Abstract
Background
Defects in mismatch repair (MMR) system or microsatellite instability (MSI) and detected in colorectal carcinoma (CRC), both in sporadic and more frequently in hereditary cases. Immunohistochemistry (IHC) is the most frequent method for MMR protein deficiency screening in CRCs. In this study, we aimed to evaluate immunohistochemical expression of MMR and Ki-67 in colorectal carcinoma with clinicopathological features.
Methods
In this study, we evaluated the immunohistochemical expression of MMR proteins including MSH6, MSH2, PMS2 and MLH1 in 50 resection materials with colorectal carcinoma. Their expression is correlated with clinicopathological features of patients together, with Ki-67 protein expression in attempt to screen the most significant predictor of microsatellite instability.
Results
Of the 50 cases of cancer colon, 28% were classified as MSI-H, 20% were MSI-L, and 52% were MSS. The most frequent pattern in MSI-H tumors was concurrent loss of MSH6 and PMS2 proteins. There was a significant correlation between MMR protein expression pattern with tumor size, grade, T-classification and stage (0.015, 0.0515, 0.0162 and 0.0391), respectively. MSI-H tumors were located more frequently in right colon, early TNM stage and poorly differentiated and infrequent distant metastases. There was a significant correlation between Ki-67 high expression and MSI status patterns in their common biological aspects distinct from MSI-negative tumors.
Conclusions
Mismatch repair defective colorectal carcinoma has characteristics clinicopathological features different from MSS tumors. The role of immunohistochemistry (IHC) for MSI evaluation is the easiest and effective way for evaluation of MMR deficiency in colorectal carcinoma.
Similar content being viewed by others
Background
Colorectal carcinoma (CRC) is the third most common cancer in men, the second most frequent in women and the second cause of cancer-related deaths globally according to the World Health Organization GLOBOCAN database (Global Cancer Observatory 2023). Almost 60% of patients are present in developed countries (Siegel et al. 2023). The most cases of cancer colon are sporadic, while about 5–10% are hereditary (Ramezani et al. 2021). Multiple genes are associated with hereditary cancer colon, but gene mutations are associated with Lynch syndrome (LS), the most frequent cause of hereditary type, also known as colorectal cancer with inherited non-polyposis (Li et al. 2020). Familial adenomatous polyposis (FAP) is another form of hereditary cancer colon. Another category that shows familial cancer cluster in a family with a recognized hereditary syndrome is also referred to as familial colorectal cancer; however, its genetic basis is still unknown (Kumar et al. 2018).
Approximately 15% of colon cancer shows mismatch repair pathway (MMR) defect that leads to microsatellite instability (MSI). Pathogenesis of MSI colon cancer is caused by mismatch repair system flaw. It is important clinically to distinguish MSI cancer from MMS malignancies. This is due to the fact that MSI cancers have more favorable prognosis than MSS tumors and respond differently to chemotherapy. It is also desirable to recognize Lynch syndrome patients (Kawakami et al. 2015).
About 15–20% of random colon cancer cases and 90% of LS patients have MSI (Lachit and Vinita 2022). Four genes that have a part in correcting errors in mismatch repair or replication are MSH2, MSH6, PMS2 and MLH1. Defects in these genes lead to microsatellite instability (MSI), which may be observed in some malignancies including colon cancers, especially LS (Lynch syndrome) (Goshayeshi et al. 2018). MSI is seen in cancer of endometrium and stomach, but it may also be seen in many other types of tumors (Lorenzi et al. 2020). In Lynch syndrome, the major underlying mechanism responsible for development of the tumor with MSI is a germline mutation in one allele of an MMR gene, together with the presence of somatic mutation, which results in loss of function of corresponding non-mutant allele. However, in sporadic cases of cancer colon, tumors occur due to aberrant methylation of promoter region of MLH1 gene that leads to transcriptional suppression and also undetectable protein production (Boland and Goel 2010).
Distinct clinicopathological parameters used by pathologists include anatomical site, age, tumor-infiltrating lymphocytes, degree of histological differentiation and other clinicopathological features that are important for MSI prediction and had a correlation with MMR gene mutations (Hashmi et al. 2017).
Molecular testing using the polymerase chain reaction (PCR) could identify and assess the MSI phenotype, but several studies recognized the use of immunohistochemistry for analyzing MMR mutation genes because of its simplicity, reproducibility, being equally informative and availability nature (Hashmi et al. 2017; McCarthy et al. 2019).
Ki-67 is a nuclear protein that binds to DNA and expressed during all phases of cell cycle except resting G0 phase. It is considered a proliferative marker that has been widely used to evaluate its expression and proliferative activity of cancer cells in colorectal cancer. There has been a significant increase in number of studies that confirmed the importance of Ki-67 in cancer grading and prognosis evaluation (Luo et al. 2019). So, to obtain reliable criteria for diagnosis and predict its prognosis, quantitative measurement of Ki-67 using image analysis system is considered a helpful method.
In this study, we aimed to investigate the relation between clinicopathological characteristics, Ki-67 proliferation marker and immunohistochemical expression of MMR proteins including MSH6, MSH2, PMS2 and MLH1 in a total of 50 resected materials with colorectal adenocarcinoma.
Materials and methods
Specimens and ethical approval
In our study, 50 cases of CRC were examined. They were retrieved consecutively from archives of private laboratories. This study was approved by ethics committee of the National Research Center (approval no.16/308).
Histopathology
The histopathological characteristics were reviewed. The histopathological diagnosis was verified by two pathologists. The clinical and pathological information was retrospectively collected from records and summarized.
Immunohistochemical interpretation
Formalin-fixed, paraffin-embedded tissue sections were cut to generate sections on charged slides, including center of tumor and tumor invasion front. The slides were incubated all night at 37°C for accurate adhesion of tissue. Deparaffinization, rehydration and epitope retrieval steps in citrate buffer were followed. The staining step and incubation time were programmed in autostainer link software (Dako Omnis version). A four-antibody panel of MMR proteins, including MLH1, MSH2, MSH6, PMS2 and Ki-67, was conducted. The used primary antibodies were as follows: anti-MLH1 (DS-0301-A; mouse monoclonal primary antibody; concentrated dilution: 1:100) (Diagnostic BioSystems, 6616 Owens Drive, Pleasanton, CA), anti-MSH2 (DS-0633-B; mouse monoclonal primary antibody; concentrated dilution: 1:200) (Diagnostic BioSystems, 6616 Owens Drive, Pleasanton, CA), anti-MSH6 (DS-0307-B; mouse monoclonal primary antibody; concentrated dilution; 1:100) (Diagnostic BioSystems, 6616 Owens Drive, Pleasanton, CA) and anti-PMS2 (DS-0161-B, mouse monoclonal primary antibody; concentrated dilution: 1:100) (Diagnostic BioSystems, 6616 Owens Drive, Pleasanton, CA) and anti-Ki-67 (DS-0276-B; rabbit polyclonal primary antibody; concentrated dilution: 1:100) (Diagnostic BioSystems, 6616 Owens Drive, Pleasanton, CA). Tissue was then blocked with Ab for 1.5 h at 22 °C (Thermo Fischer Scientific). An HRP-conjugated goat anti-rabbit antibody was used as a secondary antibody. All markers are considered positive, if nuclear staining is brown in color.
According to the CAP guidelines for immunohistochemical interpretation, any nuclear staining, no matter how patchy, is seen as “no loss of expression.” Only complete absence of nuclear staining regarded as “loss of expression” is considered negative, provided that positive internal controls. Hence, when nuclear staining was lacking for at least one protein, carcinoma was termed MSI (Yuan et al. 2015a).
When revealed that two or more markers are unstable (having negative expression), MSI-H is taken into account (lost expression). If only one marker is unstable, the MSI-low is taken into account (lost expression). When there are no loss of expression and no unstable markers, MSS occurs. We adhere to the MSI definition used in the Bethesda-approved Fujiyoshi et al. study (2017).
Immunohistomorphometric quantitative analysis of Ki-67 expression
Under the Olympus CX-41 light microscope, the immunostaining was observed and captured on camera using a DP-12 Olympus digital camera (Olympus Optical Co. Ltd., Tokyo, Japan). Pathological diagnoses had been established for all specimens. At the Pathology Lab, MRCE Unit, National Research Centre, computerized morphometric analysis was carried out using Leica Qwin DW3000 image analysis system (LEICA Imaging Systems Ltd., Cambridge, England) that consists of a Leica DM-LB microscope with a JVC color video camera attached to computer system. We selected five tumor fields with the highest expression and assessed the area percentage of the positive cells at high magnification (× 200). We detected the positively stained cells, which were masked automatically by a blue mask called binary image. We used the measure field software program that automatically measures the area percentage of the detected features in the binary image and then displays the results in a table form. Both processing-related artifacts and any areas of necrosis were neglected.
Immunohistochemical analysis
The area percentage of Ki-67 positively stained cells was calculated using interactive measuring software of the system as area per field in micrometer square, area percentage and area fraction. Table with total, standard error, mean, standard deviation and minimum and maximum areas measured immediately flashed on the monitor as results. Proliferation index (PI) of Ki-67 was computed as follows: Ki-67 > 25% was considered to have a high PI, while Ki-67 25% was regarded to have a low PI (Jahan et al. 2020).
Statistical methods
Using the Windows version of SPSS 20.0, statistical analysis was carried out (New York, IBM, USA). In order to examine association between protein expression and clinicopathological indices, Fisher's exactor Pearson Chi-square tests were utilized. Using these tests, a univariate study comparing lymph node metastasis, distant metastasis and clinicopathological indices was carried out. The expression of these factors was correlated, and Spearman's correlation analysis was used to assess this. Sex, age, differentiation, location, depth of invasion, distant metastasis, lymph node metastasis and expression of Ki-67 were included as covariates. For analysis, beta coefficients and 95% confidence intervals (CI) were utilized. P value was regarded as statistically significant, when its value is ≤ 0.05.
Results
Clinicopathological features of CRC
In this study, 50 colorectal carcinoma patients were examined. Of all patients, 30 (60%) were female. The age range was 20–71 years. These age groups consisting of < 50 years 36% of patients and ≥ 50 years were 32 (64%). Based on the pathological report of surgical specimens, 36 cases had a tumor size larger than 5 cm. The site of tumor involvement in pathological report of the patients was categorized into right colon, left colon and rectum. The most common site of involvement was left colon with 40% of reported cases. Thirty patients were classified as grade II and twenty patients as grade III. Approximately 84% of the tumors tested were stages II and III counting 18 and 24, respectively (Table 1).
Correlation of clinicopathological features with MSI status patterns
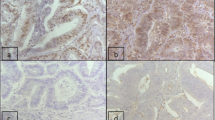
Of the 50 cases, 14 (28%,) were categorized as MSI-H (Fig. 1), 10 (20%) were MSI-L (Fig. 2) and 26 (52%) were MSS (Fig. 3), by MSI analysis. The correlations of clinicopathological parameters of CRC patients with MSI status patterns are illustrated in Table 2. Colorectal carcinomas with MSI exhibited different clinicopathological characteristics than those with MSS. The differences were statistically significant, especially for tumor size, grade, T-classification and stage as demonstrated in Table 2. When compared with MSS, MSI-H was more frequently seen in right colon, poorly differentiated, at relatively early TNM stage as well as infrequent distant metastasis. On the other hand, the clinicopathological features of MSS tumors had no significant differences in comparison with MSI-H or MSI-L in gender, tumor site, age, lymph nodes metastasis nor Duke’s classification.
Correlation between lack of MMR protein expression and MSI status patterns
We determined the proportion of tumors lacking a specific mismatch repair protein for each category of microsatellite instability (MSI-H, MSI-L or MSS) (MSH6, MSH2, MLH1 and PMS2) (Table 3). Among MSI-H tumors (Fig. 1), 8 (16%) exhibited loss of MSH2, 10 (20%) exhibited loss of MSH6, 2 (4%) exhibited loss of MLH1 and 12 (24%) exhibited loss of PMS2. Among MSI-L tumors, four (8%) exhibited loss of MSH2 (Fig. 2) and three (6%) exhibited loss of PMS2, without any loss of MSH6 or MLH1. Of total 12 tumors that showed loss of MSH2 expression, six cases were negative for PMS2 expression and four cases were negative for MSH6. Eight cases lacked expression of both MSH6 and PMS2. None of the examined 50 tumors for MLH1 showed loss of its expression except two cases (Graph 1).
Correlation between Ki-67 expression and MSI status patterns
According to the appropriate cut-off values of Ki-67 indicated above, all included patients were divided into two groups: low expression and high expression. Quantitative assessment of Ki-67 expression area was measured by image analysis system dividing them into low expressed group < 25% and high group ≥ 25% (Fig. 4). Twenty-eight cases (56%) have low expression of Ki-67 with a range of 0.193–10.478 and 22 (44%) of cases have high expression with a range of 25.351–78.504.
Among the low expression of Ki-67 tumors, 8 cases were MSI-H with the mean area percentage of expression 4.291 ± 0.59, 10 cases were MSI-L with the mean area percentage of expression 2.887 ± 0.531 and 10 cases were MSS with the mean area percentage of expression 3.711 ± 0.79. On the other hand, the high expression of Ki-67 tumors was assessed as 6 cases of MSI-H with the mean area percentage of expression 32.218 ± 1.235 and 16 cases were MSS with the mean area percentage of expression 44.545 ± 2.273. There was a negative correlation between Ki-67 low expression and all of MSI status patterns. The high expression of Ki-67 showed a positive correlation with MSI status patterns (Table 4).
Discussion
Colorectal carcinoma is considered the second most common cause of cancer-related deaths worldwide (Global Cancer Observatory 2023). Colon carcinoma caused by defects in DNA mismatch repair system commonly shows microsatellite instability, which is observed in both sporadic and more frequently inherited cases of colorectal carcinoma (Ramezani et al. 2021). PCR amplification of microsatellite genes is gold-standard approach for screening the microsatellite status; however, it is expensive, technically tricky and not predictable in routine pathology laboratories (Hashmi et al. 2017). Immunohistochemistry IHC was validated as another method for mutation screening being sensitive, available and inexpensive (Rosai and Rosai 2011). Our study aimed to assess IHC expression of MMR proteins in correlation with patients clinicopathological features and also correlation between them and Ki-67 expression.
In the present study, most patients (64%) were 50 or over 50 years with no noticeable sex dominance for CRCs. The mean age of incidence in the literature is 62 years, and it is rare to be screened under 40 years unless there are predisposing factors (Rosai and Rosai 2011). Our results complied with studies of Karahan et al. (2015) which showed that the mean age of CRC patients was 66 years and only 4.8% of cases were under 40, however, with male predominance for CRCs.
Many studies reported that rectosigmoid and proximal colon are common locations for CRCs (Yuan et al. 2015b); however, other studies showed that the tumor was mainly localized in sigmoid colon with no statistical significance (Karahan et al. 2015). In our study, the commonest site of involvement was left colon with no statistical meaning, which could be as a result of few cases.
In 15–17% of patients with colorectal carcinoma, microsatellite instability pathway is responsible for the pathogenesis (Ramezani et al. 2021; Goshayeshi et al. 2018). In both hereditary and sporadic cancer, molecular phenotype of colorectal carcinoma is tightly correlated with its biological behavior, clinicopathological features, prognosis and even patient response to treatment (Yuan et al. 2015b).
International guidelines recommended screening of MMR deficiency for all cases of colorectal carcinoma, without respect to age at diagnosis (Molecular Testing Strategies for Lynch Syndrome in People with Colorectal Cancer Diagnostics Guidance 2017). Accurate patient identification with Lynch syndrome (LS) is extremely important as it can increase survival and enhance quality of live (Stjepanovic et al. 2019; Chiaravalli et al. 2020).
In our study, 28% of cases were categorized as MSI-H, 20% were MSI-L and 52% were MSS by MSI analysis. Clinicopathological features of MSI tumors were different from MSS ones, especially for tumor size, grade, T-classification, stage and differences, which were statistically significant. Compared with MSS tumors, MSI-H cancers were more frequently located in right colon, poorly differentiated tumors, early TNM stage as well as infrequent distant metastases. On the other hand, the clinicopathological characteristics of MSS tumors had no significant differences compared to either MSI-H or MSI-L CRC in gender, age, site of the tumor, lymph nodes metastases or Duke’s classification. In study of Karahan et al. (2015) and with respect to our study, cases of CRCs that were localized in right colon did not express at least one MMR marker and had poorly differentiating carcinoma morphology. On the other hand, Soliman et al.’s study (2019) showed a significant correlation between MMR expression and lymphovascular emboli, tumor grade, N stage, T stage, tumor-infiltrating lymphocytes, signet ring component and peritumoral lesion. They also showed that right-sided location, lower grade, higher nodal stage and marked infiltrating lymphocytes were chosen as MSH-H colorectal carcinoma predictors.
The study of Garcia et al. (2022) showed significant association of MMR protein expression with right-sided colon location, poor differentiation and mucinous histology, but not with gender, age, stage, lymphocytic infiltrate or lymphovascular invasion.
Our study in accordance with that of Karahan et al. (2015) showed that none of MMR markers used had a statistically significant relationship with lymph node metastases. Also, another study of Ramezani et al. (2021) demonstrated that MSH6 had an insignificant correlation with lymph node metastases, but with better prognosis.
In our study, patients with MSI-H and MSI-L colon carcinoma have relatively less advanced stage compared to those with MSS tumors. This is similar to previous studies of Malesci et al., Ogino et al. and Yuan et al. (Karahan et al. 2015; Malesci et al. 2007; Ogino et al. 2009). These results explain relative better prognosis of MSI-correlated carcinoma compared with MSS ones.
In our current study, we investigate the expression of the four MMR proteins using IHC including MSH6, MSH2, MLH1 and PMS2. Of 50 tumors with abnormal MMR protein expression, 14 were classified as MSI-H, 10 were MSI-L and 26 were MSS by MSI analysis. Among 14 MSI-H tumors, abnormal MRR protein expression is present, with the most frequent expression pattern that was concurrent loss of MSH6 and PMS2 proteins. Among MSI-L tumors, 8% exhibited loss of MSH2 and 6% exhibited loss of PMS2 without any MSH6 or MLH1 loss. Of total 12 tumors that showed loss of MSH2 expression, six were PMS2 negative and four cases were negative for MSH6. Eight cases lacked expression of both MSH6 and PMS2, while only two cases of 50 tumors examined showed loss of MLH1 expression. In Yuan et al.’s study (2015b), concurrent loss of MLH1 and PMS2 is the most frequent. This is followed by concurrent loss of expression of MSH2 and MSH6. This study detected also that isolated MSH6 loss was present in 8.3%, followed by isolated negative expression of PMS2 (1.4%).
The results from another study demonstrated that loss of MLH1 and PMS2 expression is more common than that of MSH2 and MSH6 in colorectal carcinoma (Chunta 2019). Garcia et al. (2022) also showed loss of MMR protein expression in 8.7% of patients of caner mostly MLH1 and PMS2. These findings are consistent with the molecular properties of MMR proteins, as studies in vivo and vitro proved that MMR gene products in the cells are always present as heterodimers complex. Also, MSH2 and MLH1 are obligatory patterns, combined with their secondary patterns MSH6 and PMS2, respectively. So, if respective MMR gene is mutated and causes degeneration of the earlier patterns, the latter patterns will no longer exist (Lemos Garcia et al. 2022).
Ki-67 protein expression is associated with proliferation in tumor cells and can be used as a marker for tumor aggression. Every cell cycle's active phase (G1, S, G2 and M) contained Ki-67, whereas resting phase lacked it (G0) (Mulyawan 2019). The correlation between Ki-67 and prognosis of patients with cancer colon was still contradictory in various studies (Hayashi et al. 2015). However, other reports showed that high expression of Ki-67 was associated with poor prognosis of colon cancer patients (Luo et al. 2019). In our study, we evaluated relationship between microsatellite status and Ki-67 expression in colorectal carcinoma in order to clarify biological profile of MSI-positive tumors. Our results revealed that among low expression of Ki-67 tumors (< 25%), 8 cases were MSI-H, 10 cases were MSI-L and 10 cases were MSS, and there was a negative correlation between Ki-67 low expression and all MSI status patterns (Hampel 2018).
On the other hand, the high expression of Ki-67 (≥ 25%) was assessed in 6 cases of MSI-H and 16 cases of MSS, and the correlation is significant between Ki-67 high expression and MSI status patterns. Previous study of Takagi et al. (2022) showed that colorectal carcinoma with MSI exhibits elevated Ki-67 expression regardless of hereditary or non-familial cancer types, pointing to their shared molecular features that set them apart from MSI-negative tumors (Takagi et al. 2022). However, other study showed that MSI is not correlated with Ki-67 expression and could not improve diagnostic accuracy of colorectal carcinoma, but Ki-67 expression alone is significantly high in poorly differentiated colorectal carcinoma and correlates with the presence of metastases (Chunta 2019; Mulyawan 2019).
Conclusions
In conclusion, mismatch repair defective colorectal carcinoma has characteristics clinicopathological features different from MSS tumors. The most frequent pattern in MSI-H tumors was loss of expression of MSH6 and PMS2 proteins. This study highlights the role of immunohistochemistry in screening MMR protein status in patients with cancer colon. However, the results of our study must be supported with studies conducted in large series for detecting MMR deficiency in CRC. Accurate identification of MMR protein status is extremely important for colon cancer patients being increase survival and improve quality of life.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- MMR:
-
Mismatch repair
- MSI:
-
Microsatellite instability
- CRC:
-
Colorectal carcinoma
- MSH6:
-
MutS homolog 6
- MSH2:
-
MutS homolog 2
- PMS2:
-
Postmeiotic segregation increased 2
- MLH1:
-
MutL protein homolog 1
- MSI-L:
-
Low microsatellite instability
- MSI-H:
-
High microsatellite instability
- IHC:
-
Immunohistochemistry
- LS:
-
Lynch syndrome
- PCR:
-
Polymerase chain reaction
- PI:
-
Proliferation index
- CI:
-
Confidence intervals
References
Boland CR, Goel A (2010) Microsatellite instability in colorectal cancer. Gastroenterology 138:2073-2087.e3
Chiaravalli AM, Carnevali I, Sahnane N, Leoni E, Furlan D, Berselli M, Sessa F, Tibiletti MG (2020) Universal screening to identify Lynch syndrome: two years of experience in a Northern Italian Center. Eur J Cancer Prev 29:281–288
Chunta MA (2019) Expression and clinical significance of mismatch repair protein and Ki-67 in colorectal cancer. Chin J Gastroenterol 12:39–42
Fujiyoshi K, Yamaguchi T, Kakuta M, Takahashi A, Arai Y, Yamada M et al (2017) Predictive model for high-frequency microsatellite instability in colorectal cancer patients over 50 years of age. Cancer Med 6(6):1255–1263
Global Cancer Observatory. International Agency for Research on Cancer. World Health Organization. https://gco.iarc.fr/ (Accessed 23 Jan 2023)
Goshayeshi L, Ghaffarzadegan K, Khooei A, Esmaeilzadeh A, Rahmani Khorram M, Mosannen Mozaffari H, Kiani B, Hoseini B (2018) Prevalence and clinicopathological characteristics of mismatch repair-deficient colorectal carcinoma in early-onset cases as compared with late-onset cases: a retrospective cross-sectional study in Northeastern Iran. BMJ Open 8:e023102
Hampel H (2018) Population screening for hereditary colorectal cancer. Surg Oncol Clin N Am 27(2):319–325
Hashmi AA, Ali R, Hussain ZF, Faridi N, Khan EY, Bakar SMA et al (2017) Mismatch repair deficiency screening in colorectal carcinoma by a four-antibody immunohistochemical panel in Pakistani population and its correlation with histopathological parameters. World J Surg Oncol 15(1):4–11
Hayashi H, Beppu T, Sakamoto Y, Miyamoto Y, Yokoyama N, Higashi T, Nitta H, Hashimoto D, Chikamoto A, Baba H (2015) Prognostic value of Ki-67 expression in conversion therapy for colorectal liver-limited metastases. Am J Cancer Res 5(3):1225–1233
Jahan SI, Badruddoza SM, Asafudullah SM, Amin MN (2020) Expression of Ki-67 and its association with histological type, grade stage colorectal carcinoma. Ibrahim Card Med J 10(12):33–39
Karahan B, Argon A, Yıldırım M, Vardar E (2015) Relationship between MLH-1, MSH-2, PMS-2, MSH-6 expression and clinicopathological features in colorectal cancer. Int J Clin Exp Pathol 8(4):4044–4053
Kawakami H, Zaanan A, Sinicrope FA (2015) Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol 16(7):30
Kumar V, Abbas AK, Aster JC, Perkins JA (2018) Robbins basic pathology. In: Kumar V, Abbas AK, Aster JC, Perkins JA (Eds), 10th edition, p. 935. https://smtebooks.eu/book/7756/robbins-basic-pathology10th-edition-pdf
Lachit K, Vinita P (2022) Study of mismatch repair protein expression by using immunohistochemistry in various carcinomas with special reference to colorectal adenocarcinomas: at a tertiary referral laboratory in India. Asian Pac J Cancer Biol 7(4):341–347
Lemos Garcia J, Rosa I, Saraiva S, Marques I, Fonseca R, Lage P, Francisco I, Silva P, Filipe B, Albuquerque C et al (2022) Routine immunohistochemical analysis of mismatch repair proteins in colorectal cancer: a prospective analysis. Cancers 14:3730
Li K, Luo H, Huang L, Luo H, Zhu X (2020) Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int 20:16
Lorenzi M, Amonkar M, Zhang J, Mehta S, Liaw K-L (2020) Epidemiology of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in solid tumors: a structured literature review. J Oncol 2020:1807929
Luo ZW, Zhu MG, Luo XZ (2019) Increased expression of Ki-67 is a poor prognostic marker for colorectal cancer patients: a meta-analysis. BMC Cancer 19:1–3
Luo Z-W, Zhu M-G, Zhang Z-Q, Ye F-J, Huang W-H, Luo X-Z (2019) Increased expression of Ki-67 is a poor prognostic marker for colorectal cancer patients: a meta analysis. BMC Cancer 19:123
Malesci A, Laghi L, Bianchi P, Delconte G, Randolph A, Torri V, Carnaghi C, Doci R, Rosati R, Montorsi M, Roncalli M, Gennari L, Santoro A (2007) Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res 13:3831–3839
McCarthy AJ, Capo-Chichi JM, Spence T, Grenier S, Stockley T, Kamel-Reid S, Serra S, Sabatini P, Chetty R (2019) Heterogenous loss of mismatch repair (MMR) protein expression: a challenge for immunohistochemical interpretation and microsatellite instability (MSI) evaluation. J Pathol Clin Res. 5(2):115–129
Molecular testing strategies for lynch syndrome in people with colorectal cancer diagnostics guidance. 2017. www.nice.org.uk/guidance/dg27 Accessed 2 Jan 2022
Mulyawan I (2019) Role of Ki67 protein in colorectal cancer. Int J Res Med Sci 7(2):644–648
Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, Giovannucci EL, Fuchs CS (2009) CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut 58:90–96
Ramezani M, Aalami Aleagha Z, Almasi A, Khazaei S, Oltulu P, Sadeghi M (2021) Expression of MSH-6 immunohistochemistry marker in Colorectal cancer. World Cancer Res J 8:1989
Rosai J (2011) Gastrointestinal tract. In: Rosai J (ed) Rosai and Ackerman’s surgical pathology. Elsevier Saunders, China, pp 731–803
Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A (2023) Colorectal cancer statistics, 2023. CA Cancer J Clin 73(3):233–254
Soliman NA, Morsia DF, Helmy NA (2019) Immunohistochemical expression of MMR proteins with clinicopathological correlation in colorectal cancer in Egypt. Open Access Maced J Med Sci. 7(10):1608–1617
Stjepanovic N, Moreira L, Carneiro F, Balaguer F, Cervantes A, Balmaña J, Martinelli E, ESMO Guidelines Committee (2019) Hereditary gastrointestinal cancers: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1558–1571
Takagi S, Kumagai S, Kinouchi Y, Hiwatashi N (2022) High Ki-67 labelling index in human colorectal cancer with microsatellite instability. Anticancer Res 22(64):3241–3244
Yuan L, Chi Y, Chen W, Chen X, Wei P, Sheng W, Zhou X, Shi D (2015a) Immunohistochemistry and microsatellite instability analysis in molecular subtyping of colorectal carcinoma based on mismatch repair competency Int J. Clin Exp Med 8(11):20988–21000
Yuan L, Chi Y, Chen W, Chen X, Wei P, Sheng W, Zhou X, Shi D (2015b) Original Article Immunohistochemistry and microsatellite instability analysis in molecular subtyping of colorectal carcinoma based on mismatch repair competency. Int J Clin Exp Med 8(11):20988–21000
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NNY and MES designed the study. NNY, DMA and ASAS collected the specimen blocks, collected the data and examined the slides. NNY, MES and DMA analyzed the data. NFA and NNY wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of National Research Center (approval no.16/308).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yassen, N.N., Abouelfadl, D.M., Abbas, N.F. et al. Microsatellite instability screening in colorectal carcinoma: immunohistochemical analysis of MMR proteins in correlation with clinicopathological features and Ki-67 protein expression. Bull Natl Res Cent 47, 155 (2023). https://doi.org/10.1186/s42269-023-01132-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-023-01132-8