in mice. Osteoarthr Cartil. 2014;22:1878–85. https://doi.org/10.1016/j.joca.2014.07.019.
de Melo NR, Cunha PLR, Pinto ACMD, et al. Hylan G-F20 and galactomannan joint flares are associated to acute synovitis and release of inflammatory cytokines. Adv Rheumatol. 2020;60:26. https://doi.org/10.1186/s42358-020-00127-7.
Ghosh S, Bowen J, Jiang K, et al. Investigation of techniques for the measurement of articular cartilage surface roughness. Micron. 2013;44:179–84. https://doi.org/10.1016/j.micron.2012.06.007.
Korkala O, Karaharju E, Grönblad M, et al. 1984 Articular cartilage after meniscectomy. Rabbit knees studied with the scanning electron microscope. Acta Orthop Scand. 1984;55:273–7. https://doi.org/10.3109/17453678408992355.
Loeser RF, Olex AL, McNulty MA, et al. Disease progression and phasic changes in gene expression in a mouse model of osteoarthritis. PLoS ONE. 2013;8(1): e54633. https://doi.org/10.1016/j.joca.2014.07.019.
Newton MD, Osborne J, Gawronski K, et al. Articular cartilage surface roughness as an imaging-based morphological indicator of osteoarthritis: a preliminary investigation of osteoarthritis initiative subjects. J Orthop Res. 2017;35:2755–64. https://doi.org/10.1371/journal.pone.0054633.
Maerz T, Newton MD, Matthew HW, et al. Surface roughness and thickness analysis of contrast-enhanced articular cartilage using mesh parameterization. Osteoarthr Cartil. 2016;24:290–8. https://doi.org/10.1016/j.joca.2015.09.006.
Siebelt M, van der Windt AE, Groen HC, et al. FK506 protects against articular cartilage collagenous extra-cellular matrix degradation. Osteoarthr Cartil. 2014;22:591–600. https://doi.org/10.1016/j.joca.2014.02.003.
Iijima H, Aoyama T, Ito A, et al. Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthr Cartil. 2014;22(7):1036–43. https://doi.org/10.1016/j.joca.2014.05.009.
Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil. 2006;14:13–29. https://doi.org/10.1016/j.joca.2005.07.014.
He B, Wu JP, Kirk TB, et al. High-resolution measurements of the multilayer ultra-structure of articular cartilage and their translational potential. Arthritis Res Ther. 2014;16:205. https://doi.org/10.1186/ar4506.
Nunes RM, Girão VCC, Cunha PLR, et al. Decreased sulfate content and zeta potential distinguish glycosaminoglycans of the extracellular matrix of osteoarthritis cartilage. Front Med 2021;8:612-370. https://doi.org/10.3389/fmed.2021.612370
Kajabi AW, Casula V, Sarin JK, et al. Evaluation of articular cartilage with quantitative MRI in an equine model of post-traumatic osteoarthritis. J Orthop Res. 2021;39:63–73. https://doi.org/10.1002/jor.24780.
Kajabi AW, Casula V, Ojanen S, et al. Multiparametric MR imaging reveals early cartilage degeneration at 2 and 8 weeks after ACL transection in a rabbit model. J Orthop Res. 2020;38:1974–86. https://doi.org/10.1002/jor.24644.
Nuki G. Osteoarthritis: a problem of joint failure. Z Rheumatol. 1999;58(3):142–7. https://doi.org/10.1007/s003930050164.
Morrison EH, Bayliss MT, Ferguson MW, et al. Novel articular cartilage structure in the South American opossum, Monodelphis domestica. J Anat. 1993;182(3):321–8.
Changoor A, Nelea M, Méthot S, et al. Structural characteristics of the collagen network in human normal, degraded and repair articular cartilages observed in polarized light and scanning electron microscopies. Osteoarthr Cartil. 2011;19:1458–68. https://doi.org/10.1016/j.joca.2011.09.007.
Acknowledgements
This work was partially supported by FUNCAP (Grant # PJP-0072-00092.01.00/12) Brazilian agency and partial support for Rocha FAC by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ Grants 313860/2021-1 and 403767/2021-0). Authors are also grateful to Central Analítica—UFC/CT-INFRA/MCTI-SISNANO/Pró-Equipamentos for support with SEM.
Funding
No specific funding was obtained for this study. This work was partially supported by FUNCAP (Grant # PJP-0072-00092.01.00/12) Brazilian agency and partial support for Rocha FAC by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ Grants 313860/2021-1 and 403767/2021-0). Authors are also grateful to Central Analítica—UFC/CT-INFRA/MCTI-SISNANO/Pró-Equipamentos for support with SEM.
Author information
Authors and Affiliations
Contributions
VCCGC, ACMDP, ACRML—conception of the protocol; MMB, RMN, ACMDP, ACRML—arthritis models; FMAXP, AMCVA, CCC, GACB, VCCGC—collection of imaging data; VCCGC, MMB, FAXP, ACMD, RML, GACB, CCC, ACRML, FACR—data analysis; VCCGC, ACRML, CCC, GACB, FACR—wrote and revised the manuscript. All authors read and apporoved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the guidelines from the Brazilian Society of Laboratory Animal Science (SBCAL) and the protocol was approved by the Institutional Animal Care and Use Committee at the Faculdade de Medicina of the Universidade Federal Ceará in the city of Sobral, Ceará, Brazil (registration number 113/07).
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Girão-Carmona, V.C.C., Bezerra, M.M., Porto, F.M.A.X. et al. Quantitative evaluation of very early cartilage damage in experimental osteoarthritis using scanning electron microscopy. Adv Rheumatol 62, 42 (2022). https://doi.org/10.1186/s42358-022-00277-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-022-00277-w