Abstract
Background
Anthrax is a zoonotic disease that is still regarded as a public health issue in developing nations. This paper aims to discuss the epidemiology of anthrax in Africa, the current outbreaks in Ghana and Nigeria, clinical presentations, diagnosis, and treatment of anthrax, challenges associated with the transmission of the disease to both countries and recommendations to reduce this current outbreak and curb future outbreaks in Africa.
Main body of the abstract
Online databases (PubMed, and Google Scholar) and Nigeria Ministry of Agriculture report were used to provide detailed information on the paper. On June 1, 2023, two suspected human cases of anthrax were reported, via a letter sent to all stakeholders in the country, with one death in Binduri District, Upper East region of Ghana. The cases were due to the consumption of dead cattle. Four cattle were reported dead at the time, and eleven suspected human cases were identified through contact tracing. Afterward, on July 17, 2023, the Federal Ministry of Agriculture and Rural Development of Nigeria announced the first case of anthrax disease in Nigeria. The National Veterinary Research Institute confirmed the case from samples collected from a suspected livestock farm in Niger State, Nigeria. No human case has been reported.
Short conclusion
Anthrax poses significant challenges to public health and requires cooperation between nations, especially in regions like Ghana and Nigeria, where animal movement and ecological changes can impact disease transmission. Challenges attributed to the spread of anthrax in both countries were discussed, focusing on the role of government and the general public in addressing this public health concern. Given the endemicity of certain transboundary animal diseases such as anthrax in sub-Saharan Africa, the control of animal movement across intra- and international borders in the region needs to be tightened. Regulations governing the transboundary movement of animals should be based on the World Organisation of Animal Health Terrestrial Code and should be strictly enforced to prevent ongoing and future outbreaks in Africa.
Similar content being viewed by others
Background
Anthrax is a zoonotic disease caused by Bacillus anthracis, a Gram-positive, spore-forming, rod-shaped bacterium (Kummerfeldt 2014). Each year, between 2000 and 20,000 human cases of anthrax are reported worldwide (Amiri et al. 2021). Humans usually contract anthrax by contact with infected meat from wild animals and livestock (CDC 2022). In humans, cutaneous anthrax accounts for 95–99% of all recorded cases, while gastrointestinal and inhalation forms of anthrax are less common (Kisaakye et al. 2020).
The majority of African nations, where anthrax is endemic, report at least one human epidemic annually (Makurumidze et al. 2021). Despite having successful control programs in Botswana, Zimbabwe, and Zambia, a study from 2018 found that the disease was still endemic in at least those two nations (Asante et al. 2019). According to Yamtitina and Makarov (2019), anthrax is a disease that affects domestic animals and wildlife in several nations throughout sub-Saharan Africa. In savanna environments, where significant livestock grazing and animal husbandry are practiced, outbreaks are most common (Dudley et al. 2016).
Recently, the Federal Ministry of Agriculture and Rural Development confirmed one case of the anthrax disease in a mixed livestock farm in Niger State, Nigeria. Since the West Africa outbreak started in Ghana in June 2023, this is the first case involving an animal that has been reported in Nigeria. There were eight abrupt livestock deaths recorded at the farm on July 13, 2023. The deceased animals also appeared to be bleeding from external orifices without blood clotting. This paper aims to discuss the epidemiology of anthrax in Africa, the current outbreaks in Ghana and Nigeria, clinical presentations, diagnosis, and treatment of anthrax, challenges associated with the transmission of the disease to both countries and recommendations to reduce this current outbreak and curb future outbreaks in Africa.
Main text
Recent Anthrax outbreaks in Ghana and Nigeria
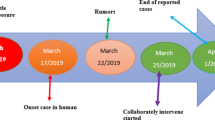
On June 1, 2023, two suspected human cases of anthrax were reported, via a letter sent to all stakeholders in the country, with one death in Binduri District, Upper East region of Ghana (NCDC 2023a). The cases were due to the consumption of dead cattle (NCDC 2023a). Four cattle were reported dead at the time (Weekly Bulletin on Outbreaks and Other Emergencies 2023). Eleven suspected human cases were identified through contact tracing (Weekly Bulletin on Outbreaks and Other Emergencies 2023).
Subsequently, on July 17, 2023, the Federal Ministry of Agriculture and Rural Development (FMARD) of Nigeria announced the first case of anthrax disease in Nigeria (NCDC 2023). After the report, the samples were collected from multiple species of animal and transported to the National Veterinary Research Institute (NVRI), Plateau State, Nigeria for laboratory confirmation of the disease, and the results were positive for anthrax. No human case has been reported (NCDC 2023). Efforts are being made to reveal the source of the infection and to curb the spread of the disease.
Anthrax status in Ghana and Nigeria
Anthrax was reported in Ghana in 1972, 1997, 2018, and 2019 (FAO 1972; WOAH 1997), before the resurgence of the disease in 2023. Nigeria has not recorded any Anthrax cases in the past. The 2023 outbreak is the first recorded case of Anthrax in Nigeria.
Epidemiology of Anthrax in Africa
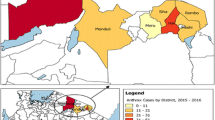
Anthrax is endemic to Africa, with numerous outbreaks across several countries, especially in sub-Saharan Africa. Here, we present reported anthrax cases in humans and animals across African countries (Table 1). The reports in the table were extracted from the scientific articles published during the period of the outbreak in each country.
Clinical presentations of Anthrax in humans and animals
There are five conventional clinical manifestations of naturally occurring anthrax: inhalational, gastrointestinal, cutaneous, injectional, and oropharyngeal (Table 2) (Savransky et al. 2020). According to Sweeney et al. (2011), the patient’s innate and specific immunity, virulence, and number of infecting bacteria all affect the severity of anthrax infection. With a fatality rate of 9–33%, injectional anthrax is a recently identified clinical form that has been observed in drug users because of injecting spore-contaminated morphine (Sweeney et al. 2011; Mariam 2023). Most Anthrax cases (> 95%) have a cutaneous origin, and the availability of good antibiotics used widely has reduced the fatality rate to 3–5% recently (Savransky et al. 2020). Occasionally occurring side effects include sepsis and meningoencephalitis brought on by the spread of the cutaneous lesion (Elbahr et al. 2022). As for the other types of clinical manifestations, Doganay et al. (2023) reported that the incidence rate of inhalation anthrax, and gastrointestinal anthrax are 12% and 5%, respectively.
Diagnosis of Anthrax in humans and animals
The following steps are taken in order to diagnose anthrax: patient history taking, clinical examination, and laboratory testing.
The WHO Guidance states that probable cases should be verified by taking appropriate samples from the patient’s lesions and then having a laboratory evaluation (Forbes et al. 2018). In cases where inhalational anthrax is suspected, these samples may comprise swabs from skin lesions, blood, sputum, pulmonary effusion, or bronchial biopsy specimens. Samples from oropharyngeal lesions, ascites fluid, feces, and vomit in probable intestinal anthrax cases, as well as cerebral fluid in suspected meningitis cases, are also included.
According to biochemical and blood characteristics, cutaneous anthrax cases often have a leukocyte count of less than 10 × 103 cells/µL (CDC 2015). Elevations of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) may be observed in cases of chronic cutaneous infections, toxic shock, systemic anthrax, leukocytosis with neutrophilia, hypoalbuminemia, and hyponatremia (Doganay et al. 2010). Disseminated intravascular coagulation (DIC), thrombocytopenia, and leukopenia may arise if severe sepsis progresses (Stearns-Kurosawa et al. et al. 2006).
According to Pillai et al. (2019), a number of easy first-line laboratory tests can be used to distinguish B. anthracis from other Bacillus species. These tests include susceptibility to penicillin, lack of motility, hemolytic activity when cultured on blood agar, and gamma phage susceptibility. The best method to detect the bacteria is using microbiological techniques, although often these methods produce confounding findings, especially when trying to distinguish the pathogen from closely related strains of Bacillus cereus. Certain B. cereus strains may also exhibit certain B. anthracis phenotypic traits. For instance, reports of hemolytic B. anthracis strains resistant to the gamma phage and penicillin have also been documented.
B. anthracis can be definitively and quickly diagnosed in clinical and environmental specimens using Real-time PCR testing and DNA amplification-based PCR (Polymerase Chain Reaction) (Zasada 2020). In the study by Wang et al. (2021), certain DNA regions of the genes pagA (pXO1), capB (pXO2), capC (pXO2), and Ba813 (chromosomal) are diagnostic targets. More recently, reports have indicated that more advanced isothermal DNA amplification methods, including HDA (helicase-dependent amplification), LAMP (loop-mediated isothermal amplification), and RPA (recombinase polymerase amplification), can be applied. Apart from DNA-based techniques, immunological methods can also be utilized to anthrax pathogens. Luminex test, MPFIA (magnetic particle fluorogenic immunoassay), FRET (Förster resonance energy transfer), ELISA (enzyme-linked immunosorbent assay), ABICAP (antibody immuno column for analytical processes) immunofiltration, and flow cytometry analysis using fluorescently labeled antibodies are a few of these.
The creation of biosensors that can identify B. anthracis quickly and precisely has received attention in recent years (Kim and Yoon 2010; Wang et al. 2021). Genosensors (nucleic acid probes), immunosensors (antibody probes), aptasensors (aptamers), and peptide-nucleic acid chimera probes (PNAs) are the four different types of biosensor systems that are currently available. They use a variety of signal production techniques, including as optical, piezoelectric, and electrochemical (amperometric, conductometric, and potentiometric). Genosensors operate on the basis that a signal is produced when pathogen-specific DNA binds to a probe. Thus far, pagA, lef, and BA813 have been the targets of developed genosensors (Yordanov and Dimitrova 2023). Additionally, B. anthracis spore simulants have been identified in a single step using impedimetric aptasensors (Mazzaracchio et al. 2019). A magnesium niobate-lead titanate/tin (PMN-PT/Sn) piezoelectric microcantilever sensor (PEMS) and an ultrasensitive portable capillary biosensor (UPAC) have both been equipped with antibody probes that identify the antigenic structure unique to B. anthracis (Zasada 2020). The pathogen has also been found in clinical and environmental specimens using matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) (Seng et al. 2013; Tsuchida et al. 2020).
Treatment of Anthrax in humans and animals
In Africa, all forms of anthrax are treated by antibiotics and antitoxin after proper conduction of antibiotic sensitivity testing. Antibiotics can also be used a prophylaxis to prevent anthrax manifestation in people who have been exposed but have not shown clinical symptoms. The major two antibiotics that are being used are ciprofloxacin and doxycycline to prevent anthrax.
Challenges associated with the spread of anthrax in Ghana and Nigeria
Anthrax is a zoonotic disease and its spread poses significant challenges to public health and requires cooperation between nations, especially in regions like Ghana and Nigeria, where animal movement and ecological changes can impact disease transmission. The following points discuss the challenges attributed to the spread of anthrax in both countries, focusing on the role of government and the general public in addressing this public health concern.
-
1.
Animal/wildlife movement: Animal migration plays a crucial role in the spread of anthrax, as infected animals can carry the bacterium to new locations. Especially in the case of Ghana and Nigeria, where the outbreak of anthrax was first reported in Ghana and the porosity of the two countries’ border. The movement of infected animals across borders poses a significant challenge in containing the spread of anthrax. Lack of coordinated surveillance and tracking mechanisms can make it difficult to identify and quarantine potentially infected animals.
-
2.
Trade of livestock and animal products: The trade of livestock that is not being regulated can facilitate the introduction of infected animals into new areas, increasing the risk of anthrax outbreaks. In these countries, the importation of animal products is large because of what the products are being used for locally such as food (Pomo), leather (for bags, belts, shoes, etc.), and other decorative house materials.
-
3.
Human travel: Human travel can contribute to the spread of anthrax, especially if infected individuals unknowingly carry the bacterium. Frequent travel between Ghana and Nigeria, whether for trade, tourism, or work, can possibly lead to the transmission of anthrax between the two countries.
-
4.
Environmental changes: Environmental changes, such as deforestation or climatic shifts can alter the distribution of anthrax in the environment, making it more challenging to predict and control outbreaks (Hugh-Jones and Blackburn 2009; Steenkamp et al. 2018). One of the transmissions of Anthrax is through spores, there is a possibility of the spores being transmitted to Nigeria through the Niger River coupled with the recent flood breakout that has been occurring in some southern and western parts of Nigeria.
-
5.
Public health: Swift and effective containment measures are essential to prevent further spread of anthrax. Limited resources and infrastructure in some regions of Ghana and Nigeria can hinder the implementation of robust containment measures. Quick identification of anthrax cases, timely reporting, and adequate isolation of infected individuals and animals are critical but may be impeded by limited healthcare facilities and reduced trained health personnel.
-
6.
International Cooperation: Collaborative efforts between Ghana and Nigeria are crucial to effectively combat the spread of anthrax. Disparities in healthcare systems and priorities between the two countries can make cooperation challenging. Effective communication and sharing of vital information between health authorities are important for early detection and coordinated responses to outbreaks. Building trust and fostering a spirit of collaboration can be hindered by political or economic factors.
Recommendation to curb the future outbreak in Ghana and Nigeria
Because of their porous land borders, Nigeria and Ghana enable unrestricted inflow of livestock, occasionally wild animals, and/or wildlife items (Bouslikhane 2015). Due to this, there is a high risk of zoonotic disease epidemics affecting both humans and animals in the two countries. Given the endemicity of certain transboundary animal diseases (TADs) such as anthrax in sub-Saharan Africa, the control of animal movement across intra- and international borders in the region needs to be tightened. Regulations governing the transboundary movement of animals should be based on the WOAH Terrestrial Code and should be strictly enforced. In order to increase surveillance and monitoring capability for TADs, veterinary quarantine services at borders should be upgraded on all fronts.
Additionally, given that zoonotic diseases make up around 75% of emerging infectious diseases (Rahman et al. 2020), Nigeria and Ghana need to take a multidisciplinary approach to cross-border animal disease surveillance and monitoring. According to the One Health framework, all institutions, disciplines, and sectors with a stake in protecting public health should work together and pool their resources to combat the threat posed by zoonoses (Markotter et al. 2023). If the One Health concept is not utilized in addressing the core causes of zoonotic spillovers at the human–animal interface, individual sectors and disciplines will simply continue in cycles (Willcox and Steele 2021).
We have reached a point where vaccination against anthrax and other vaccine-preventable TADs should be made absolutely mandatory for livestock farmers in Ghana and Nigeria. Animals that are entering the country need also receive vaccinations under the supervision of a professional. A partnership between the public and private sectors would go a long way toward assisting in the establishment of a sustainable vaccination program in both countries in order to achieve this.
Finally, it is crucial to emphasize the role that technology plays in the implementation of successful disease surveillance and monitoring programs. Real-time communication and activity tracking prompted by personnel should be done using modern technologies. This would support the accountability process and aid in reducing corruption of any form in the system.
Limitations of the study
The data provided in this write-up represent the past and current distribution of anthrax in Africa. These data were sourced from online databases. Some of the regions where the anthrax outbreaks occurred but were not reported online were excluded. However, due to the poor documentation system available in Africa, the link between the human and animal cases of anthrax was not available.
Conclusions
Based on the presence of introduction and exposure pathways that aid the transmission of TADs in Nigeria and Ghana, there is a potential link between the recent outbreaks of anthrax in both countries. These recent anthrax epidemics in Ghana and Nigeria might be a sign that the government is unable to adequately restrict the movement of animals across international borders. While livestock mobility is essential for regional trade and sustainable livelihood for the rural populace, there is a need to use a multidisciplinary approach to mitigate the health hazards that it brings to both animals and people. Government parastatals that work to protect public health should work together to create policies on transboundary animal movement and firmly enforce them.
Availability of data and materials
Not applicable.
Abbreviations
- NVRI:
-
National Veterinary Research Institute
- WOAH:
-
World Organizations for Animal Health
- TADs:
-
Transboundary Animal Diseases
- PEMS:
-
Piezoelectric Microcantilever Sensor
- UPAC:
-
Ultrasensitive Portable Capillary Biosensor
- MALDI-TOF:
-
Matrix-assisted Laser Desorption Ionization Time-of-flight
- MS:
-
Mass Spectrometry
- WHO:
-
World Health Organization
- CDC:
-
Centers for Disease Control and Prevention
- AST:
-
Aspartate Aminotransferase
- ALT:
-
Alanine Aminotransferase
- PCR:
-
Polymerase Chain Reaction
- MPFIA:
-
Magnetic Particle Fluorogenic Immunoassay
- FRET:
-
Förster Resonance Energy Transfer
- ELISA:
-
Enzyme Linked Immunosorbent Assay
- ABICAP:
-
Antibody Immuno Column for Analytical Processes
References
Amiri B, Ghaderi E, Mohamadi P, Shirzadi S, Afrasiabian S, Zand HS et al (2021) Geographical distribution of Anthrax using Geographic Information System (GIS) during 2010–2015 in Iran. Med J Islam Repub Iran 35:36
Asante J, Noreddin A, El Zowalaty ME (2019) Systematic review of important bacterial zoonoses in Africa in the last decade in light of the ‘One Health’concept. Pathogens 8(2):50
Bangura FI, Leno A, Hann K, Timire C, Nair D, Bah MA et al (2022) An update on the surveillance of livestock diseases and antimicrobial use in Sierra Leone in 2021—an operational research study. Int J Environ Res Public Health 19(9):5294
Bekker JL, Jooste PJ, Hoffman LC (2012) Wildlife-associated zoonotic diseases in some southern African countries in relation to game meat safety: a review. Onderstepoort J Vet Res 79(1):1–12
Bouslikhane M (2015) Cross border movements of animals and animal products and their relevance to the epidemiology of animal diseases in Africa. [Available at: https://www.woah.org/app/uploads/2021/03/2015-afr2-bouslikhane-a.pdf]
Centers for Disease Control and Prevention (CDC) (2015) Clinical Framework and Medical Countermeasure Use During an Anthrax Mass-Casualty Incident. [Available: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cdc.gov/mmwr/pdf/rr/rr6404.pdf] [Accessed on February 13th 2024]
Centers for Disease Control and Prevention (CDC) (2022) What is Anthrax? [Available at: https://www.cdc.gov/anthrax/basics/index.html] [Accessed on July 23rd, 2023]
Chavwanga V (2014) The Department of Veterinary Services and Control of contagious Cattle Diseases in Zambia 1907–1990 (Doctoral dissertation).
Cossaboom CM, Khaiseb S, Haufiku B, Katjiuanjo P et al (2019) Anthrax epizootic in wildlife, Bwabwata National Park, Namibia, 2017. Emerg Infect Dis 25(5):947
Doganay M, Metan G, Alp E (2010) A review of cutaneous anthrax and its outcome. J Infect Public Health 3(3):98–105
Doganay M, Dinc G, Kutmanova A, Baillie L (2023) Human anthrax: update of the diagnosis and treatment. Diagnostics 13(6):1056
Dudley JP, Hang’Ombe BM, Leendertz FH, Dorward LJ (2016) Carnivory in the common hippopotamus H ippopotamus amphibius: implications for the ecology and epidemiology of anthrax in African landscapes. Mammal Rev 46(3):191–203
de Garine-Wichatitsky M, Fritz H, Chaminuka P, Caron A et al (2017) Consequences of animals crossing the edges of transfrontier parks. In: Transfrontier conservation areas 137–162.
Elbahr US, Tekin R, Papić M, Pandak N, Erdem H, Can FK et al (2022) Factors leading to dissemination of cutaneous anthrax: an international ID-IRI study. New Microbes New Infect 48:101028
Food & Agriculture Organization (1972) Animal Health Yearbook, 1971. Rome, FAO. Italy: iv+201pp
Forbes BA, Hall GS, Miller MB, Novak SM, Rowlinson MC, Salfinger M (2018) Practical guidance for clinical microbiology laboratories: mycobacteria. Clin Microbiol Rev 31(2):10–128
Hugh-Jones M, Blackburn J (2009) The ecology of Bacillus anthracis. Mol Aspects Med 30(6):356–367
Kim J, Yoon MY (2010) Recent advances in rapid and ultrasensitive biosensors for infectious agents: lesson from Bacillus anthracis diagnostic sensors. Analyst 135(6):1182–1190
Kisaakye E, Ario AR, Bainomugisha K, Cossaboom CM et al (2020) Outbreak of anthrax associated with handling and eating meat from a cow, Uganda, 2018. Emerg Infect Dis 26(12):2799
Kummerfeldt CE (2014) Raxibacumab: potential role in the treatment of inhalational anthrax. Infection and drug resistance 101–109
Makurumidze R, Gombe NT, Magure T, Tshimanga M (2021) Investigation of an anthrax outbreak in Makoni District, Zimbabwe. BMC Public Health 21:1–10
Mariam DT (2023) Study on status, Zoonoses, Biowarfare, Economic and public health importance of anthrax. Int J Vet Sci Res 9(2):027–040
Markotter W, Mettenleiter TC, Adisasmito WB, Almuhairi S et al (2023) Prevention of zoonotic spillover: from relying on response to reducing the risk at source. [Available at: https://cdn.who.int/media/docs/default-source/one-health/ohhlep/ohhlep-prevention-of-zoonotic-spillover.pdf]
Mazzaracchio V, Neagu D, Porchetta A, Marcoccio E, Pomponi A, Faggioni G (2019) A label-free impedimetric aptasensor for the detection of Bacillus anthracis spore simulant. Biosens Bioelectron 126:640–646
Muhindo DS, Pongombo BL, Mulinda ÉB et al (2023) Verbal autopsy and outbreak investigation of anthrax in Livestock and Wildlife at the Virunga National Park Interface Area, Democratic Republic of the Congo. https://doi.org/10.21203/rs.3.rs-2697987/v1
Mukarati NL, Matope G, de Garine-Wichatitsky M et al (2020) The pattern of anthrax at the wildlife-livestock-human interface in Zimbabwe. PLoS Negl Trop Dis 14(10):e0008800
Muturi M, Gachohi J, Mwatondo A, Lekolool I et al (2018) Recurrent anthrax outbreaks in humans, livestock, and wildlife in the same locality, Kenya, 2014–2017. Am J Trop Med Hyg 99(4):833
Mwakapeje ER, Ndimuligo SA, Mosomtai G et al (2019) Ecological niche modeling as a tool for prediction of the potential geographic distribution of Bacillus anthracis spores in Tanzania. Int J Infect Dis 79:142–151
National Institute for Communicable Disease (2019) Anthrax outbreak in Lesotho and measures to prevent human exposure in South Africa, 2019. [Available at: https://www.nicd.ac.za/anthrax-outbreak-in-lesotho-and-measures-to-prevent-human-exposure-in-south-africa/] (accessed 22 July 2023).
Nigeria Centre for Disease Control and Prevention (2023) Confirmation of Anthrax Outbreak in Nigeria. [Available at: https://ncdc.gov.ng/news/491/confirmation-of-anthrax-outbreak-in-nigeria] (accessed 22 July 2023).
Omodo M, Gardela J, Namatovu A, Okurut RA (2023) Anthrax bio-surveillance of livestock in Arua District, Uganda, 2017–2018. Acta Trop 240:106841
Pillai SP, Prentice KW, Ramage JG, DePalma L, Sarwar J, Parameswaran N et al (2019) Rapid presumptive identification of Bacillus anthracis isolates using the Tetracore RedLine Alert™ test. Health Security 17(4):334–343
Rahman MT, Sobur MA, Islam MS, Ievy S, Hossain MJ et al (2020) Zoonotic Diseases: etiology, impact, and control. Microorganisms 8(9):1405
Raza A, Baleanu D, Yousaf M, Akhter N et al (2022) Modeling of anthrax disease via efficient computing techniques. Intell Autom Soft Comput 32(2):1109–1124
Savransky V, Ionin B, Reece J (2020) Current status and trends in prophylaxis and management of anthrax disease. Pathogens 9(5):370
Seng P, Abat C, Rolain JM, Colson P, Lagier JC, Gouriet F et al (2013) Identification of rare pathogenic bacteria in a clinical microbiology laboratory: impact of matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 51(7):2182–2194
Seyoum AF, Bitew AB, Negussie H (2022) A Retrospective Study on the epidemiology of anthrax among livestock from 2011 to 2020 in Awi Administrative Zone, Amhara Region, Northwest Ethiopia. Vet Med Res Rep 3(13):313–321
Stearns-Kurosawa DJ, Lupu F, Taylor FB Jr, Kinasewitz G, Kurosawa S (2006) Sepsis and pathophysiology of anthrax in a nonhuman primate model. Am J Pathol 169(2):433–444
Steenkamp PJ, van Heerden H, van Schalkwyk OL (2018) Ecological suitability modeling for anthrax in the Kruger National Park, South Africa. PLoS ONE 13(1):e0191704
Sweeney DA, Hicks CW, Cui X, Li Y, Eichacker PQ (2011) Anthrax infection. Am J Respir Crit Care Med 184(12):1333–1341
Thapa NK, Wangdi K, Dorji T, Dorjee J et al (2014) Investigation and control of anthrax outbreak at the human–animal interface, Bhutan, 2010. Emerg Infect Dis 20(9):1524
Tournier JN, Rougeaux C, Biot FV, Goossens PL (2019) Questionable efficacy of therapeutic antibodies in the treatment of anthrax. Msphere 4(3):10–1128
Tsuchida S, Umemura H, Nakayama T (2020) Current status of matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF MS) in clinical diagnostic microbiology. Molecules 25(20):4775
Wang DB, Cui MM, Li M, Zhang XE (2021) Biosensors for the Detection of Bacillus anthracis. Acc Chem Res 54(24):4451–4461
Wilcox BA, Steele JA (2021) One health and emerging zoonotic diseases. In: Kickbusch I, Ganten D, Moeti M (eds) Handbook of global health. Springer, Cham 1–49 https://doi.org/10.1007/978-3-030-05325-3_88-2
Wondmnew T, Asrade B (2023) Case-control study of human anthrax outbreak investigation in farta woreda, South Gondar Northwest Ethiopia. BMC Infect Dis 23:167. https://doi.org/10.1186/s12879-023-08136-9
World Health Organization Africa (2021) Weekly bulletin on outbreaks and other emergencies. Available at: https://www.afro.who.int/health-topics/disease-outbreaks/outbreaks-and-other-emergencies-updates?postCat=in-thenews&fwd=false%2525252525253Ffwd&page=13. Accessed 25 Apr 2024
World Health Organization Africa (2022) Weekly bulletin on outbreaks and other emergencies. Available at https://www.afro.who.int/health-topics/disease-outbreaks/outbreaks-and-other-emergencies-updates. Accessed 25 Apr 2024
World Health Organization Africa (2023) Weekly bulletin on outbreaks and other emergencies. [Available at: https://www.afro.who.int/health-topics/disease-outbreaks/outbreaks-and-other-emergencies-updates] (accessed 21 July 2023)
World Organisation for Animal Health (WOAH) Terrestrial Code. [Available at: https://www.woah.org/en/what-we-do/animal-health-and-welfare/animal-diseases/]
Yamtitina MN, Makarov VV (2019) Anthrax Global Epizootology. 1. Susceptible Animals. Veterinary Science Today. https://doi.org/10.29326/2304-196x-2018-4-27-49-52
Yordanov S, Dimitrova A (2023) Anthrax in animals-manifestation, diagnostics and prevention. Bulg J Anim Husband 60(3):37–47
Zasada AA (2020) Detection and identification of Bacillus anthracis: from conventional to molecular microbiology methods. Microorganisms 8(1):125
Acknowledgements
None declared.
Funding
No funding was available.
Author information
Authors and Affiliations
Contributions
ROA, VCO, AH, DOA, and JFA conceptualized the topic and developed the manuscript. The final manuscript was reviewed and approved by all authors before being submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adesola, R.O., Okeke, V.C., Hamzat, A. et al. Unraveling the binational outbreak of anthrax in Ghana and Nigeria: an in-depth investigation of epidemiology, clinical presentations, diagnosis, and plausible recommendations toward its eradication in Africa. Bull Natl Res Cent 48, 45 (2024). https://doi.org/10.1186/s42269-024-01203-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-024-01203-4