Abstract
Background
Systemic sclerosis (SSc) is a chronic disease characterized by autoimmunity, vasculopathy, and visceral and cutaneous fibrosis. Vitamin D has several functions in the immunological system, and different studies have suggested a potential role in triggering autoimmune diseases. Patients with SSc may present with low serum levels of vitamin D, but the association between hypovitaminosis D and disease onset or any clinical manifestation is still obscure. Our goal was to verify the causal relationship between hypovitaminosis D and SSc onset or any particular clinical manifestation in the literature.
Methods
A systematic literature review was performed through February 24th, 2021 on Pubmed, Lilacs/BIREME, and Cochrane databases. The eligible studies were read in full text, and, in the absence of exclusion criteria, were included in this review after consensus between two reviewers.
Results
Forty articles met the eligibility criteria and the main results of each study are described. In most studies, SSc patients showed a higher prevalence of vitamin D deficiency and insufficiency compared to controls. Additionally, in some reports serum levels of vitamin D were inversely correlated with the severity of SSc. Oral supplementation did not seem to affect serum levels of vitamin D. Four of the included studies were with experimental models.
Conclusion
In conclusion, vitamin D deficiency seems to have a role in susceptibility to SSc, as well as in the clinical manifestations of the disease.
Similar content being viewed by others
Introduction
Systemic sclerosis (SSc) is a multisystemic autoimmune disease with a complex pathophysiology. SSc is characterized by the presence of autoantibodies and arises from the interrelation between vascular dysfunction, adaptive and innate immunity dysregulation, and excess activation of fibroblasts and similar cells, resulting in the development of progressive tissue fibrosis [1,2,3]. With a multifactorial etiology, several environmental and genetic factors seem to trigger the onset of SSc and its outcomes [1]. SSc affects predominantly young adults and has an important impact on quality of life and mortality rates [4]. Treatment is multidisciplinary, with a focus on symptoms management and predominant visceral injuries. A better understanding of the pathophysiology behind the disease could help advance research on this area and allow for the development of new therapeutic options [4].
Vitamin D is a steroid hormone known for its function on the regulation of calcium homeostasis and bone metabolism. The active metabolite of vitamin D (calcitriol) is formed by the activation of a precursor on skin exposed to ultraviolet B (UVB) radiation [5]. Serum vitamin D levels may vary according to factors such as food intake (although to a lesser extent), bowel absorption, exposure to sunlight, and renal and hepatic metabolisms [5,6,7]. Vitamin D receptors (VDR) are present in antigen-presenting cells, natural killer cells, and activated B- and T-cell lymphocytes [5], which reinforces the evidence of its multiple immunomodulatory actions on adaptive and innate immune responses [8]. Vitamin D has a predominantly immunosuppressive effect, inhibiting proinflammatory cytokines production (IL6 and IL-17), stimulating anti-inflammatory cytokines production (IL4 and IL-10), and polarizing the Th1 response (IL-1, TNF-α, IFN-γ) into a Th2 response (autoantibodies, TGF-β) [5, 6]. Additionally, vitamin D inhibits the Th17 response and stimulates regulatory T cells. The immunomodulatory and tolerant effects of vitamin D on the adaptive and innate immune systems seem to play a protective role in the development of autoimmunity [6].
Hypovitaminosis D has been reported in several autoimmune diseases. Serum vitamin D levels are significantly lower in patients with SSc compared to healthy individuals [6]. Some studies have reported vitamin D insufficiency (< 30 ng/ml) in over 80% of patients with SSc [9, 10], usually in association with worse quality of life and lower bone mass [10,11,12,13,14]. The role of hypovitaminosis D in SSc pathophysiology and its possible therapeutic impact have been the subject of various studies in different scenarios. Therefore, this systematic review aims to verify the causal relationship between hypovitaminosis D and SSc onset or any particular clinical manifestation of the disease.
Methods
A comprehensive search in PubMed, Lilacs/BIREME, and Cochrane Library databases was performed through Fabruary 24th, 2021. The keywords used were “systemic sclerosis” and “vitamin D”. No restrictions for language, year or type of publication were added (Supplemental file 1).
After excluding duplicate studies, two reviewers independently reviewed all the articles found in different databases. Initially, titles and summaries were analyzed according to the eligibility criteria and all studies on the association between serum vitamin D levels and the clinical and pathophysiological aspects of SSc or on the role of vitamin D in the pathophysiology of SSc were selected. The selected articles were read in full and, in the absence of exclusion criteria, were included in this review after consensus between two reviewers. If no consensus were reached, a third reviewer would decide on study inclusion. The question that guided studies selection was: “What is the role of vitamin D in the pathophysiology and in the clinical manifestations of SSc?”, so all observation or intervention studies assessing vitamin D levels and its correlation with clinical and/or pathophysiological manifestations were eligible for inclusion in this review. We included all clinical and experimental studies published after peer-review, that would address the question guiding paper selection. Articles describing vitamin D levels on SSc patients without evaluation of clinical and/or pathophysiological manifestations were excluded, as well as other review articles and research on SSc therapy or treatment. Preprint studies were not included. In addition, the reference sections of the relevant articles were scanned for additional references that could have been missing from the databases.
Most of the studies included in this review defined vitamin D deficiency as a serum level < 10 ng/ml and insufficiency as a serum level of 10–30 ng/ml.
Results
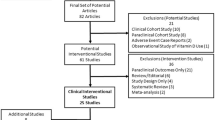
Overall, 391 publications were identified in the different databases: 162 in PubMed, 211 in Lilacs/BIREME, and 10 in Cochrane Library. Of these, 118 were excluded after summary analysis, 205 were duplicate studies, two were not available online, and one was an erratum. Additionally, 17 publications were excluded after full text review for not meeting the eligibility criteria, and 12 were excluded due to being review articles. Eight additional articles were included after reference review. At the end of the selection process, 40 articles were included in this systematic review. Four included studies were experimental models on the effect of vitamin D or a vitamin D analogue. These data are shown in the flowchart on Fig. 1.
Characteristics of the selected articles, number of subjects, serum levels of vitamin D, proportion of patients with vitamin D < 10 and < 30 ng/mL and the main results of each study are presented in Table 1. Most studies showed vitamin D deficiency and insufficiency in SSc patients, regardless of season or oral vitamin D supplementation. Some studies identified association of vitamin D levels with clinical and pathophysiological manifestations, such as an inverse correlation of vitamin D levels with disease severity. Other reports did not identified an association between SSc manifestations and vitamin D.
The methodology and the main results of the experimental studies included in this review are summarized in Table 2.
Discussion
In the last two decades, several authors have reported lower levels of vitamin D in patients with SSc compared to healthy individuals [11,12,13,14, 16, 21, 25, 38, 48]. Despite the well-known seasonal variations in serum vitamin D levels, with peak values in summer [41], SSc patients present consistently lower levels throughout all seasons [38, 41]. Initially, we intended to estimate the mean serum vitamin D level among SSc patients across studies, but the great population heterogeneity could lead this estimation to substantial misinterpretation and inaccuracy. Seasonal variation, age, gender, comorbidities, life habits and drug treatment were some of the factors that could falsely impact this measure.
The evidence behind the association between hypovitaminosis D and SSc onset is scarce [15, 29,30,31, 34, 38]. So far, no published study has properly demonstrated this cause-effect relationship. Future research needs to add more on biologic plausibility, temporality, intensity, consistency and dose-response effect of hypovitaminosis D and SSc onset. An inception cohort study would be a suitable design. However, it may not be feasible due to the very low incidence of SSc in the general population. Longitudinal case-control studies would be the alternative; nonetheless vitamin D levels may not be generally available for comparison between groups.
Recently, An et al. (2017) have performed a meta-analysis to investigate the association between vitamin D and SSc, which included six case-control studies with a total of 554 SSc patients and 321 healthy controls. Meta-analysis results showed that SSc patients suffered from decreased vitamin D levels compared with healthy controls and in diffused SSc patients the vitamin D levels were significantly lower than those in limited SSc. Vitamin D level was not associated with Rodnan score, systolic pulmonary pressure, gastrointestinal ulcer, or pulmonary involvement, so the authors suggested that lower vitamin D levels in SSc patients may not be a factor accelerating disease severity [49].
On the other hand, some studies have shown an association between vitamin D deficiency and severity of SSc [9, 16, 41]. Hypovitaminosis D is common in SSc and patients with vitamin D deficiency have a greater frequency of pulmonary disease, lower diffusion and higher estimated pulmonary hypertension compared to patients with vitamin D insufficiency [8, 22, 37]. Additionally, these patients have higher inflammatory markers [9, 22] and Cruz-Domínguez et al. (2017) have demonstrated lower vitamin D levels in patients with and without calcinosis [24]. Park et al. (2017) demonstrated that vitamin D deficiency is an independent risk factor for digital ulcers in SSc, suggesting an involvement with the microangiopathic manifestations of the disease. However, the study could not demonstrate an association with macrovascular conditions in SSc patients, such as arterial stiffness and atherosclerosis [35], which suggests that vitamin D may act differently on micro- and macrovascular mechanisms in SSc. Caimmi et al. (2019) found in a retrospective cohort study that a decrease in 25-hydroxyvitamin D (25(OH)D) is associated with the risk of developing digital ulcers after 5 years [20]. In spite of this, no consensus has been reached, and further studies are needed to clarify the association between low vitamin D levels and the vascular involvement of the disease [18, 21, 43, 49].
Hypovitaminosis D diagnosis is based on serum 25(OH) D levels, and there is no consensus regarding the ideal values that should be adopted. Several experts agree to define vitamin D deficiency as serum levels below 10 ng/ml [9, 10, 16, 18, 22, 38]. However, this definition is not universal, with some authors suggesting deficiency levels to be lower than 20 ng/ml (50 nmol/l) [39, 41], taking in consideration the regulatory variations of PTH. In turn, vitamin D insufficiency is commonly defined as serum levels between 10 and 30 ng/ml, with levels > 30 ng/ml being considered sufficient [9, 22, 24, 27]. Higher levels of vitamin D do not seem to provide any additional benefits [50]. Nevertheless, the optimal concentration of vitamin D for proper immune system function is not yet well-defined. Studies have demonstrated that genetic polymorphisms in the vitamin D binding protein, in enzymes or even in the VDR could determine variations in 25(OH) D serum levels, further complicating the definition of adequate serum levels [51,52,53].
Vitamin D deficiency in patients with SSc seems to be multifactorial. In a retrospective cohort comprising 327 SSc patients and 141 controls, Arnson et al. (2011) substantiate an inverse relation between serum vitamin D levels and the extent of cutaneous involvement, they showed that patients with a Rodnan score higher than 10 had a significantly lower serum vitamin D level compared to other patients (17.7 ± 10.4 ng/ml versus 8 ± 10.1 ng/ml; p = 0.02) [16], a result recently replicated in another study [17]. Since then, other authors also demonstrated an inverse correlation between serum vitamin D levels and the extent of cutaneous involvement [11, 12, 28, 42]. On the other hand, some studies did not evidence the same correlation between cutaneous involvement and vitamin D levels [8, 26, 32, 35, 40, 49]. These authors suggest that the epidermal synthesis could remain virtually normal, and that the hepatic and renal hydroxylation mechanisms of vitamin D could also work normally in this disease. Gastrointestinal involvement in SSc seems to contribute to vitamin D malabsorption, although these data are still controversial [19, 22]. Furthermore, it is possible that some medications used by SSc patients, such as corticosteroids or anticonvulsants for neuropathic pain control, could interfere with vitamin absorption [16]. In spite of this, not all authors were able to evidence differences in vitamin D levels between patients on and off these medications [41]. In addition to the cutaneous and gastrointestinal involvement, reduced sun exposure due to physical incapacity, self-image disorders, depression, and social and psychological limitations could compound the deficit [9, 34]. As one might expect, serum vitamin D levels could simply reflect a better health in general due to better life habits, such as exercise, outdoor activities and a healthier diet. This hypothesis is corroborated by previous research showing an association between low levels of 25(OH) D and a worse quality of life and physical function in SSc patients [10, 33].
Many authors have explored the association between lower vitamin D levels and SSc phenotype. Sampaio-Barros et al. (2016) demonstrated an association between lower serum vitamin D levels, underlying vascular involvement and the production of anti-Scl70 autoantibodies [10]. In addition, lower levels of vitamin D were described in patients with diffuse cutaneous SSc when compared with limited cutaneous disease [12, 49]. On the other hand, Vacca et al. (2009) showed an association between lower serum vitamin D levels and the presence of anti-centromere autoantibodies [9]. Other authors, however, have failed to demonstrate any association between serum vitamin D levels and disease subtype or specific autoantibodies [18, 43]. Therefore, the role of vitamin D in the clinical and serological phenotypes of SSc is not yet well known. Further studies should seek to clarify the possible associations between hypovitaminosis D and disease subtype, also considering the prognostic impact of these findings.
Considering the pathogenesis of SSc, several studies have suggested that vitamin D is related to the regulation of cellular immunity mechanisms [6,7,8]. The synthesis of 1,25 dihydroxyvitamin D (1,25(OH)2D) and presence of VDR in activated immune cells have attracted the attention of several researchers. Vitamin D acts as a regulator of innate and adaptive immune responses, promoting monocyte differentiation into macrophages, modulating macrophage response and inhibiting production of chemokines and inflammatory cytokines [54]. Polymorphisms in the VDR gene also seem to be associated with vitamin D deficiency, as suggested by Kamal et al. (2016). The authors found no association between VDR polymorphisms (ApaI and TaqI) and susceptibility to SSc, but reported an association between the ApaI polymorphism and disease subtype [31]. In a recent study, an association between some VDR single nucleotide polymorphisms and SSc susceptibility was stablished [55].
Interestingly, Carmel et al. (2015) reported a higher frequency and an increased level of anti-25(OH) D IgM antibodies in SSc patients when compared to controls, similarly to what has been described in patients with systemic lupus erythematosus (SLE). However, the same study reported lower levels of anti-25(OH) D IgG antibodies in SSc patients than in controls [23]. The pathophysiological significance and clinical relevance of these autoantibodies in SSc remains controversial.
Vitamin D also seems to have an important role in modulating TGF-β activity, an essential factor in collagen production by fibroblasts. Zerr et al. (2015) analyzed VDR expression in fibroblasts of SSc patients and in a murine model of bleomycin-induced fibrosis. The authors characterized the VDR as a negative regulator of TGF-β signaling. Furthermore, they demonstrated that treatment with a vitamin D analogue (paricalcitol) was able to restore VDR signaling and reduce the pro-fibrotic effects of TGF-β on fibroblasts [47]. Similar findings were described in a study on skin fibroblast cultures from SSc patients and controls, which demonstrated the inhibitory effects of vitamin D in collagen and hyaluronate production induced by TGF [44]. The research of Boelsma et al. (1995) supports these findings by demonstrating evidence of cutaneous fibrosis in humans, which was also correlated with lower serum levels of vitamin D [56]. However, this finding could also result from reduced vitamin D production due to cutaneous fibrosis [57]. When assessing the therapeutic use of topical vitamin D analogues on SSc patients with hypovitaminosis D, a reduction in cutaneous fibrosis was observed. These results may be due to the role of vitamin D in reducing pro-fibrotic signaling by TGF-β and in inducing a polarization of the local immune response by producing potentially pro-fibrotic Th2 cytokines [45, 46]. A study by Ahmadi et al. (2017) analyzed the serum levels of fibroblast growth factor 23 (FGF-23), which has an essential role in the kidney-bone axis, and its membrane co-receptor Klotho. Klotho is a protein predominantly expressed in the renal tubules and possibly involved in calcium and phosphate homeostasis, prevents hyperphosphatemia, fibrosis, arteriosclerosis and inflammation [15]. A deficiency in α-Klotho expression has been studied for its potential involvement in endothelial dysfunction, microangiopathy, calcinosis and fibrosis, which are common features of scleroderma. Serum levels of soluble Klotho tend to decrease with age, and a defect in its gene increases the risk for age-related diseases. Significantly lower serum levels of Klotho in SSc patients were identified [29]. It is believe that an increase in inflammatory markers could reduce the renal expression of this protein [15].
In two independent European cohorts, Vacca et al. (2011) assessed the frequency of vitamin D insufficiency and deficiency in SSc patients. These studies found no difference in serum levels between SSc patients under the standard dose of vitamin D supplementation (800 IU/day) or under placebo [58]. In a Spanish retrospective cohort, Rios-Fernández et al. (2010) also described a high prevalence of vitamin D deficiency in SSc patients and corroborated the finding that supplementation with the usual dose of vitamin D was insufficient (60.4% of patients with low vitamin D levels were taking supplements) [36]. Therefore, conventional vitamin D supplementation (800 IU/day) does not seem to correct deficiency in SSc patients. Higher doses could be needed, especially in patients with severe disease or high inflammatory activity.
In a cross-sectional study with 140 SSc patients, 91 patients did not receive vitamin D supplementation, while 49 received 8000 to 12,500 IU of 25(OH) D weekly for a minimum period of 6 months. Non-supplemented patients had lower serum levels of vitamin D (9.8 ± 4.1 vs. 26 ± 8.1 ng/ml, p < 0.0001), although less than one-third of supplemented patients had reached normal levels by the end of the study [27]. In contrast, a retrospective cohort by Trombetta et al. (2017) was not able to demonstrate any changes on serum vitamin D levels in SSc patients supplemented with 1000 IU/day of cholecalciferol for 6 to 12 months [41]. The authors raised several hypotheses to justify these findings, with the strongest ones pointing to an interference in vitamin D activation caused by cutaneous fibrosis and to cholecalciferol malabsorption [27]. In spite of the variation in results for studies on vitamin D supplementation and the need for further research, most experts still recommend correcting vitamin D deficiency. The dose required to achieve and maintain adequate 25(OH) D levels depends on several factors. Some authors have suggested that a raise of 1 ng/ml of 25(OH) D in serum levels would require about 100 IU/day of vitamin D intake for approximately 3 months to reach a steady state once supplementation is initiated [59, 60]. In spite of this estimate, individual responses can vary, and the known risk factors for vitamin D deficiency should be considered for a personalized therapeutic approach.
Our study has some limitations. The major one is the lack of prospective cohort studies or randomized controlled trials assessing the role of vitamin D in the pathophysiology and in the clinical manifestations of SSc, so no definitive conclusions could be drawn regarding cause-effect relationship between hypovitaminosis D and SSc onset or any clinical manifestation. Besides, literature on Medline/EMBASE was not included in this systematic literature review due to access restrictions, however, considering the great number of overlaps between Medline/EMBASE and Medline/PubMed, it is not likely that any major study was left out of this review. Also, we have chosen to include as many peer-reviewed articles as possible, in this regard, a quality assessment of included articles was not performed and methodologically distinct studies were included. Our article selection strategy was based on the fact that systematic reviews evaluate areas where evidence may be too heterogeneous for comparison and a meta-analysis study is not possible [61, 62].
Conclusion
In conclusion, vitamin D deficiency is frequent in SSc patients and seems to be associated with some clinical and serological features of the disease. However, to date, there is no evidence that the usual supplementation interferes with vitamin D deficiency, and its clinical impact remains uncertain. Further randomized trials on the clinical effects of vitamin D supplementation are necessary to verify the effects of vitamin D on the clinical characteristics of SSc, as well as to identify the potential role of vitamin D on immunomodulation and treatment of the disease. Thus, the optimal dosage of vitamin D supplementation and the need for monitoring of 25(OH) D serum levels in SSc patients are still open fields for research. Further studies are also needed to clarify the relationship between serum vitamin D levels and the pathophysiology of SSc. It remains unclear if vitamin D deficiency is an epiphenomenon or if it actually determines an increase in susceptibility and a worse prognosis for this complex disease.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 1,25(OH)2D:
-
1,25 dihydroxyvitamin D
- 25(OH)D:
-
25-hydroxyvitamin D
- ANA:
-
Antinuclear antibodies
- APS:
-
Antiphospholipid syndrome
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- DLCO:
-
Diffusion capacity of carbon monoxide
- DM:
-
Dermatomyositis
- DU:
-
Digital ulcers
- EDAS:
-
European Disease Activity Score
- ESR:
-
Erythrocyte sedimentation rate
- ET-1:
-
Endotelina 1
- FGF-23:
-
Fibroblast growth factor 23
- GERD:
-
Gastroesophageal reflux disease
- HAQ:
-
Health Assessment Questionnaire
- mRSS:
-
Modified Rodnan skin score
- OA:
-
Osteoarthritis
- PASP:
-
Pulmonary arterial systolic pressure on Doppler echocardiography
- PM:
-
Polymyositis
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- SF-36:
-
Short-Form-36 Questionnaire
- SLE:
-
Systemic lupus erythematosus
- SSc:
-
Systemic sclerosis
- TGF-β:
-
Transforming growth factor β
- Tregs:
-
Regulatory T-cells
- UVB:
-
Ultraviolet B
- VDR:
-
Vitamin D receptor
References
Murdaca G, Contatore M, Gulli R, Mandich P, Puppo F. Genetic factors and systemic sclerosis. Autoimmun Rev. 2016;15(5):427–32. https://doi.org/10.1016/j.autrev.2016.01.016.
Denton CP, Black CM, Abraham DJ. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2(3):134–44. https://doi.org/10.1038/ncprheum0115.
Zimmermann AF, Pizzichini MM. Update on etiopathogenesis of systemic sclerosis. Rev Bras Reumatol. 2013;53(6):516–24. https://doi.org/10.1016/j.rbr.2013.01.001.
Desbois AC, Cacoub P. Systemic sclerosis: An update in 2016. Autoimmun Rev. 2016;15(5):417–26. https://doi.org/10.1016/j.autrev.2016.01.007.
Bivona G, Agnello L, Pivetti A, Milano S, Scazzone C, Sasso BL, et al. Association between hypovitaminosis D and systemic sclerosis: true or fake? Clin Chim Acta. 2016;458:115–9. https://doi.org/10.1016/j.cca.2016.04.026.
Rosen Y, Daich J, Soliman I, Brathwaite E, Shoenfeld Y. Vitamin D and autoimmunity. Scand J Rheumatol. 2016;45(6):439–47. https://doi.org/10.3109/03009742.2016.1151072.
Pelajo CF, Lopez-Benitez JM, Miller LC. Vitamin D and autoimmune rheumatologic disorders. Autoimmun Rev. 2010;9(7):507–10. https://doi.org/10.1016/j.autrev.2010.02.011.
Groseanu L, Bojinca V, Gudu T, Saulescu I, Predeteanu D, Balanescu A, et al. Low vitamin D status in systemic sclerosis and the impact on disease phenotype. Eur J Rheumatol. 2016;3(2):50–5. https://doi.org/10.5152/eurjrheum.2015.0065.
Vacca A, Cormier C, Piras M, Mathieu A, Kahan A, Allanore Y. Vitamin D deficiency and insufficiency in 2 independent cohorts of patients with systemic sclerosis. J Rheumatol. 2009;36(9):1924–9. https://doi.org/10.3899/jrheum.081287.
Sampaio-Barros MM, Takayama L, Sampaio-Barros PD, Bonfa E, Pereira RM. Low vitamin D serum levels in diffuse systemic sclerosis: a correlation with worst quality of life and severe capillaroscopic findings. Rev Bras Reumatol Engl Ed. 2016;56(4):337–44. https://doi.org/10.1016/j.rbre.2016.05.006.
Corrado A, Colia R, Mele A, Di Bello V, Trotta A, Neve A, et al. Correction: relationship between body mass composition, bone mineral density, skin fibrosis and 25(OH) vitamin D serum levels in systemic sclerosis. PLoS One. 2015;10(11):e0142748. https://doi.org/10.1371/journal.pone.0142748.
Corrado A, Colia R, Mele A, Di Bello V, Trotta A, Neve A, et al. Relationship between body mass composition, bone mineral density, skin fibrosis and 25(OH) vitamin D serum levels in systemic sclerosis. PLoS One. 2015;10(9):e0137912. https://doi.org/10.1371/journal.pone.0137912.
Ibn Yacoub Y, Amine B, Laatiris A, Wafki F, Znat F, Hajjaj-Hassouni N. Bone density in Moroccan women with systemic scleroderma and its relationships with disease-related parameters and vitamin D status. Rheumatol Int. 2012;32(10):3143–8. https://doi.org/10.1007/s00296-011-2150-1.
Atteritano M, Lasco A, Mazzaferro S, Macri I, Catalano A, Santangelo A, et al. Bone mineral density, quantitative ultrasound parameters and bone metabolism in postmenopausal women with depression. Intern Emerg Med. 2013;8(6):485–91. https://doi.org/10.1007/s11739-011-0628-1.
Ahmadi R, Hajialilo M, Ghorbanihaghjo A, Mota A, Raeisi S, Bargahi N, et al. FGF-23, Klotho and vitamin D levels in scleroderma. Iran J Public Health. 2017;46(4):530–6.
Arnson Y, Amital H, Agmon-Levin N, Alon D, Sanchez-Castanon M, Lopez-Hoyos M, et al. Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: a retrospective cohort study and review of the literature. Autoimmun Rev. 2011;10(8):490–4. https://doi.org/10.1016/j.autrev.2011.02.002.
Atteritano M, Santoro D, Corallo G, Visalli E, Buemi M, Catalano A, et al. Skin involvement and pulmonary hypertension are associated with vitamin D insufficiency in scleroderma. Int J Mol Sci. 2016;17(12).
Belloli L, Ughi N, Marasini B. Vitamin D in systemic sclerosis. Clin Rheumatol. 2011;30(1):145–6. https://doi.org/10.1007/s10067-010-1564-6.
Braun-Moscovici Y, Furst DE, Markovits D, Rozin A, Clements PJ, Nahir AM, et al. Vitamin D, parathyroid hormone, and acroosteolysis in systemic sclerosis. J Rheumatol. 2008;35(11):2201–5. https://doi.org/10.3899/jrheum.071171.
Caimmi C, Bertoldo E, Pozza A, Caramaschi P, Orsolini G, Gatti D, et al. Vitamin D serum levels and the risk of digital ulcers in systemic sclerosis: a longitudinal study. Int J Rheum Dis. 2019;22(6):1041–5. https://doi.org/10.1111/1756-185X.13554.
Calzolari G, Data V, Carignola R, Angeli A. Hypovitaminosis D in systemic sclerosis. J Rheumatol. 2009;36(12):2844 author reply 5.
Caramaschi P, Dalla Gassa A, Ruzzenente O, Volpe A, Ravagnani V, Tinazzi I, et al. Very low levels of vitamin D in systemic sclerosis patients. Clin Rheumatol. 2010;29(12):1419–25. https://doi.org/10.1007/s10067-010-1478-3.
Carmel NN, Rotman-Pikielny P, Lavrov A, Levy Y. Vitamin D antibodies in systemic sclerosis patients: findings and clinical correlations. Isr Med Assoc J. 2015;17(2):80–4.
Cruz-Dominguez MP, Garcia-Collinot G, Saavedra MA, Medina G, Carranza-Muleiro RA, Vera-Lastra OL, et al. Clinical, biochemical, and radiological characterization of the calcinosis in a cohort of Mexican patients with systemic sclerosis. Clin Rheumatol. 2017;36(1):111–7. https://doi.org/10.1007/s10067-016-3412-9.
Di Liberto D, Scazzone C, La Rocca G, Cipriani P, Lo Pizzo M, Ruscitti P, et al. Vitamin D increases the production of IL-10 by regulatory T cells in patients with systemic sclerosis. Clin Exp Rheumatol. 2019;37(Suppl 119(4)):76–81.
Gambichler T, Chrobok I, Hoxtermann S, Kreuter A. Significantly decreased serum 25-hydroxyvitamin d levels in a large german systemic sclerosis cohort. J Rheumatol. 2011;38(11):2492–3 author reply 4.
Giuggioli D, Colaci M, Cassone G, Fallahi P, Lumetti F, Spinella A, et al. Serum 25-OH vitamin D levels in systemic sclerosis: analysis of 140 patients and review of the literature. Clin Rheumatol. 2017;36(3):583–90. https://doi.org/10.1007/s10067-016-3535-z.
Gupta S, Mahajan VK, Yadav RS, Mehta KS, Bhushan S, Chauhan PS, et al. Evaluation of serum vitamin D levels in patients with systemic sclerosis and healthy controls: results of a pilot study. Indian Dermatol Online J. 2018;9(4):250–5. https://doi.org/10.4103/idoj.IDOJ_328_17.
Hajialilo M, Noorabadi P, Tahsini Tekantapeh S, Malek MA. Endothelin-1, alpha-Klotho, 25(OH) Vit D levels and severity of disease in scleroderma patients. Rheumatol Int. 2017;37(10):1651–7. https://doi.org/10.1007/s00296-017-3797-z.
Hax V, Gasparin AA, Schneider L, Monticielo OA, Soares HMF, Streit MDA, et al. Vitamin D and cytokine profiles in patients with systemic sclerosis. J Clin Rheumatol. 2020;26(7):289–94. https://doi.org/10.1097/RHU.0000000000001112.
Kamal A, Gamal SM, Elgengehy FT, Alkemary AK, Siam I. Association of VDR ApaI and TaqI gene polymorphisms with the risk of scleroderma and Behcet's disease. Immunol Investig. 2016;45(6):531–42. https://doi.org/10.1080/08820139.2016.1180302.
Matsuoka LY, Dannenberg MJ, Wortsman J, Hollis BW, Jimenez SA, Varga J. Cutaneous vitamin D3 formation in progressive systemic sclerosis. J Rheumatol. 1991;18(8):1196–8.
Montabone E, Data V, Carignola R. Vitamin D status and quality of life in systemic sclerosis patients. J Clin Rheumatol. 2016;22(4):229–30. https://doi.org/10.1097/RHU.0000000000000406.
Orbach H, Zandman-Goddard G, Amital H, Barak V, Szekanecz Z, Szucs G, et al. Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann N Y Acad Sci. 2007;1109(1):385–400. https://doi.org/10.1196/annals.1398.044.
Park EK, Park JH, Kweon SM, Kim GT, Lee SG. Vitamin D deficiency is associated with digital ulcer but not with atherosclerosis or arterial stiffness in patients with systemic sclerosis: a pilot study. Clin Rheumatol. 2017;36(6):1325–33. https://doi.org/10.1007/s10067-017-3622-9.
Rios Fernandez R, Fernandez Roldan C, Callejas Rubio JL, Ortego CN. Vitamin D deficiency in a cohort of patients with systemic scleroderma from the south of Spain. J Rheumatol. 2010;37(6):1355; author reply 6. https://doi.org/10.3899/jrheum.091143.
Rios-Fernandez R, Callejas-Rubio JL, Fernandez-Roldan C, Simeon-Aznar CP, Garcia-Hernandez F, Castillo-Garcia MJ, et al. Bone mass and vitamin D in patients with systemic sclerosis from two Spanish regions. Clin Exp Rheumatol. 2012;30(6):905–11.
Seriolo B, Molfetta L, Cutolo M. Seasonal variations in serum levels of 25-hydroxyvitamin D in patients with systemic sclerosis. Clin Rheumatol. 2011;30(3):445–6. https://doi.org/10.1007/s10067-011-1684-7.
Shinjo SK, Bonfa E, de Falco CV, Pereira RM. Low bone mass in juvenile onset sclerosis systemic: the possible role for 25-hydroxyvitamin D insufficiency. Rheumatol Int. 2011;31(8):1075–80. https://doi.org/10.1007/s00296-010-1421-6.
Taylan A, Birlik M, Kenar G, Toprak B, Gundogdu B, Gurler O, et al. Osteoprotegrin interacts with biomarkers and cytokines that have roles in osteoporosis, skin fibrosis, and vasculopathy in systemic sclerosis: a potential multifaceted relationship between OPG/RANKL/TRAIL and Wnt inhibitors. Mod Rheumatol. 2019;29(4):619–24. https://doi.org/10.1080/14397595.2018.1500736.
Trombetta AC, Smith V, Gotelli E, Ghio M, Paolino S, Pizzorni C, et al. Vitamin D deficiency and clinical correlations in systemic sclerosis patients: a retrospective analysis for possible future developments. PLoS One. 2017;12(6):e0179062. https://doi.org/10.1371/journal.pone.0179062.
Ursini F, D'Angelo S, Padula A, Leccese P, Abignano G, Mennillo GA, et al. Vitamin D deficiency in systemic sclerosis: a possible role of subclinical liver fibrosis? Retrospective analysis from an Italian cohort. Clin Rheumatol. 2017;36(12):2871–2. https://doi.org/10.1007/s10067-017-3709-3.
Zhang L, Duan Y, Zhang TP, Huang XL, Li BZ, Ye DQ, et al. Association between the serum level of vitamin D and systemic sclerosis in a Chinese population: a case control study. Int J Rheum Dis. 2017;20(8):1002–8. https://doi.org/10.1111/1756-185X.12794.
Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, et al. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab. 2013;98(2):E298–303. https://doi.org/10.1210/jc.2012-3074.
Terao M, Yang L, Matsumura S, Yutani M, Murota H, Katayama I. A vitamin D analog inhibits Th2 cytokine- and TGFbeta -induced periostin production in fibroblasts: a potential role for vitamin D in skin sclerosis. Dermatoendocrinol. 2015;7(1):e1010983. https://doi.org/10.1080/19381980.2015.1010983.
Usategui A, Criado G, Del Rey MJ, Fare R, Pablos JL. Topical vitamin D analogue calcipotriol reduces skin fibrosis in experimental scleroderma. Arch Dermatol Res. 2014 Oct;306(8):757–61. https://doi.org/10.1007/s00403-014-1466-6.
Zerr P, Vollath S, Palumbo-Zerr K, Tomcik M, Huang J, Distler A, et al. Vitamin D receptor regulates TGF-beta signalling in systemic sclerosis. Ann Rheum Dis. 2015;74(3):e20. https://doi.org/10.1136/annrheumdis-2013-204378.
Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12(2):127–36. https://doi.org/10.1016/j.autrev.2012.07.007.
An L, Sun MH, Chen F, Li JR. Vitamin D levels in systemic sclerosis patients: a meta-analysis. Drug Des Devel Ther. 2017;11:3119–25. https://doi.org/10.2147/DDDT.S144860.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8. https://doi.org/10.1210/jc.2010-2704.
Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood). 2004;229(11):1136–42. https://doi.org/10.1177/153537020422901108.
McGrath JJ, Saha S, Burne TH, Eyles DW. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2010;121(1–2):471–7. https://doi.org/10.1016/j.jsbmb.2010.03.073.
Hewison M. An update on vitamin D and human immunity. Clin Endocrinol. 2012;76(3):315–25. https://doi.org/10.1111/j.1365-2265.2011.04261.x.
Helming L, Bose J, Ehrchen J, Schiebe S, Frahm T, Geffers R, et al. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106(13):4351–8. https://doi.org/10.1182/blood-2005-03-1029.
Li J, Chen SY, Liu HH, Yin XD, Cao LT, Xu JH, et al. Associations of vitamin D receptor single nucleotide polymorphisms with susceptibility to systemic sclerosis. Arch Med Res. 2019;50(6):368–76. https://doi.org/10.1016/j.arcmed.2019.09.006.
Boelsma E, Pavel S, Ponec M. Effects of calcitriol on fibroblasts derived from skin of scleroderma patients. Dermatology. 1995;191(3):226–33. https://doi.org/10.1159/000246550.
Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE. Pathogenesis of systemic sclerosis. Front Immunol. 2015;6:272.
Vacca A, Cormier C, Mathieu A, Kahan A, Allanore Y. Vitamin D levels and potential impact in systemic sclerosis. Clin Exp Rheumatol. 2011;29(6):1024–31.
Hollis BW. Editorial: the determination of circulating 25-hydroxyvitamin D: no easy task. J Clin Endocrinol Metab. 2004;89(7):3149–51. https://doi.org/10.1210/jc.2004-0682.
Schneider L, Dos Santos AS, Santos M, da Silva Chakr RM, Monticielo OA. Vitamin D and systemic lupus erythematosus: state of the art. Clin Rheumatol. 2014;33(8):1033–8.
Gurevitch J, Koricheva J, Nakagawa S. S Gavin. Meta-analysis and the science of research synthesis. Nature. 2018;555(7695):175–82. https://doi.org/10.1038/nature25753.
Lee YH. An overview of meta-analysis for clinicians. Korean J Intern Med. 2018;33(2):277–83. https://doi.org/10.3904/kjim.2016.195.
Acknowledgements
We would like to thank Fernanda Igansi, biologist and clinical research coordinator at the Department of Cardiology of the Hospital de Clínicas de Porto Alegre, for her assistance in reviewing and selecting the articles. We are grateful to the Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (FIPE/HCPA) for the financial support.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LS, VH, TFM and RKMB acquired the data, conceived and designed the research, and drafted the manuscript. OM, RC and NAM made a critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
search strategy and paper selection.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schneider, L., Hax, V., Monticielo, O. et al. Dualities of the vitamin D in systemic sclerosis: a systematic literature review. Adv Rheumatol 61, 34 (2021). https://doi.org/10.1186/s42358-021-00192-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-021-00192-6