Abstract
Sjogren's syndrome (SS) is an autoimmune disease characterized by lymphocytic infiltration of the exocrine glands and other organs. Women with SS often experience gynecological symptoms due to the disease and need extra care regarding their sexual activity, reproductive health and during pregnancy, conditions that are not properly conducted in the clinical practice. To cover this gap, a panel of experts from the Brazilian Society of Rheumatology conducted a systematic review and meta-analysis on the identification of symptoms, diagnosis, monitoring, prognosis, and treatment of these manifestations. A Focus Group meeting was held and included experts in the field and methodologists, based on a previously developed script, with themes related to the objective of the study. The most important topics were summarized and 11 recommendations were provided.
Similar content being viewed by others
Background
Sjogren's syndrome (SS) is an autoimmune disease characterized by lymphocytic infiltration of the exocrine glands and other organs [1]. The disease may occur in isolation, when it is called primary Sjogren's syndrome (pSS), or in conjunction with rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), or another rheumatic disease, when it is called secondary Sjogren's syndrome [2]. pSS is a common disease that affects 0.04–0.08% of people worldwide and has a female to male ratio of 9–14 to 1 [3]. As the process leads to progressively reduced or absent glandular secretion along with mucosal dryness, SS is characterized by symptoms ranging from xerophthalmia, xerostomia, fatigue, myalgia, and arthralgia to severe systemic symptoms with cutaneous, vascular, renal, pulmonary, or neurological involvement [2]. Besides these well-known symptoms, women with SS often experience vaginal dryness and dyspareunia, which result in a substantial disease burden as well as reduced quality of life [4]. Despite this, little attention has been given to gynecological and obstetric variables in women with SS [5]. Knowledge of the main gynecological and obstetric characteristics is required for early diagnosis, careful monitoring, and multidisciplinary programs.
To address these gaps, the Sjögren's syndrome Committee of the Brazilian Society of Rheumatology conducted a broad systematic review of the literature on population-based studies investigating gynecological symptoms and obstetric morbidities in Sjogren’s patients. The Brazilian Society of Rheumatology gathered the experts in the field and developed recommendations for the screening and management of women with these manifestations. Therefore, the current study represents an effort by this committee with the objective of retrieving the best available evidence and providing guidance for the identification of symptoms, diagnosis, monitoring, prognosis, and treatment of gynecological and obstetric manifestations in women with SS.
Methods
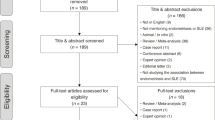
A systematic literature review was conducted of papers on the identification of symptoms, diagnosis, monitoring, prognosis, and treatment of gynecological and obstetric manifestations in women with Sjogren’s syndrome. We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, LILACS, and Trip Database for studies published up to January 7th, 2021 (Fig. 1). A search strategy was designed for MEDLINE (Additional file 1: Appendix 1) and adapted for the main electronic databases. The search was conducted without language, date, or any other type of restriction. The methodological quality of studies reporting prevalence data was evaluated using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist. We pooled clinical data by extracting the number of events and total patients to perform proportion meta-analysis. For studies that presented continuous data as medians and inter-quartile ranges, or as medians and ranges, we estimated means and standard deviations following the method described by Wan et al. [6]. To present pooled results with their respective 95% confidence intervals (CI), we used the “meta” and “metafor” packages from R software (version 3.6.1). We used a generalized linear mixed model (GLMM) method with a random-effects model for pooling the results. To calculate an overall proportion, we used logit transformation. For continuous data, we pooled results of means with their respective 95% CI by the inverse variance method with a random-effects model. A panel composed by specialists in SS and members of the Sjögren's syndrome Committee of Brazilian Society of Rheumatology elaborated question and recommendations. Agreement between the recommendations was achieved in a Focus Group meeting occurred on March 10th, 2020.
Important points—gynecological care
-
Women with SS experience more genital discomfort related to dryness of the mucosa, which can be the cause of dyspareunia and decreased satisfaction with sexual activity. There is a prevailing association between these complaints and indices of poor quality of life.
-
The coincidence between age of onset and onset of climacteric symptoms in Sjogren's Syndrome can worsen the framework.
-
These women are also more predisposed to reproductive disorders that harm fertility, although reduced gonadal reserve and premature ovarian failure are unusual.
-
The impact on fertility can be evidenced by oocyte quality and quantity rates, reduced serum levels of anti-mullerian hormone, and increased Luteinizing Hormone (LH) levels. Long menstrual cycles (> 35 days) may be associated with infertility.
Gynecological recommendations
-
1.
Active inquiry regarding genital and sexual complaints is recommended, since they are not spontaneously reported to the rheumatologist. (Hundred percent agreement).
-
2.
Patients should be referred for follow-up with the gynecologist. (Hundred percent agreement).
Gynecological involvement
Vaginal dryness, dyspareunia, genital infections
Women with SS experience vaginal discomfort related to mucosal dryness, which can be the cause of higher rates of dyspareunia and decreased satisfaction with sexual activity in this population [7,8,9,10]. The complaints become more relevant in the climacteric period, adding vaginal atrophy related to hypoestrogenism to the preexisting dryness [11]. The most common manifestations were vaginal dryness (prevalence of 64%) and dyspareunia (62%) (Figs. 2, 3; Table 1 on Additional file 1).
Despite the changes occurring in the genital mucosa as a result of local dryness and the increased use of lubricant, there seems to be no high risk of vaginal infections in these cases [12]. Previous data show that premenopausal SS women are not more exposed to vaginal infections than healthy women of the same age, although according to some authors they exhibit more inflammation and vaginal atrophy [12, 13]. Some studies indicate that the pH and composition of the vaginal microbiota are similar between reproductive-age SS patients and healthy controls and the most prevalent genera in this flora (Lactobacillus, Gardnerella, and Streptococcus) are equally found in both cases [14]. In contrast, the changes in vaginal microbiota that occur postmenopause can be explained by hypoestrogenism and worsened by sicca due to SS. Lactobacilli use the breakdown products of glycogen to produce lactic acid, which contributes to low vaginal pH and thereby inhibits the growth of other bacteria. This phenomenon occurs through the influence of estrogen (premenopausal) and makes the health of this epithelium less dependent on dryness [15, 16]. Contradictorily, the oral dryness leads to proliferation of Lactobacillus in the oral mucosa, which contributes to increased dental caries and Candida infection rates [17]. Despite this, additional genital infections can be expected in SS patients considering the set of dryness, hypoestrogenism, therapeutic regimens, and degree of immunosuppression to which they are submitted.
Sexual dysfunction
Sexual dysfunction refers to a multifactorial etiology of symptoms that compromise the quality of general and sexual lives of several individuals, including rheumatic patients, proportionally related to the chronicity of the disease [18]. The evaluation of sexual dysfunction is heterogeneous in different societies, arising from cultural and religious influences. The female sexual function index (FSFI), in Rosen et al. [19], is a female inventory of symptoms that takes into account six main domains: desire, arousal, lubrication, orgasm, satisfaction, and pain. The lower the score achieved, the worse the quality of sexual life reported by the individuals [19]. Pooled results from studies evaluating sexual dysfunction in women with Sjogren’s syndrome are displayed in Fig. 4.
Studies on sexual dysfunction conducted in patients with SS and controlled by healthy women in the same age group using this specific instrument demonstrate significant impairment in the sexual life of SS patients. The results show lower means of total score, a lower frequency of sexual intercourse, and a worse index in the domains of lubrication and dyspareunia [5, 20,21,22].
There is a clear relationship of greater sexual dysfunction with age, with the degree of vaginal dryness, and with the physical impact of the disease (intensity of pain and fatigue measured by ESSPRI) in SS patients. The use of vaginal lubricants, apparently, improves sexual satisfaction in these cases [21].
A considerable proportion of patients do not raise complaints of a gynecological or sexual nature in rheumatological appointments, since they do not associate them with the underlying disease or because of embarrassment [21, 23].
Fertility
Sjogren's syndrome preferably affects women of near-menopausal age and sometimes women of reproductive age. As in other autoimmune diseases (systemic lupus erythematosus, Hashimoto's thyroiditis, etc.), reproductive dysfunctions like reduced gonadal reserve and early ovarian failure may occur [7, 8]. Two studies assessing reproductive dysfunction in women with Sjogren’s syndrome were pooled and the results are shown in Additional file 1: Appendix 2 (Fig. 5).
Nevertheless, Lehrer et al. [7], applied questionnaires on gynecological symptoms to 539 patients and the resulting data did not show statistical differences between Sjogren’s syndrome females and the healthy American population with respect to fertility rate (10% vs. 10–15%), miscarriage (17% vs. 12–16%), or premature ovarian failure [7]. Skopouli et al., in a Greek cohort study from the same year, supported these findings of no impact on fertility, parity, or age at onset of menopause [8].
Currently, the reduction in fertility can be measured by several parameters. The decrease in serum anti-mullerian hormone, rise in LH serum levels, and reduced amount in quantity and quality of oocytes are some of the methods employed. However, there is no consensus that patients with SS might submit the complete investigation routinely [24]. Prolactin levels may be higher in primary SS patients but it is unclear if there is any association to clinical, hormonal or immunological outcomes [25,26,27]. Long menstrual cycles lasting more than 35 days seem to have some relationship with infertility in SS [7].
Endometriosis
Endometriosis is an estrogen-dependent inflammatory disease caused by the implantation of endometrial tissue outside the uterus. It affects 2 to 10% of women of childbearing age and can cause pelvic pain, dysmenorrhea, and infertility [28, 29]. The gold standard of diagnosis is laparoscopy, only indicated in women with relevant symptoms. The phenomenon may occur through a combination of retrograde menstruation and disturbances in immune surveillance. A number of immunological changes have been described in endometriosis since the 80s, leading to the proposal of possible associations with cases of fibromyalgia, thyroiditis, multiple sclerosis, and arthritis. Apparently, SLE and SS are diseases with a higher risk ratio [30].
It remains poorly defined, however, if the immunological alterations described, such as the circulating immunoglobulin surge, detection of anti-endometrial antibodies, and changes in the chemokine profile, could be inducing endometriosis or are simply a consequence of its presence.
It is not possible to stipulate the frequency of the association of endometriosis and SS, due both to different protocols used for diagnosing the first and to the lack of data in SS cohorts.
The majority of registry studies with analysis of a large number of patients with endometriosis in Spain, Denmark, and Israel [31,32,33] failed to demonstrate an unequivocal association.
Contraception and hormonal replacement therapy
The use of contraceptive methods sometimes includes potential interactions among hormonal contraceptives and other medications, increase in infection risk with intrauterine devices and thrombosis [34] Menopausal hormone therapy (HT) has not been studied in SS patients. Decisions about which contraceptive methods and about the risks and benefits of HT use should be individualized and take into consideration patient´s medical status, tobacco use, family history of hormonal-dependent cancers, laboratory features such as antiphospholipid antibody, and stage of reproductive live [34].
Important points—obstetric care
-
Neonatal lupus syndrome (NLS) results from the trans placental passage of maternal anti-SSA/ Ro antibodies around the eleventh week of pregnancy. The most severe manifestation is congenital heart block (CHB) which may require cardiac pacemaker implantation.
-
In addition to cardiac conduction system injuries in the newborn, fetal hydrops, liver dysfunctions, cytopenias, and transient skin lesions may also occur.
Obstetric recommendations
-
3.
All pregnant women with SS should be assisted by a multidisciplinary team in a high-risk prenatal care center, regardless of the controversy in literature data on gestational outcomes such as spontaneous abortion rates, fetal growth restriction, or prematurity. (Hundred percent agreement).
-
4.
It is recommended that the disease be well controlled in the 6 months before pregnancy and that the profile of antiphospholipid and anti-SSA/Ro antibodies be updated. (Hundred percent agreement).
-
5.
Adjustment of pregnancy compatible drugs and specific vitamin supplements for pregnant women are recommended. The use of hydroxychloroquine should be encouraged in positive anti-SSA/Ro pregnant women and it is mandatory in pregnant women with a previous history of fetal heart block or other forms of neonatal lupus. (Hundred percent agreement).
Obstetric recommendations in the presence of Neonatal Lupus Syndrome risk
-
6.
Prenatal care and delivery should be accomplished in a referral Hospital. (Hundred percent agreement).
-
7.
Hydroxychloroquine (5 mg/kg/d) should be prescribed for all positive anti-SSA/Ro pregnant women because of its impact on reducing the recurrence of NLS (which can reach 20%) in subsequent pregnancies, compared to patients who do not use the drug. (Hundred percent agreement).
-
8.
In these pregnant women, the effectiveness of treatment with corticosteroids, human immunoglobulin, β-sympathomimetics, or plasmapheresis is controversial. Dexamethasone may be useful in reversing carditis and incomplete blocks in addition to improving the hemodynamic conditions of the fetus. It is recommended that the decision for treatment be shared and adjusted considering each case. (Hundred percent agreement).
-
9.
Electrocardiogram and weekly fetal echocardiography are recommended in the interval of greatest risk for the onset of heart block (16–26 weeks) and in the newborn. (Hundred percent agreement).
-
10.
All newborns should also be evaluated with a blood count and liver assessment. The risk of these children developing an autoimmune disease in the future is not increased. (Hundred percent agreement).
-
11.
Breastfeeding should be encouraged. (Hundred percent agreement).
Obstetric and fetal manifestations
Obstetric
Although fertility disorders are rare, the likelihood that women with autoimmune inflammatory diseases will have complicated pregnancies is much more significant. Forest plots for the incidence of obstetric and fetal outcomes in patients with Sjogren’s syndrome or rheumatic diseases with reactive anti-SSA/Ro antibodies are presented in Figs. 5, 6, 7, 8 and 9. The effects of underlying disease on pregnancy and of pregnancy on maternal health differ depending on the disease, pre-existing systemic damage, profile of autoantibodies, and type of prescription regime [35]. Data on pregnancies in primary Sjogren's syndrome are scarce [5]. In preliminary studies, with retrospective data collection, anti-SSA/Ro antibodies appear as a possible factor causing pregnancy loss [36, 37]. Considering the high frequency of these antibodies in SS patients (60–90%) compared to other rheumatic diseases such as SLE (30–50%) and RA (11%), we could assume higher rates of spontaneous abortions in pSS and in pregnant women with anti-SSA/Ro [38,39,40]. However, the literature does not show clinical or statistical differences between the outcomes of pregnancy in seropositive or seronegative patients [38,39,40,41,42].
Likewise, for some authors there is a higher rate of premature births and a lower neonatal average weight, explained by pathological restriction on intrauterine growth in SSA-positive pregnant women [43, 44]. Contradictorily, other studies do not show increased rates of prematurity or fetal growth restriction [8, 42, 45]. Even if the literature data are controversial regarding gestational outcomes, all pregnant women should be assisted in a high-risk prenatal care centre [46].
Furthermore, several authors agree that there is a higher rate of cesarean sections in Sjogren's syndrome (see Additional file 1: Table 2).
For a safe pregnancy, SS patients must be well controlled for at least 6 months [46]. Adjustments for tapering and discontinuing some medications that require washout periods should be provided [47]. Mycophenolate mofetil, leflunomide, cyclophosphamide, methotrexate, and biologic disease modifying anti-rheumatic drugs like rituximab, belimumab and tocilizumab are the main drugs to be avoided [47,48,49]. Dietary supplementation with vitamins, minerals and folic acid should be indicated according to the specifics of each patient, by the gynecologist, in prenatal care. The use of hydroxychoroquine should be encouraged in positive anti-SSA/Ro pregnant women and it is mandatory in pregnant women with a previous history of fetal heart block or other forms of neonatal lupus [50] It´s also recommended to update the profile of antiphospholipid, anti-SSB/La and anti-SSA/Ro antibodies at this time [46, 50].
Neonatal lupus syndrome (NLS)
One of the most feared gestational complications in patients with SS is neonatal lupus syndrome, especially congenital heart block (CHB) [42].
NLS occurs as a result of passive transplacental passage of maternal anti-SSA and/or anti-SSB antibodies at the beginning of pregnancy (~ 11 weeks) [42]. It affects similarly male and female fetuses and can cause cardiac disease [42,43,44,45], skin lesions, cytopenias, neurological and hepatobiliary manifestations in the newborn [51,52,53,54]. All the extracardiac injuries, usually mild and self-limited, may be present at birth or develop during the first months of life [51, 55]. Laboratory abnormalities in asymptomatic babies can be identified in up to one third of cases [51, 52, 54].
Among the most significant cardiac injuries are arrhythmogenic damage to the conduction system, such as congenital autoimmune heart block (AVBc), endocardial fibroelastosis, and myocarditis, which can progress to dilated cardiomyopathy [55,56,57,58,59,60,61,62,63]. The increased risk period for the development of fetal heart injury is between 16 and 26 weeks of pregnancy and it can be detected by fetal echocardiography [42, 58, 64,65,66]. In some studies, the prevalence of congenital autoimmune heart block (defined as atrioventricular block diagnosed in utero, at birth, or in the neonatal period) reaches 1–2% [46, 55, 56].
Irreversible third degree CBH is the most serious manifestation of NLS, requiring cardiac pacemaker implantation and beings associated with higher rates of intrauterine and perinatal mortality [57, 67]. Electric conduction blocks, regardless of degree, can be detected by prenatal ultrasound between 18 and 24 weeks of gestation [68]. Fetal bradycardia is the main finding, often the only one. Due to the existence of the gap between the transfer of maternal antibodies and development of the electric fetal conduction system (~ 12 weeks) and the late clinical observation of CHB (~ 20 weeks), without other structural fetal abnormalities, it becomes very difficult to fix any effective therapeutic intervention [68].
Sinus bradycardia, atrioventricular nodal dysfunction, and increased QT interval are also included electrocardiographic disorders [57, 58, 68].
Some fetuses (6% of all cases of AVBc) may develop dilated cardiomyopathy and die from congestive heart failure or still require a heart transplant. Valve malformations, pulmonary artery stenosis, and atrial or ventricular septal defects have been reported occasionally [59,60,61,62]. Fetal necropsy findings also reveal unsuspected fibrotic lesions of the sinoatrial node [62].
Interestingly, asymptomatic fetuses and newborn infants with incomplete AV changes can evolve with a variable spectrum of cardiac injuries, even later and in the absence of circulating maternal antibodies, suggesting that an intrinsic factor (the fetal itself or the intrauterine environment) might be relevant in the progression of lesions [63].
A cardiac pacemaker is implanted in most of these fetuses in the neonatal period. Even asymptomatic patients with AVBc have an indication for prophylactic pacemaker implantation, due to the unpredictable risk of Stokes-Adams attacks [63, 64]. Intrauterine and neonatal mortality due to AVBc affects up to 30% of cases when associated with fetal hydrops and prematurity, even with advanced intensive care support [64].
The literature is controversial regarding the effectiveness of pharmacological therapy, be it the use of fluorinated corticosteroids that are resistant to the placental enzyme action or IVIG for the prevention or treatment of CHB. The rationale for this type of management in an attempt to obtain better outcomes for the fetus would be to: 1—reduce circulating maternal antibodies and placental transfer; 2—reduce cardiac tissue inflammation before fibrosis and irreversible AVBc [69,70,71,72,73]. However, there is not enough evidence that this actually occurs or that the therapy can prevent AVBc, and safety of some regimes remains a concern [74,75,76]. In selected cases, studies support the idea that dexamethasone (but not prednisone) can reverse carditis and incomplete blocks in addition to improving the hemodynamic conditions of the fetus [71,72,73,74, 77, 78]. Sympathomimetic agent improves bradycardia but does not influence the prognosis [68, 79]. Plasmapheresis has been reported as a therapeutic option in a few case reports [80].
The multicenter study by Cuneo et al. [81], followed 315 pregnant women with anti SSA/Ro antibodies through home monitoring, using a device for measuring the fetal heart rate and rhythm twice a day, and showed that the window of opportunity for therapeutic intervention is very slight [81].
The recurrence of AVBc in a subsequent pregnancy is approximately 10 times greater [78, 82] and the rate of NLS reaches 20% [68, 83]. Hydroxychloroquine (HCQ) impacts on reducing these outcomes and it has not been related to fetal malformations or hearing and visual risks evaluated in the first year of life [74, 75, 83, 84]. Several retrospective studies suggest that women taking hydroxychloroquine are less likely to have a fetus with second- or third-degree heart block [50, 84]. Most authors recommend the administration of HCQ before conception and another part as soon as the patient with positive anti-SSA becomes pregnant [50, 83, 84].
Management of the newborn with NLS
Specific procedures are adopted according to the manifestation presented by each child [85].
Skin lesion A transient, erythematous-desquamative or erythematous-annular and photosensitive skin rash similar to that found in SLE can appear at birth (20%) and up to 2–3 months later (80%). The lesions last for the period of clearance of the circulating maternal antibodies and usually resolve spontaneously without leaving scars, and therefore do not require drug treatment [86, 87]. Histological and immunofluorescence findings are similar to subacute cutaneous lupus. Newborns with skin lesions should be protected from sun exposure until complete regression [85, 88].
Cytopenias: Thrombocytopenia, anemia, and neutropenia are described in NLS. According to Cimaz et al., [52], inconspicuous hematological variations can occur in up to 27% of those born to positive anti-SSA mothers and revert spontaneously. Cases of anemia or severe thrombocytopenia may require blood transfusions, the use of corticosteroids, and intravenous human immunoglobulin in their treatment [52, 89, 90].
Hepatic Liver disorders range from a slight increase in serum transaminases to severe cholestatic syndrome, hepatomegaly and, rarely, splenomegaly [52, 53, 91].
Neurological Non-specific CNS anomalies on cerebral image, in absent of clinical neurological findings, are reported in newborns with NLS [51, 54]. The anomalies resolve during follow-up and do not present any clinical correlate.
Cardiac The most common involvement is AVBc of the first, second, and third degrees usually without structural cardiac lesions. The treatment of intrauterine fetal atrioventricular block is often disappointing and more than 90% of affected newborns undergo definitive pacemaker implantation. Fortunately, these children can lead practically normal lives if there is no associated cardiomyopathy [92].
After birth, a progressive reduction of anti SSA/Ro blood titers occurs over 6 months.
A clinical and laboratorial follow-up should be performed in all infants, until the first year of life [51]. The risk of children with NLS developing autoimmune diseases in the future is not superior than the risk of asymptomatic children born to mothers with autoimmune diseases. There is no contraindication to breastfeeding [52, 92, 93].
The topic of neonatal lupus syndrome is vast, especially in the field of fetal heart block, and goes beyond Sjogren´s syndrome. We do not intend to exhaust all the content in this approach (Table 1).
Conclusions
Despite its importance, the gynecological and obstetric care of patients with SS are not as properly incorporated in the clinical practice, as sicca symptoms or extra glandular manifestations are. A significative prevalence of gynecological symptoms and obstetric care needs were identified in this systematic review and meta-analysis as well a high morbidity associated with them. To attend these demands and improve pSS women´s health care, our panel of specialists developed eleven recommendations, all of them with high agreement between the members. A limitation of our guideline was the absence of a patient representative or a gynecologist/obstetric in the voting panel, a strategy that will be used in future guidelines.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its Additional file 1).
Change history
20 March 2022
A Correction to this paper has been published: https://doi.org/10.1186/s42358-022-00242-7
References
Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017;69(2326–5205 (Electronic)):35–45.
Patel R, Shahane A. The epidemiology of Sjogren’s syndrome. Clin Epidemiol. 2014;30(1179–1349 (Print)):247–55.
Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of primary Sjögren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(11):1983. https://doi.org/10.1136/annrheumdis-2014-205375.
Fox RI. Sjögren’s syndrome. Lancet. 2005;366(9482):321–31. https://doi.org/10.1016/S0140-6736(05)66990-5.
van Nimwegen JF, Arends S, van Zuiden GS, Vissink A, Kroese FG, Bootsma H. The impact of primary Sjögren’s syndrome on female sexual function. Rheumatology (Oxford). 2015;54(7):1286–93. https://doi.org/10.1093/rheumatology/keu522.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. https://doi.org/10.1186/1471-2288-14-135.
Lehrer S, Bogursky E, Yemini M, Kase NG, Birkenfeld A. Gynecologic manifestations of Sjögren’s syndrome. Am J Obstet Gynecol. 1994;170(3):835–7. https://doi.org/10.1016/s0002-9378(94)70294-2.
Skopouli FN, Papanikolaou S, Malamou-Mitsi V, Papanikolaou N, Moutsopoulos HM. Obstetric and gynaecological profile in patients with primary Sjögren’s syndrome. Ann Rheum Dis. 1994;53(9):569–73. https://doi.org/10.1136/ard.53.9.569.
Mulherin DM, Sheeran TP, Kumararatne DS, Speculand B, Luesley D, Situnayake RD. Sjögren’s syndrome in women presenting with chronic dyspareunia. BJOG Int J Obstet Gynaecol. 1997;104(9):1019–23. https://doi.org/10.1111/j.1471-0528.1997.tb12060.x.
Bloch KJ, Buchanan WW, Wohl MJ, Bunim JJ. Sjogren’s Syndrome. A clinical, pathological, and serological study of sixty-two cases. Medicine (Baltimore). 1965;44:187–231.
Marchesoni D, Mozzanega B, De Sandre P, Romagnolo C, Gambari PF, Maggino T. Gynaecological aspects of primary Sjogren’s syndrome. Eur J Obstet Gynecol Reprod Biol. 1995;63(1):49–53. https://doi.org/10.1016/0301-2115(95)02224-u.
van der Meulen TA, van Nimwegen JF, Harmsen HJM, Liefers SC, van der Tuuk K, Kroese FGM, et al. Normal vaginal microbiome in women with primary Sjögren’s syndrome-associated vaginal dryness. Ann Rheum Dis. 2019;78(5):707–9. https://doi.org/10.1136/annrheumdis-2018-214404.
Capriello P, Barale E, Cappelli N, Lupo S, Teti G. Sjögren’s syndrome: clinical, cytological, histological and colposcopic aspects in women. Clin Exp Obstet Gynecol. 1988;15(1–2):9–12.
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci. 2011;108(Supplement 1):4680–7. https://doi.org/10.1073/pnas.1002611107.
Hummelen R, Macklaim JM, Bisanz JE, Hammond JA, McMillan A, Vongsa R, et al. Vaginal microbiome and epithelial gene array in post-menopausal women with moderate to severe dryness. PLoS ONE. 2011;6(11): e26602. https://doi.org/10.1371/journal.pone.0026602.
van Nimwegen JF, van der Tuuk K, Liefers SC, Verstappen GM, Visser A, Wijnsma RF, Vissink A, Hollema H, et al. Vaginal dryness in primary Sjögren’s syndrome: a histopathological case–control study. Rheumatology. 2020;59(10):2806–15. https://doi.org/10.1093/rheumatology/keaa017.
van der Meulen TA, Harmsen HJM, Bootsma H, Liefers SC, Vich Vila A, Zhernakova A, et al. Dysbiosis of the buccal mucosa microbiome in primary Sjögren’s syndrome patients. Rheumatology (Oxford). 2018;57(12):2225–34. https://doi.org/10.1093/rheumatology/key215.
Ferreira C, da Mota LM, Oliveira AC, de Carvalho JF, Lima RA, Simaan CK, et al. Frequency of sexual dysfunction in women with rheumatic diseases. Rev Bras Reumatol. 2013;53(1):35–46. https://doi.org/10.1016/s2255-5021(13)70004-x.
Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. https://doi.org/10.1080/009262300278597.
Yildiz Ç, Karakuş S, Bozoklu Akkar Ö, Şahin A, Bozkurt B, Yanik A. Primary Sjögren’s syndrome adversely affects the female sexual function assessed by the female sexual function index: a case–control study. Arch Rheumatol. 2017;32(2):1238. https://doi.org/10.5606/ArchRheumatol.2017.6066.
Isik H, Isik M, Aynioglu O, Karcaaltincaba D, Sahbaz A, Beyazcicek T, et al. Are the women with Sjögren’s Syndrome satisfied with their sexual activity? Rev Bras Reumatol. 2017;57:210–6.
Priori R, Minniti A, Derme M, Antonazzo B, Brancatisano F, Ghirini S, et al. Quality of sexual life in women with primary Sjögren syndrome. J Rheumatol. 2015;42(8):1427–31. https://doi.org/10.3899/jrheum.141475.
Maddali Bongi S, Del Rosso A, Orlandi M, Matucci-Cerinic M. Gynaecological symptoms and sexual disability in women with primary Sjögren’s syndrome and sicca syndrome. Clin Exp Rheumatol. 2013;31(5):683–90.
Karakus S, Sahin A, Durmaz Y, Aydin H, Yildiz C, Akkar O, et al. Evaluation of ovarian reserve using anti-müllerian hormone and antral follicle count in Sjögren’s syndrome: preliminary study. J Obstet Gynaecol Res. 2017;43(2):303–7. https://doi.org/10.1111/jog.13216.
El Miedany YM, Ahmed I, Moustafa H, El Baddini M. Hyperprolactinemia in Sjogren’s syndrome: a patient subset or a disease manifestation? Joint Bone Spine. 2004;71(3):203–8. https://doi.org/10.1016/S1297-319X(03)00151-9.
Haga HJ, Rygh T. The prevalence of hyperprolactinemia in patients with primary Sjögren’s syndrome. J Rheumatol. 1999;26(6):1291–5.
Gutiérrez MA, Anaya JM, Scopelitis E, Citera G, Silveira L, Espinoza LR. Hyperprolactinaemia in primary Sjögren’s syndrome. Ann Rheum Dis. 1994;53(6):425. https://doi.org/10.1136/ard.53.6.425-a.PMID:8037502;PMCID:PMC1005360.
Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–79. https://doi.org/10.1056/NEJMra0804690.
Shigesi N, Kvaskoff M, Kirtley S, Feng Q, Fang H, Knight JC, et al. The association between endometriosis and autoimmune diseases: a systematic review and metaanalysis. Hum Reprod Update. 2019;25(4):486–503. https://doi.org/10.1093/humupd/dmz014.
Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715–24. https://doi.org/10.1093/humrep/17.10.2715.
Matorras R, Ocerin I, Unamuno M, Nieto A, Peiró E, Burgos J, et al. Prevalence of endometriosis in women with systemic lupus erythematosus and Sjögren’s syndrome. Lupus. 2007;16(9):736–40. https://doi.org/10.1177/0961203307081339.
Nielsen NM, Jørgensen KT, Pedersen BV, Rostgaard K, Frisch M. The cooccurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjogren syndrome. Hum Reprod. 2011;26(6):1555–9. https://doi.org/10.1093/humrep/der105.
Greenbaum H, Weil C, Chodick G, Shalev V, Eisenberg VH. Evidence for an association between endometriosis, fibromyalgia, and autoimmune diseases. Am J Reprod Immunol. 2019;81(4): e13095. https://doi.org/10.1111/aji.13095.
Ammaritano LR. Therapy insight: guidelines for selection of contraception in women with rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3(5):273–81. https://doi.org/10.1038/ncprheum0484 (quiz 305-6).
Carvalheiras G, Faria R, Braga J, Vasconcelos C. Fetal outcome in autoimmune diseases. Autoimmun Rev. 2012;11(6–7):A520–30. https://doi.org/10.1016/j.autrev.2011.12.002.
Watson RM, Braunstein BL, Watson AJ, Hochberg MC, Provost TT. Fetal wastage in women with anti-Ro(SSA) antibody. J Rheumatol. 1986;13(1):90–4.
Hull R, Harris E, Morgan S, Hughes G. Anti-Ro antibodies and abortions in women with SLE. Lancet. 1983;2:1138.
Routsias JG, Tzioufas AG. Sjögren’s syndrome-study of autoantigens and autoantibodies. Clin Rev Allergy Immunol. 2007;32:238–51. https://doi.org/10.1007/s12016-0078003-8.
Franceschini F, Cavazzana I. Anti-Ro/SSA and La/SSB antibodies. Autoimmunity. 2005;38(1):55–63. https://doi.org/10.1080/08916930400022954.
Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64(7):2319–27. https://doi.org/10.1002/art.34380.
Mavragani CP, Dafni UG, Tzioufas AG, Moutsopoulos HM. Pregnancy outcome and anti-Ro/SSA in autoimmune diseases: a retrospective cohort study. Br J Rheumatol. 1998;37(7):740–5. https://doi.org/10.1093/rheumatology/37.7.740.
Brucato A, Cimaz R, Caporali R, Ramoni V, Buyon J. Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clin Rev Allergy Immunol. 2011;40(1):27–41. https://doi.org/10.1007/s12016-009-8190-6.
Priori R, Gattamelata A, Modesti M, Colafrancesco S, Frisenda S, Minniti A, et al. Outcome of pregnancy in Italian patients with primary Sjögren syndrome. J Rheumatol. 2013;40(7):1143–7. https://doi.org/10.3899/jrheum.121518.
Hussein SZ, Jacobsson LT, Lindquist PG, Theander E. Pregnancy and fetal outcome in women with primary Sjogren’s syndrome compared with women in the general population: a nested case-control study. Rheumatology (Oxford). 2011;50(9):1612–7. https://doi.org/10.1093/rheumatology/ker077.
Martínez-Sánchez N, Pérez-Pinto S, Robles-Marhuenda Á, Arnalich-Fernández F, Martín Cameán M, Hueso Zalvide E, et al. Obstetric and perinatal outcome in anti Ro/SSA-positive pregnant women: a prospective cohort study. Immunol Res. 2017;65(2):487–94. https://doi.org/10.1007/s12026-016-8888-5.
Gupta S, Gupta N. Sjögren Syndrome And Pregnancy: a literature review. Perm J. 2017;21:16–047. https://doi.org/10.7812/TPP/16-047.
Korpen CG, Hoeltzenbein M, Tincani A, Fischer-betz R, Elefant E, Chambers C, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheumatic Dis. 2016. https://doi.org/10.1136/annrheumdis-2015-208840.
Flint J, Panchal S, Hurrell A, van de Venne M, Gayed M, Schreiber K, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016. https://doi.org/10.1093/rheumatology/kev404.
Flint J, Panchal S, Hurrell A, van de Venne M, Gayed M, Schreiber K, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part II: analgesics and other drugs used in rheumatology practice. Rheumatology (Oxford). 2016. https://doi.org/10.1093/rheumatology/kev405.
Tunks RD, Clowse MEB, Miller SG, Brancazio LR, Barker PCA. Maternal autoantibody levels in congenital heart block and potential prophylaxis with antiinflammatory agents. Am J Obstet Gynecol. 2013;208(1):64.e1-7. https://doi.org/10.1016/j.ajog.2012.09.020.
Zuppa AA, Riccardi R, Frezza S, Gallini F, Luciano RM, Alighieri G, Romagnoli C, De Carolis S. Neonatal lupus: Follow-up in infants with anti-SSA/Ro antibodies and review of the literature. Autoimmun Rev. 2017;16(4):427–32. https://doi.org/10.1016/j.autrev.2017.02.010.
Cimaz R, Spence DL, Hornberger L, Silverman ED. Incidence and spectrum of neonatal lupus erythematosus: A prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr. 2003;142(6):678–83. https://doi.org/10.1067/mpd.2003.233.
Motta M, Rodriguez-Perez C, Tincani A, Lojacono A, Chirico G. Outcome of infants from mothers with anti-SSA/Ro antibodies. J Perinatol. 2007;27(5):278–83. https://doi.org/10.1038/sj.jp.7211688.
Boros CA, Spence D, Blaser S, Silverman ED. Hydrocephalus and macrocephaly: new manifestations of neonatal lupus erythematosus. Arthritis Rheum. 2007;57(2):261–6. https://doi.org/10.1002/art.22543.
Julkunen H, Kaaja R, Kurki P, Palosuo T, Friman C. Fetal outcome in women with primary Sjögren’s syndrome. A retrospective case-control study. Clin Exp Rheumatol. 1995;13(1):65–71.
Costedoat-Chalumeau N, Amoura Z, Lupoglazoff JM, Huong DL, Denjoy I, Vauthier D, et al. Outcome of pregnancies in patients with anti-SSA/Ro antibodies: a study of 165 pregnancies, with special focus on electrocardiographic variations in the children and comparison with a control group. Arthritis Rheum. 2004;50(10):3187–94. https://doi.org/10.1002/art.20554.
Gerosa M, Cimaz R, Stramba-Badiale M, Goulene K, Meregalli E, Trespidi L, et al. Electrocardiographic abnormalities in infants born from mothers with autoimmune diseases—a multicentre prospective study. Rheumatology. 2007;46(8):1285–9. https://doi.org/10.1093/rheumatology/kem073.
Brucato A, Frassi M, Franceschini F, Cimaz R, Faden D, Pisoni MP, et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum. 2001;44(8):1832–5. https://doi.org/10.1002/1529-0131(200108)44:8%3c1832::Aidart320%3e3.0.Co;2-c.
Eronen M, Sirèn MK, Ekblad H, Tikanoja T, Julkunen H, Paavilainen T. Short- and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics. 2000;106(1 Pt 1):86–91. https://doi.org/10.1542/peds.106.1.86.
Moak JP, Barron KS, Hougen TJ, Wiles HB, Balaji S, Sreeram N, et al. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol. 2001;37(1):238–42. https://doi.org/10.1016/s07351097(00)01048-2.
Udink ten Cate FE, Breur JM, Cohen MI, Boramanand N, Kapusta L, Crosson JE, et al. Dilated cardiomyopathy in isolated congenital complete atrioventricular block: early and long-term risk in children. J Am Coll Cardiol. 2001;37(4):1129–34. https://doi.org/10.1016/s0735-1097(00)01209-2.
Ho SY, Esscher E, Anderson RH, Michaëlsson M. Anatomy of congenital complete heart block and relation to maternal anti-Ro antibodies. Am J Cardiol. 1986;58(3):291–4. https://doi.org/10.1016/0002-9149(86)90064-0.
Askanase AD, Friedman DM, Copel J, Dische MR, Dubin A, Starc TJ, et al. Spectrum and progression of conduction abnormalities in infants born to mothers with anti-SSA/Ro anti-SSB/La antibodies. Lupus. 2002;11(3):145–51. https://doi.org/10.1191/0961203302lu173oa.
Altman CA, Sheth SS. Could timing be everything for antibody-mediated congenital heart? J Am Coll Cardiol. 2018;72(16):1952–4. https://doi.org/10.1016/j.jacc.2018.08.1039.
Glickstein JS, Buyon JP, Friedman D. The fetal PR interval: pulsed Doppler echocardiographic assessment. Am J Cardiol. 2000;86:236–9.
Friedman D, Buyon J, Kim M, Glickstein JS. Fetal cardiac function assessed by Doppler myocardial performance index (Tei index). Ultrasound Obstet Gynecol. 2003;21:33–6.
Buyon JP, Hiebert R, Copel J, et al. Autoimmune-associated congenital heart block: mortality, morbidity, and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66.
Friedman DM, Kim MY, Copel JA, Davis C, Phoon CK, Glickstein JS, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117(4):485–93. https://doi.org/10.1161/circulationaha.107.707661.
Rosenthal D, Druzin M, Chin C, Dubin A. A new therapeutic approach to the fetus with congenital complete heart block: preemptive, targeted therapy with dexamethasone. Obstet Gynecol. 1998;92(4 Pt 2):689–91. https://doi.org/10.1016/s00297844(98)00149-5.
Yang CH, Chen JY, Lee SC, Luo SF. Successful preventive treatment of congenital heart block during pregnancy in a woman with systemic lupus erythematosus and anti Sjögren’s syndrome A/Ro antibody. J Microbiol Immunol Infect. 2005;38(5):365–9.
Copel JA, Buyon JP, Kleinman CS. Successful in utero therapy of fetal heart block. Am J Obstet Gynecol. 1995;173(5):1384–90. https://doi.org/10.1016/0002-9378(95)906215.
Saleeb S, Copel J, Friedman D, Buyon JP. Comparison of treatment with fluorinated glucocorticoids to the natural history of autoantibody-associated congenital heart block: retrospective review of the research registry for neonatal lupus. Arthritis Rheum. 1999;42(11):2335–45. https://doi.org/10.1002/15290131(199911)42:11%3c2335.
Kaaja R, Julkunen H. Prevention of recurrence of congenital heart block with intravenous immunoglobulin and corticosteroid therapy. Arthritis Rheum. 2003;48(1):280–1.
Izmirly PM, Saxena A, Sahl SK, Shah U, Friedman DM, Kim MY, et al. Assessment of fluorinated steroids to avert progression and mortality in anti-SSA/Ro-associated cardiac injury limited to the fetal conduction system. Ann Rheum Dis. 2016;75(6):11615. https://doi.org/10.1136/annrheumdis-2015-208311.
Costedoat-Chalumeau N, Amoura Z, Le Thi HD, Wechsler B, Vauthier D, Ghillani P, et al. Questions about dexamethasone use for the prevention of anti-SSA related congenital heart block. Ann Rheum Dis. 2003;62(10):1010–2. https://doi.org/10.1136/ard.62.10.1010.
Levesque K, Morel N, Maltret A, Baron G, Masseau A, Orquevaux P, et al. Description of 214 cases of autoimmune congenital heart block: results of the French neonatal lupus syndrome. Autoimmun Rev. 2015;14(12):1154–60. https://doi.org/10.1016/j.autrev.2015.08.005.
Buyon JP, Clancy RM. Neonatal lupus: review of proposed pathogenesis and clinical data from the US-based Research Registry for Neonatal Lupus. Autoimmunity. 2003;36(1):41–50. https://doi.org/10.1080/0891693031000067340.
Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31(7):1658–66. https://doi.org/10.1016/s0735-1097(98)00161-2.
Miyoshi T, Maeno Y, Sago H, Inamura N, Yasukohchi S, Kawataki M, et al. Evaluation of transplacental treatment for fetal congenital bradyarrhythmia: nationwide survey in Japan. Circ J. 2012;76:469–76.
Barclay CS, French MA, Ross LD, Sokol RJ. Successful pregnancy following steroid therapy and plasma exchange in a woman with anti-Ro (SS-A) antibodies. Case report Br J Obstet Gynaecol. 1987;94(4):369–71. https://doi.org/10.1111/j.14710528.1987.tb03107.x.
Cuneo BF, Sonesson SE, Levasseur S, Moon-Grady AJ, Krishnan A, Donofrio MT, et al. Home monitoring for fetal heart rhythm during anti-Ro pregnancies. J Am Coll Cardiol. 2018;72(16):1940–51. https://doi.org/10.1016/j.jacc.2018.07.076.
Julkunen H, Eronen M. The rate of recurrence of isolated congenital heart block: a population-based study. Arthritis Rheum. 2001;44(2):487–8. https://doi.org/10.1002/1529-0131(200102)44:2%3c487::Aid-anr70%3e3.0.Co;2-d.
Levy RA, Vilela VS, Cataldo MJ, Ramos RC, Duarte JL, Tura BR, et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus. 2001;10(6):401–4. https://doi.org/10.1191/096120301678646137.
Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, Khamashta MA, Kim MY, Saxena A, et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012;126(1):76–82. https://doi.org/10.1161/circulationaha.111.089268.
Vanoni F, Lava SA, Fossali EF, Cavalli R, Simonetti GD, Bianchetti MG, et al. Neonatal systemic lupus erythematosus syndrome: a comprehensive review. Clin Rev Allergy Immunol. 2017;53(3):469–76. https://doi.org/10.1007/s12016-017-8653-0.
Lee LA, Weston WL. Cutaneous lupus erythematosus during the neonatal and childhood periods. Lupus. 1997;6(2):132–8. https://doi.org/10.1177/096120339700600208.
Neiman AR, Lee LA, Weston WL, Buyon JP. Cutaneous manifestations of neonatal lupus without heart block: characteristics of mothers and children enrolled in a national registry. J Pediatr. 2000;137(5):674–80. https://doi.org/10.1067/mpd.2000.109108.
Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365–81.
Watson R, Kang JE, May M, Hudak M, Kickler T, Provost TT. Thrombocytopenia in the neonatal lupus syndrome. Arch Dermatol. 1988;124(4):560–3.
Kanagasegar S, Cimaz R, Kurien BT, Brucato A, Scofield RH. Neonatal lupus manifests as isolated neutropenia and mildly abnormal liver functions. J Rheumatol. 2002;29(1):187–91.
Laxer RM, Roberts EA, Gross KR, Britton JR, Cutz E, Dimmick J, et al. Liver disease in neonatal lupus erythematosus. J Pediatr. 1990;116(2):238–42. https://doi.org/10.1016/s0022-3476(05)82880-x.
Martin V, Lee LA, Askanase AD, Katholi M, Buyon JP. Long-term followup of children with neonatal lupus and their unaffected siblings. Arthritis Rheum. 2002;46(9):2377–83. https://doi.org/10.1002/art.10638.
Cimaz R. Any increased risk of autoimmune disease? Lupus. 2004;13(9):736–9. https://doi.org/10.1191/0961203303lu1093oa.
Haga HJ, Gjesdal CG, Irgens LM, Ostensen M. Reproduction and gynaecological manifestations in women with primary Sjögren’s syndrome: a case-control study. Scand J Rheumatol. 2005;34(1):45–8. https://doi.org/10.1080/03009740510017959.
Siamopoulou-Mavridou A, Manoussakis MN, Mavridis AK, Moutsopoulos HM. Outcome of pregnancy in patients with autoimmune rheumatic disease before the disease onset. Ann Rheum Dis. 1988;47(12):982–7. https://doi.org/10.1136/ard.47.12.982.
Takaya M, Ichikawa Y, Shimizu H, Uchiyama M, Moriuchi J, Arimori S. Sjögren’s syndrome and pregnancy. Tokai J Exp Clin Med. 1991;16(2):83–8.
De Carolis S, Salvi S, Botta A, Garofalo S, Garufi C, Ferrazzani S, et al. The impact of primary Sjogren’s syndrome on pregnancy outcome: our series and review of the literature. Autoimmun Rev. 2014;13(2):103–7. https://doi.org/10.1016/j.autrev.2013.09.003.
Brucato A, Doria A, Frassi M, Castellino G, Franceschini F, Faden D, et al. Pregnancy outcome in 100 women with autoimmune diseases and anti-Ro/SSA antibodies: a prospective controlled study. Lupus. 2002;11(11):716–21. https://doi.org/10.1191/0961203302lu252oa.
Acknowledgements
Not applicable.
Funding
There wasn’t financial sponsor for this paper.
Author information
Authors and Affiliations
Contributions
All authors made contributions to the acquisition of data, have been involved in drafting the manuscript or revising it critically for important intellectual content, participated in the voting rounds, gave final approval of the version to be published and have participated sufficiently in the work to take public responsibility for appropriate portions of the content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors have no competing interests for this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
the authors identified an error in point 9, section: Obstetric recommendations in the presence of Neonatal Lupus Syndrome risk. The original article has been corrected.
Supplementary Information
Additional file 1.
Table 1: Gynecological symptoms in Sjogren´s syndrome patients. Table 2: Gestational outcomes in patients with Sjogren's syndrome or rheumatic diseases with reactive anti-SSA / Ro antibodies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oliveira, F.R., Valim, V., Pasoto, S.G. et al. 2021 recommendations of the Brazilian Society of Rheumatology for the gynecological and obstetric care of patients with Sjogren’s syndrome. Adv Rheumatol 61, 54 (2021). https://doi.org/10.1186/s42358-021-00208-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-021-00208-1