Abstract
Background
Preharvest and postharvest conditions of medicinal plants strongly affect the quantity and quality of their secondary metabolites. Here, the effect of different harvesting times (5 am, 12 noon and 5 pm) and different drying methods (shade at 25 °C, oven at 60 °C and microwave at 180 W) on essential oil content and components, total phenol content and antioxidant activity of rosemary was monitored.
Results
Harvesting time only had a significant effect on the essential oil content and components; however, all investigated traits were significantly affected by drying methods. The essential oil content of plants harvested at 5 am and 5 pm was higher than those harvested at 12 noon. The highest amount of essential oil was obtained from shade (0.85–1.12%) and oven (0.66–1.04%) dried plants, respectively. Total phenol and antioxidant activity of oven dried plants were significantly lower than shade and microwave-dried plants. The number, type and amount of essential oil components were affected by harvesting time and drying method. α-Pinene, verbenone and camphor were the major components of the essential oils. Oxygenated monoterpenes were the main group of essential oil compounds in all treatments. The results of biplot and cluster analysis, based on the measured traits, placed the treatments in three separate clusters; I: shade dried, II: oven-dried, and III: microwave-dried plants.
Conclusion
Generally, to obtain the higher essential oil content, phenol and antioxidant activity, harvesting at 5 am and 5 pm and also shade drying are recommended; however, for reaching to essential oil with specific quality, different harvesting time and drying method could be used.
Similar content being viewed by others
Background
Rosemary (Rosmarinus officinalis L.) is one of the most widely used medicinal herbs belonging to the Lamiaceae family. Rosemary essential oil and extract have been used in cosmetics, food and pharmaceutical industries (González-Minero et al. 2020; Nieto et al. 2018; Rašković et al. 2014). Antifungal (Özcan and Chalchat 2008), anti-inflammatory (De Melo et al. 2011), antioxidant (Borrás-Linares et al. 2014; Nieto et al. 2018), antimicrobial (Al Fadel and Al Laham 2013; Angioni et al. 2004; Mekonnen et al. 2016), antiangiogenesis (Kayashima and Matsubara 2012), anticancer (Ho et al. 1994; Petiwala et al. 2013; Tai et al. 2012; Wang et al. 2012), antidiabetic (Bakirel et al. 2008; Khalil et al. 2012), antidepressant (Machado et al. 2013), neuroprotective (Kayashima and Matsubara 2012) and antiobesity activities (Cui et al. 2012; Ibarra et al. 2011) are the most important medicinal properties of rosemary. Flavonoids, phenolic acids and terpenoids are the most important secondary metabolites of this plant (Andrade et al. 2018; Fiume et al. 2018). The most important compounds of the rosemary essential oil are α-pinene, camphor, camphene, 1,8-cineole, borneol and linalool (Bajalan et al. 2017; Borges et al. 2018; Hussain et al. 2010; Mohammed et al. 2020; Takayama et al. 2016). The amount of production and accumulation of secondary metabolites in the medicinal plants are significantly affected by instinct and extrinsic conditions (Verma et al. 2012). Genetics and environmental conditions are the main factors determining the quantity and quality of secondary metabolites in medicinal plants (Mahmoud et al. 2018; Verma et al. 2010). The quantity and quality of effective ingredients are greatly affected by organ type (Gershenzon et al. 2000), temperature, day length, relative humidity and light (Kakaraparthi et al. 2014), water and soil properties (Santos et al. 2016), harvesting time (Rustaiee et al. 2010), drying method (Sellami et al. 2011), storage conditions (Da Silva et al. 2005), and extraction method (Nikkhah et al. 2009). Harvesting time during the different seasons, developmental stages or day hours are also affected quantity and quality of the active ingredient of medicinal plant. Many studies have been done on the effect of harvesting time on quantity and quality of the active ingredient of medicinal plants such as Ocimum basilicum L. (Da Silva et al. 2005), Rosmarinus officinalis L. (Diab et al. 2002), Micromeria fruticosa L. (Dudai et al. 2001), Mentha piperita L. (Gershenzon et al. 2000), Ocimum basilicum L. (Hussain et al. 2008), Cymbopogon winterianus Jowitt. (Kakaraparthi et al. 2014), Salvia lanceolata (Kamatou et al. 2008), Melissa officinalis L. (Khalid et al. 2009), Marrubium vulgare (Mahmoud et al. 2018), Thymus vulgaris (Omidbaigi et al. 2008), Origanum onites L. (Ozkan et al. 2010), Foeniculum vulgare Mill. (Pouryousef 2015), Lippia gracilis Schauer (Santos et al. 2016), Rhodococcus erythropolis DCL14 (Van Der Werf and Boot 2000), Murraya koenigii (L.) Spreng (Verma et al. 2012) and Cinnamomum camphora (Zhang et al. 2019). Harvesting time affects the production and accumulation of secondary metabolites by affecting photosynthesis and plant growth factors (Mahmoud et al. 2018). Therefore, determining the most appropriate harvesting time can help us to achieve the highest quantity and quality of active ingredients (Verma et al. 2012). In addition to preharvest conditions, postharvest conditions also affect the content and components of the active ingredient of medicinal plants. Drying is one of the most important postharvest processes, which has a great impact on maintaining the quality of medicinal plants and their compounds (Mirhosseini et al. 2015). Reducing the volume and transportation cost (Sellami et al. 2013), reducing crop decay and increasing storage life (Prusinowska and Śmigielski 2015; Xing et al. 2017), and maintaining the quantity and quality of active ingredients (Ashafa and Pitso 2014) are the most important benefits of plant drying. Different drying methods have different effects on the appearance and phytochemical properties of the plants. Therefore, depending on the advantages and disadvantages of the drying method, plant species and its phytochemical compounds, the most appropriate drying method should be chosen (Soysal 2004; Soysal and Öztekin 2001). The effect of drying on the phytochemical properties has been studied in several medicinal plants such as Artemisia afra Jacq. (Ashafa and Pitso 2014), Thymus vulgaris L. (Calín-Sánchez et al. 2013), Thymus daenensis Celak. (Dehghani Mashkani et al. 2018), Artemisia annua L. (Ferreira and Luthria 2010), Coriandrum sativum L. (Pirbalouti et al. 2017), Salvia officinalis L. (Sellami et al. 2013), Kelussia odoratissima Mozaff. (Mirhosseini et al. 2015), Origanum vulgare L. (Ozdemir et al. 2018), Laurus nobilis L. (Sellami et al. 2011), Petroselinum crispum Mill. (Soysal 2004), Coriandrum sativum L. (Soysal and Öztekin 2001), Rosmarinus officinalis (Szumny et al. 2010) and Perilla frutescens (Xing et al. 2017). The results of these studies indicate the remarkable effects of drying on the content and components of the secondary metabolites. The study aimed to investigate the effect of different harvesting times and drying methods on essential oil content and components as well as the total phenol content and antioxidant activity of rosemary extract for obtaining the best harvesting time and drying method.
Methods
Plant material preparation
This experiment was conducted during 2018 at the University of Kurdistan (Sanandaj, Iran). In this experiment, stem cuttings of rosemary (10 cm in length) were prepared from maternal plant and then planted in the plastic pots containing soli and sand (ratio 1:1) in the greenhouse conditions with 60% relative humidity and 25 °C mean temperature. Then, three-month-old seedlings were transplanted to the main field in early spring and planted at intervals 50 cm between rows and 30 cm above the rows. The experimental design was performed as a randomized complete block. The field was located in Sanandaj city (Iran) with geographical coordinates as follows: latitude 35° 15′ 28" N, longitude 47° 01′ 29" E, and elevation 1411 m. Physical and chemical properties of field soil are shown in Table 1. Aerial parts of plants were harvested at full flowering stage in different times including 5 am, 12 noon and 5 pm and then dried with three different methods shade at 25 °C, oven at 60 °C and microwave at 180 W until their humidity reached to 10% based on wet weight.
Essential oil extraction
The essential oil was extracted by the water distillation method using a Clevenger-type apparatus. For this purpose, to 50 g of plant sample, 150 mL of distilled water was added and heated for 3 h. After the mentioned time, the volume of extracted essential oil was recorded and the essential oil content (v/w %) was calculated. The essential oils were dried over anhydrous sodium sulfate and then sealed in a glass vials and refrigerated at 4 °C until analysis.
Essential oil analysis
To determine the type and percent of essential oil constituents, essential oils were analyzed using GC-FID and GC-MS apparatus. The specifications of GC-FID and GC-MS and their working conditions were as follows:
Gas chromatography with flame ionization detection (GC-FID)
Gas chromatography was performed with an Agilent technology apparatus (Agilent 7990B, USA), equipped to a flame ionization detector with 30 m capillary column, 0.25 mm internal diameter and 0.5 µm film thicknesses. Helium was used as carrier gas with flow rate of 1 mL/min. The oven temperature was held at 60 °C for 5 min and then raised to 240 °C with rate 3 °C/min and kept at 240 °C for 10 min. The injector and detector temperatures were 230 and 250 °C, respectively.
Gas chromatography mass spectrometry (GC-MS)
For identification of essential oil components, an Agilent gas chromatograph equipped with mass spectrometer (5977A, USA) (HP-5 column, 30 m × 0.25 mm, film thickness 0.1 µm) was used. Carrier gas was Helium with the flow rate of 1 mL/min. The oven temperature program was the same as GC. Injector temperature was set at 260 °C, ionization voltage was 70 eV and the spectral scanning range was 40–400 m/z.
Identification of essential oil components
The components of essential oil were identified by comparing their retention indices to published literature, digital library of system and also with spectra mass of standard compounds. The quantity of components was obtained by calculating the areas under the curve in chromatograms.
Extraction
Hot continues extraction method using Soxhlet apparatus was performed for extraction. Methanol (99%, Merck) was used as the solvent. After 3 h, the obtained extracts (about 5 g) were condensed by rotary evaporator and finally dried in the oven at 60 °C.
Assessment of total phenol
Folin–Ciocalteu method was used for the identification of the total phenol content of extracts. Briefly, 1 mg dry extract was mixed with 1 mL methanol, and then, 100 µL of this solution was mixed with 100 µL Folin–Ciocalteu (10%), 200 µL sodium carbonate (7.5%) and 1.6 mL distilled water. The obtained mixture was allowed for incubation at room temperature (25 °C) and dark condition for 1 h, and then, absorbance was measured at 760 nm by spectrophotometer (S2100 suv New Jersey). Gallic acid with different concentrations (50, 100, 150, 200 and 250 ppm) was used as standard. Finally, the total phenol content of extracts was expressed as mg of gallic acid per g dry extract (Morshedloo et al. 2012).
Assessment of antioxidant activity
The antioxidant activity of the dried extracts was determined based on the scavenging activities of the DPPH free radicals. Briefly, 1 mg of dry extract was mixed with 1 mL methanol, and then, 50 µL of this solution mixed with 950 µL methanol solution of DPPH (0.2 mmol) and incubated in room temperature and dark condition for 30 min. After the mentioned time, the absorbance was read at 517 nm by spectrophotometer, and the inhibition percentage of DPPH is calculated using Eq. 1 (Khorshidi et al. 2020).
AOA is antioxidant activity; Ab is the absorbance of the blank reaction (containing DPPH and methanol); and As is the absorbance of the sample reaction (containing DPPH, methanol and plant extract).
Finally, the antioxidant activity of the extracts was calculated based on IC50.
Statistical analysis
Data analyzed using SPSS software (ver.21) and Duncan’s multiple range test at probability level 5% were used to compare the mean of data. SPSS, GraphPad Prism (ver.8) and Statgraphics (ver.19) software were used to cluster analysis and draw the heat map and biplot, respectively.
Results
Essential oil content and components
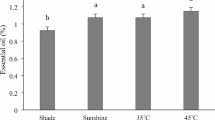
Harvesting time had a significant effect on the essential oil content of plants, so that the essential oil content of plants harvested at 5 am and 5 pm was significantly higher than plants harvested at 12 noon. At all harvest times, shade dried plants had the highest essential oil content, while the lowest content was obtained in microwave-dried plants. Generally, the highest essential oil content (1.12%) was obtained at 5 am harvest and shade dried plants, followed by 5 pm harvest and shade dried (1.08%), and the lowest essential oil content (0.58%) was obtained at 12 noon harvest and microwave-dried plants (Fig. 1). The number, type and total of identified compounds were varied in the essential oils of plants harvested at different times and drying methods (Table 2). The highest number of identified compounds (27 compounds) was observed in the essential oils of plants harvested at 12 noon and dried in the shade, as well as plants harvested at 5 pm and dried in the oven. The highest total of identified compounds was belonged to the plants harvested at 5 am and dried in the shade (84.57%), while the lowest compounds were in the plants harvested at 12 noon and dried in the oven (78.76%). The dominant components of essential oil in all treatments (except plants harvested at 12 noon and dried in the microwave) were α-pinene, verbenone and camphor, although the amount of these compounds varied in different treatments. In the essential oils of plants harvested at 12 noon and microwave-dried, the main constituents of the essential oil were α-pinene, borneol and camphor. Plants harvested at 5 am and dried in the oven had the highest α-pinene content (21.64%), while plants harvested at 12 noon and dried in the shade had the lowest α-pinene content (12.09%). Oxygenated monoterpenes followed by monoterpene hydrocarbons were the main classes of essential oil compounds in all treatments. The highest amount of oxygenated monoterpenes (52.95%) was identified in the essential oils of plants harvested at 12 noon and dried in the shade, and the highest amount of monoterpene hydrocarbons (34.83%) was observed in the essential oils of plants harvested at 5 pm and dried in the oven. Generally, the essential oils of plants dried in the oven were poorer in viewpoint of oxygenated monoterpenes but richer in monoterpene hydrocarbons than the essential oils of plants dried in shade and microwave. The essential oils of dried plants in the microwave were richer in the viewpoint of sesquiterpenes (oxygenated sesquiterpenes and sesquiterpene hydrocarbons) than the essential oils of dried plants in shade and oven. Harvested plants at 12 noon had a higher content of sesquiterpenes than harvested plants at 5 am and 5 pm.
Essential oil content (A), total phenol of extract (B), antioxidant activity of extract (C) and drying time (D) of Rosmarinus officinalis as affected by different harvesting times (5 am, 12 noon and 5 pm), and drying methods (shade, oven and microwave). Means with the same letter are not significantly different at p < 0.05 (Duncan’s test)
Total phenol content and antioxidant activity
Harvesting time did not affect the total phenol content, but the drying method had a significant effect on total phenol, so that shade and microwave-dried plants had more phenol compared to oven dried plants. The highest total phenol (215.2 mg GA/g dry extract) was observed in plants harvested at 12 noon and shade dried, although there were no significant differences with other shade and microwave plants. The lowest total phenol (111.2 mg GA/g dry extract) was belonged to the plants harvested at 5 am and oven-dried, although there was no significant difference with other oven-dried plants (Fig. 1). The antioxidant activity of the plant extracts varied from 82.48 µg/ml in the plants harvested at 5am and dried in the oven to 31.85 µg/mL in plants harvested at 12 noon and dried in the shade. Harvesting time had no significant effect on the antioxidant activity of plant extracts, but the antioxidant activity of the extracts was significantly affected by the drying method, so that the lowest antioxidant activity (maximum IC50) was observed in oven-dried samples. The extracts of dried plants in the shade and microwave were not significantly different in the viewpoint of antioxidant activity (Fig. 1).
Drying time
Harvesting time had no significant effect on drying time, but the drying time was significantly affected by the drying method. The shortest drying time was belonged to the microwave method, followed by oven and shade drying. The time required to dry the plants varied from 0.34 h (plants harvested at 5 am and microwave-dried) to 165.3 h (plants harvested at 12 noon and shade-dried) (Fig. 1).
Correlation between traits, biplot and cluster analysis
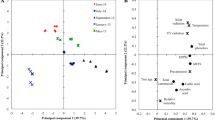
The strong positive correlation was observed between essential oil content with drying time and monoterpene hydrocarbons (r = 0.63 and r = 0.79, respectively). Plants with higher essential oil content were poorer in the viewpoint of oxygenated monoterpenes, sesquiterpene hydrocarbons and oxygenated sesquiterpenes (r = − 0.65, r = − 0.84 and r = − 0.71, respectively). Plants with higher phenol content had lower IC50 (higher antioxidant activity) (r = − 0.96). Essential oils with higher monoterpene hydrocarbons had lower oxygenated monoterpenes, sesquiterpene hydrocarbons and oxygenated sesquiterpenes (r = − 0.97, r = − 0.54 and r = − 0.68, respectively). The strong positive correlation was observed between oxygenated sesquiterpenes with oxygenated monoterpenes (r = 0.58) and sesquiterpene hydrocarbons (r = 0.89). Also, the negative correlation between drying time with sesquiterpene hydrocarbons (r = − 0.70) and antioxidant activity (r = − 0.56) was the other remarkable correlations (Fig. 2).
Based on the biplot and cluster analysis results, plants harvested at different times and dried in the shade due to similarity in the time required for drying, total phenol and essential oil content were placed in the same cluster. Plants harvested at different times and dried in the microwave were similar in the viewpoint of the oxygenated monoterpenes, oxygenated sesquiterpenes and sesquiterpene hydrocarbons content and were placed in the other cluster. Finally, the plants harvested at different times and dried in the oven were placed in the same cluster due to their high similarity in the viewpoint of monoterpene hydrocarbons content and antioxidant activity (Figs. 3 and 4).
Discussion
Essential oils are volatile compounds, which evaporate from the plant at high temperatures (Pouryousef 2015). Therefore, to achieve the maximum amount of essential oil, it is better to harvest them at times of the day when the air temperature is lower. Daily temperature affects the production and accumulation of essential oils by affecting photosynthesis, respiration and the activity of enzymes related to the production of essential oils (Burbott and Loomis 1969). Ayanoglu et al. (2005) evaluated the effect of harvesting times (6 am, 12 noon and 7 pm) on the essential oil content of Melissa officinalis and concluded that the highest essential oil content was belonged to the plants harvested at 6 am. In another study, the amount of Melissa officinalis essential oil was evaluated at different times of the day (8, 9, 10, 11 and 12 am and 1, 2, 3, 4 and 5 pm), and the results indicated that the plants harvested at 5 pm had the highest amount of essential oil (Khalid et al. 2009). In contrast, in Ocimum basilicum, the essential oil content of plants harvested at different times of the day was not significantly different (Da Silva et al. 2005). A negative correlation between essential oil content and relative humidity has been reported. So that with increasing relative humidity, the amount of essential oil has decreased, and with decreasing relative humidity, the amount of essential oil has increased (Ayanoglu et al.2005). The effect of harvesting time on the essential oil content also depends on the plant species. In some plant species, the secretory structures are deep and their essential oils evaporate only under high temperatures, but in some other species, the secretory structures are superficial and their essential oils evaporate and leave the plant even at low temperatures (Gang et al. 2001). It seems that the lower essential oil content of the plants harvested at 12 noon compared with 5 am and 5 pm is due to higher temperatures, which caused the essential oils to evaporate from the plant. Drying, depending on the method, applied temperature, drying time and plant species, could affect the essential oil content (Mirhosseini et al. 2015). In general, increasing the temperature and time of drying will increase the essential oil evaporation and finally the obtained essential oil will decrease (Morshedloo et al. 2021). At high temperatures, the secretory structures of the essential oil rupture and the essential oil evaporate (Sellami et al. 2011). In the shade drying, although the drying time increased due to lower temperature, less essential oil evaporated from the plant, and therefore, the dried plants under those conditions had the highest amount of essential oil. In contrast, although drying in microwave was very quick, but due to exposure to high temperatures, a large amount of essential oil evaporated and after drying the lowest amount of essential oil was obtained (Fig. 1). These results are in agreement with other findings on Coriandrum sativum L. (Pirbalouti et al. 2017), Kelussia odoratissima (Mirhosseini et al. 2015), Mentha longifolia L. (Saeidi et al. 2016) and Artemisia afra Jacq. (Ashafa and Pitso2014) and in contrast to findings on Laurus nobilis (Sellami et al. 2011), which may be due to differences in plant species, temperatures applied and the type of secretory structures. Drying time and applied temperature can lead to the loss of some components and the formation of novel constituents in essential oil (Sellami et al. 2011). The dominant component of rosemary essential oil usually is α-pinene, although their amount varies depending on the growing and processing conditions (Flamini et al. 2002; Szumny et al. 2010). Preharvest and postharvest conditions affect the type and amount of essential oil components. Processes such as decomposition, conversion, hydration and oxidation cause changes in the type and amount of essential oil components (Ashafa and Pitso 2014). The relative content of essential oil constituents is strongly influenced by the drying method (Mirhosseini et al. 2015). α-Pinene is one of the most important components of rosemary essential oil that has many applications in various industries, so its high content indicates the high quality of the essential oil (Risner et al. 2020). Monoterpenes, followed by alcohols and sesquiterpenes, are the main constituents of rosemary essential oil (Szumny et al. 2010). Changes in the essential oil components during the drying process can occur due to oxidation, hydrolysis or esterification of compounds (Pirbalouti et al. 2013). The high temperature of microwave can lead to the vaporization or conversion of some monoterpenes to sesquiterpenes (Ashafa and Pitso 2014). Microwave drying is faster than shade and oven-drying, so sesquiterpenes will not have enough time to evaporate and leave the plant due to their higher molecular weight and boiling point compared with the monoterpenes. Therefore, microwave-dried plants will have more sesquiterpenes than shade- and oven-dried plants. Some essential oil compounds, such as sesquiterpenes, require longer time to evaporate and release from the plant due to their lower volatility than monoterpenes (Díaz-Maroto et al. 2003); therefore, their amount in plants dried with faster methods such as microwave will be more than plants dried with other slow methods. An increase in the ratio of sesquiterpenes to monoterpenes due to the increase in drying temperature has been reported in Artemisia annua (Khangholil and Rezaeinodehi 2008). Harvesting at noon raise the ratio of sesquiterpenes to monoterpenes due to easy evaporation of lower molecular weight of monoterpenes. Changes in the type and amount of essential oil constituents can be influenced by genetical or environmental conditions such as temperature, light intensity and day length (Verma et al. 2010). Diurnal variation in the essential oil components of Melissa officinalis has been reported (Ayanoglu et al. 2005). The diurnal temperature has an important effect on the essential oil components (Kakaraparthi et al. 2014; Prakasa Rao et al. 1995). In contrast to our results, the lowest ratio of sesquiterpenes to monoterpenes in Melissa officinalis essential oil was obtained from plants harvested at 5 pm (Khalid et al. 2009); these opposite findings may be due to differences in growth conditions, plant species and secretory structures.
Changes in photoperiod, light intensity and temperature strongly affect the amount of phenolic compounds in the plant (Yao et al. 2005). Phenolic compounds were produced in higher concentrations in seasons with less difference between the duration of day and night (Yang and Seib 2000). Other factors such as carbohydrates, nutrients, water quality, developmental stage and stress can also affect the amount of phenolic compounds in the plant (Brasileiro et al. 2015; Mhamdi et al. 2010). Under stress conditions, the plant accumulates phenolic compounds to prepare itself for the lignification process (Souhila et al. 2019). Several studies showed that changes in total phenol content in a long time are noticeable, but its diurnal variations not significant (Brasileiro et al. 2015; Mhamdi et al. 2010; Ozkan et al. 2010; Souhila et al. 2019; Yao et al. 2005). Decreases or increases in the amount of phenolic compounds due to increase in drying temperatures have been reported in previous studies (Chan et al. 2009; Sellami et al. 2013; Kammoun Bejar et al. 2011; López et al. 2010; Özcan et al. 2020; Sathishkumar et al. 2009). Previous study attributed the increase in total phenol at low temperatures due to the reduction in the degradation of phenolic compounds (Lim and Murtijaya 2007). Other finding showed that low temperature decreased the total phenolic compounds and returned this effect to the increase in time required for biochemical reactions that cause the degradation of these compounds (Xing et al. 2017). Meanwhile, the reduction in phenolic compounds at high temperatures can be attributed to the decomposition or degradation of these compounds (Al-Farsi et al. 2007; Besbes et al. 2004; Rakić et al. 2007). On the other hand, high temperature may increase vapor pressure and temperature inside plant tissue, resulting in the destruction of cell wall. Consequently, the cell wall phenolic compounds can be released and causing more total phenol to be extracted (Inchuen et al. 2010). In addition, the increase in total phenol at relatively high temperatures can be attributed to the inactivation of enzymes that breakdown phenolic compounds (Ghasemnezhad et al. 2013). The differences between these results might be related to variation in plant species, drying method and temperature, type of phenolic compounds, extraction and analysis method.
The low antioxidant activity of oven-dried plants may be due to their low phenol content. The high temperature of the oven causes the destruction of phenolic compounds and eventually can reduce the antioxidant activity (Dorman et al. 2004; Wojdylo et al. 2007). Phenolic compounds are one of the most important compounds that scavenge free radicals or in other words increase the antioxidant activity (Prior and Cao 2000). A positive correlation between the amount of phenolic compounds and the antioxidant activity of plants has been reported in numerous studies (Abdille et al. 2005; Brasileiro et al. 2015; Ozkan et al. 2010). In contrast to our result, it has been reported that antioxidant activity does not have a significant correlation with phenol content, but depends on the nature of the compounds (Mhamdi et al. 2010).
In the microwave-drying method, the energy of microwave is absorbed by water molecules located inside the plant tissue. This energy causes the water molecules to vibrate, and as a result, the temperature rises inside the plant, and eventually, the water evaporates. But in other drying methods, due to difference between the temperature of surface and interior of plant samples, the heat gradually transferred from surface to the inside the plant. Therefore, the different heat transfer mechanism in the microwave method compared with other methods is the reason for the faster drying of plants in this method (Çelen 2019; Szumny et al. 2010). The low temperature of the shade method (25 °C) compared with other methods reduced the rate of water evaporation from the plant and finally increased the time required for drying. In methods that require a short time to dry the plants, less energy is used, which is economically important (Soysal and Öztekin 2001), and also less undesirable changes occur in appearance, quality and secondary metabolites of plants (Dehghani Mashkani et al. 2018). In most studies, the minimum time required for drying plant samples was reported by the microwave method (Khorramdel et al. 2013; Ebadi et al. 2013, 2011).
Conclusions
According to the results, only essential oil content and components were affected significantly by harvesting time, but the effect of drying method was significant on all measured traits. To achieve more essential oil, harvest at 5 am and 5 pm is recommended, but to achieve a specific quality of essential oil based on the type and amount of essential oil components, the appropriate harvest time can be different. Shade-dried plants had higher quality in the viewpoint of essential oil content, total phenol and antioxidant activity, but as with harvest time, if the purpose is to obtain the essential oil with specific quality, the proper drying method can be different.
Availability of data and materials
Data are available from the corresponding author.
Abbreviations
- GC-FID:
-
Gas chromatography with flame ionization detector
- GC-MS:
-
Gas chromatography mass spectrometry
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- AOA:
-
Antioxidant activity
- GA:
-
Gallic acid
- IC50:
-
The concentration of extract needed to scavenge 50% of DPPH
- RT:
-
Retention time
- RI:
-
Retention indices
- T1:
-
Harvest at 5 am and shade drying
- T2:
-
Harvest at 5 am and oven drying
- T3:
-
Harvest at 5 am and microwave drying
- T4:
-
Harvest at 12 noon and shade drying
- T5:
-
Harvest at 12 noon and oven drying
- T6:
-
Harvest at 12 noon and microwave drying
- T7:
-
Harvest at 5 pm and shade drying
- T8:
-
Harvest at 5 pm and oven drying
- T9:
-
Harvest at 5 pm and microwave drying
- EO:
-
Essential oil
- DT:
-
Drying time
- TPh:
-
Total phenol
- OM:
-
Oxygenated monoterpenes
- OS:
-
Oxygenated sesquiterpenes
- SH:
-
Sesquiterpene hydrocarbons
- MH:
-
Monoterpene hydrocarbons
References
Abdille MH, Singh RP, Jayaprakasha GK, Jena BS (2005) Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem 90:891–896
Al Fadel FM, Al Laham SA (2013) Antibacterial efficacy of variety plants against the resistant streptococcus which cause clinical mastitis in cows. Asian J Pharm Res Health Care 5:32–41
Al-Farsi M, Alasalvar C, Al-Abid M, Al-Shoaily K, Al-Amry M, Al-Rawahy F (2007) Compositional and functional characteristics of dates, syrups, and their by-products. Food Chem 104:943–947
Andrade JM, Faustino C, Garcia C, Ladeiras D, Reis CP, Rijo P (2018) Rosmarinus officinalis L.: an update review of its phytochemistry and biological activity. Futur Sci OA 4(4):FSO283
Angioni A, Barra A, Cereti E, Barile D, Coïsson JD, Arlorio M, Dessi S, Coroneo V, Cabras P (2004) Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J Agric Food Chem 52:3530–3535
Ashafa AOT, Pitso TR (2014) Effects of microwave drying on the yield and chemical composition of essential oils of Artemisia afra Jacq. Ex willd from the eastern free state South Africa. J Essent Oil-Bear Plants 17:1087–1093
Ayanoglu F, Arslan M, Hatay A (2005) Effects of harvesting stages, harvesting hours and drying methods on essential oil content of lemon balm grown in eastern mediterranean. Int J Bot 1:138–142
Bajalan I, Rouzbahani R, Pirbalouti AG, Maggi F (2017) Antioxidant and antibacterial activities of the essential oils obtained from seven Iranian populations of Rosmarinus officinalis. Ind Crops Prod 107:305–311
Bakirel T, Bakirel U, Keleş OÜ, Ülgen SG, Yardibi H (2008) In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J Ethnopharmacol 116:64–73
Besbes S, Blecker C, Deroanne C, Bahloul N, Lognay G, Drira NE, Attia H (2004) Date seed oil: Phenolic, tocopherol and sterol profiles. J Food Lipids 11:251–265
Borges RS, Keita H, Ortiz BLS, dos Santos Sampaio TI, Ferreira IM, Lima ES, da Silva MDJA, Fernandes CP, Oliveira AEMDFM, da Conceição EC, Rodrigues ABL, Filho ACMP, Castro AN, Carvalho JCT (2018) Anti-inflammatory activity of nanoemulsions of essential oil from Rosmarinus officinalis L.: in vitro and in zebrafish studies. Inflammopharmacology 26:1057–1080
Borrás-Linares I, Stojanović Z, Quirantes-Piné R, Arráez-Román D, Švarc-Gajić J, Fernández-Gutiérrez A, Segura-Carretero A (2014) Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int J Mol Sci 15:20585–20606
Brasileiro BG, Leite JPV, Casali VWD, Pizziolo VR, Coelho OGL (2015) Efeito da época de plantio e colheita sobre o teor de fenólicos totais e atividade antioxidante de Talinum triangulare (jacq.) willd. Acta Sci Agron 37:249–255
Burbott AJ, Loomis WD (1969) Evidence for metabolic turnover of monoterpenes in peppermint. Plant Physiol 44:173–179
Calín-Sánchez Á, Figiel A, Lech K, Szumny A, Carbonell-Barrachina ÁA (2013) Effects of drying methods on the composition of thyme (Thymus vulgaris L.) essential oil. Dry Technol 31:224–235
Çelen S (2019) Effect of microwave drying on the drying characteristics, color, microstructure, and thermal properties of Trabzon persimmon. Foods 8:7–9
Chan EWC, Lim YY, Wong SK, Lim KK, Tan SP, Lianto FS, Yong MY (2009) Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem 113:166–172
Cui L, Kim MO, Seo JH, Kim IS, Kim NY, Lee SH, Park J, Kim J, Lee HS (2012) Abietane diterpenoids of Rosmarinus officinalis and their diacylglycerol acyltransferase-inhibitory activity. Food Chem 132:1775–1780
Da Silva F, Silva Santos RH, De Andrade NJ, Almeida Barbosa LC, Dias Casali VW, De Lima RR, Passarinho RVDM (2005) Basil conservation affected by cropping season, harvest time and storage period. Pesqui Agropecu Bras 40:323–328
De Melo GAN, Grespan R, Fonseca JP, Farinha TO, Silva EL, Romero AL, Bersani-Amado CA, Cuman RKN (2011) Rosmarinus officinalis L. essential oil inhibits in vivo and in vitro leukocyte migration. J Med Food 14:944–949
Dehghani Mashkani MR, Larijani K, Mehrafarin A, Naghdi Badi H (2018) Changes in the essential oil content and composition of Thymus daenensis Celak. under different drying methods. Ind Crops Prod 112:389–395
Diab Y, Auezova L, Chebib H, Chalchat JC, Figueredo G (2002) Chemical composition of Lebanese rosemary (Rosmarinus officinalis L.) essential oil as a function of the geographical region and the harvest time. J Essent Oil Res 14:449–452
Díaz-Maroto MC, Soledad Pérez-Coello M, González Viñas MA, Dolores Cabezudo M (2003) Influence of drying on the flavor quality of spearmint (Mentha spicata L.). J Agric Food Chem 51:1265–1269
Dorman HJD, Bachmayer O, Kosar M, Hiltunen R (2004) Antioxidant properties of aqueous extracts from selected lamiaceae species grown in Turkey. J Agric Food Chem 52:762–770
Dudai N, Larkov O, Ravid U, Putievsky E, Lewinsohn E (2001) Developmental control of monoterpene content and composition in Micromeria fruticosa (L.). Druce Ann Bot 88:349–354
Ebadi MT, Rahmati M, Azizi M, Hassanzadeh Khayyat M (2011) Effects of different drying methods (natural method, oven and microwave) on drying time, essential oil content and composition of Savory (Satureja hortensis L.). Iran J Med Aromat Plants 26:477–489
Ebadi MT, Rahmati M, Azizi M, Hassanzadeh Khayyat M, Dadkhah A (2013) The effects of different drying methods on drying time, essential oil content and composition of basil (Ocimum basilicum L.) M.T. Iran J Med Aromat Plants 29:425–437
Ferreira JFS, Luthria DL (2010) Drying affects artemisinin, dihydroartemisinic acid, artemisinic acid, and the antioxidant capacity of Artemisia annua L. leaves. J Agric Food Chem 58:1691–1698
Fiume MM, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG, Shank RC, Slaga TJ, Snyder PW, Gill LJ, Heldreth B (2018) Safety assessment of Rosmarinus officinalis (rosemary)-derived ingredients as used in cosmetics. Int J Toxicol 37:12–50
Flamini G, Cioni PL, Morelli I, Macchia M, Ceccarini L (2002) Main agronomic-productive characteristics of two ecotypes of Rosmarinus officinalis L. and chemical composition of their essential oils. J Agric Food Chem 50:3512–3517
Gang DR, Wang J, Dudareva N, Nam KH, Simon JE, Lewinsohn E, Pichersky E (2001) An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol 125:539–555
Gershenzon J, McConkey ME, Croteau RB (2000) Regulation of monoterpene accumulation in leaves of peppermint. Plant Physiol 122:205–213
Ghasemnezhad A, Bagherifard A, Asghari A (2013) Study on the effect of drying temperature on some phytochemical characteristics of Artichoke (Cynara scolymus L.) leaves. Eco-Phytochem J Med Plants 1:10–21
González-Minero FJ, Bravo-Díaz L, Ayala-Gómez A (2020) Rosmarinus officinalis L. (rosemary): an ancient plant with uses in personal healthcare and cosmetics. Cosmetics 7:1–17
Ho CT, Ferraro T, Chen Q, Rosen RT, Huang MT (1994) Phytochemicals in teas and rosemary and their cancer-preventive properties. American Chemical Society, Washington
Hussain AI, Anwar F, Hussain Sherazi ST, Przybylski R (2008) Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem 108:986–995
Hussain AI, Anwar F, Chatha SAS, Jabbar A, Mahboob S, Nigam PS (2010) Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Brazilian J Microbiol 41:1070–1078
Ibarra A, Cases J, Roller M, Chiralt-Boix A, Coussaert A, Ripoll C (2011) Carnosic acid-rich rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and improves cholesterol levels and glycaemia in mice on a high-fat diet. Br J Nutr 106:1182–1189
Inchuen S, Narkrugsa W, Pornchaloempong P (2010) Effect of drying methods on chemical composition, color and antioxidant properties of Thai red curry powder. Kasetsart J Nat Sci 44:142–151
Kakaraparthi PS, Srinivas KVNS, Kumar JK, Kumar AN, Rajput DK, Sarma VUM (2014) Variation in the essential oil content and composition of citronella (Cymbopogon winterianus Jowitt.) in relation to time of harvest and weather conditions. Ind Crops Prod 61:240–248
Kamatou GPP, Van Zyl RL, Van Vuuren SF, Figueiredo AC, Barroso JG, Pedro LG, Viljoen AM (2008) Seasonal variation in essential oil composition, oil toxicity and the biological activity of solvent extracts of three South African Salvia species. S Afr J Bot 74:230–237
Kammoun Bejar A, Kechaou N, Mihoubi NB (2011) Effect of microwave treatment on physical and functional properties of orange (Citrus sinensis) peel and leaves. J Food Process Technol 2:1–7
Kayashima T, Matsubara K (2012) Antiangiogenic effect of carnosic acid and carnosol, neuroprotective compounds in rosemary leaves. Biosci Biotechnol Biochem 76:115–119
Khalid KA, Hu W, Cai W, Hussien S (2009) Influence of cutting and harvest day time on the essential oils of lemon balm (Melissa officinalis L.). J Essent Oil-Bear Plants 12:348–357
Khalil OA, Ramadan K, Danial E, Alnahdi H, Ayaz N (2012) Antidiabetic activity of Rosmarinus officinalis and its relationship with the antioxidant property. African J Pharm Pharmacol 6:1031–1036
Khangholil S, Rezaeinodehi A (2008) Effect of drying temperature on essential oil content and composition of sweet wormwood (Artemisia annua) growing wild in Iran. Pakistan J Biol Sci 11:934–937
Khorramdel S, Shabahang J, Asadi GhA (2013) Effect of drying methods on drying time, essential oil quantitative and qualitative of some of medicinal plants. Eco-Phytochem J Med Plants 1:36–48
Khorshidi J, Morshedloo MR, Moradi Sh (2020) Comparison of growth properties, essential oil content, total phenol, and antioxidant potential of different populations of three Hypericum species (H. scabrum L., H. asperulum Jaub. & Spach. and H. vermiculare) gathered from Kurdistan province. Iran J Med Aromat Plants Res 36:655–669
Lim YY, Murtijaya J (2007) Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Sci Technol 40:1664–1669
López J, Uribe E, Vega-Gálvez A, Miranda M, Vergara J, Gonzalez E, Di Scala K (2010) Effect of air temperature on drying kinetics, vitamin c, antioxidant activity, total phenolic content, non-enzymatic browning and firmness of blueberries variety O’Neil. Food Bioprocess Technol 3:772–777
Machado DG, Cunha MP, Neis VB, Balen GO, Colla A, Bettio LEB, Oliveira Á, Pazini FL, Dalmarco JB, Simionatto EL, Pizzolatti MG, Rodrigues ALS (2013) Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem 136:999–1005
Mahmoud AA, Gendy ASH, Said-Al Ahl HAH, Grulova D, Astatkie T, Abdelrazik TM (2018) Impacts of harvest time and water stress on the growth and essential oil components of horehound (Marrubium vulgare). Sci Hortic 232:139–144
Mekonnen A, Yitayew B, Tesema A, Taddese S (2016) In vitro antimicrobial activity of essential oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int J Microbiol 1:1–8
Mhamdi B, Aidi Wannes W, Sriti J, Jellali I, Ksouri R, Marzouk B (2010) Effect of harvesting time on phenolic compounds and antiradical scavenging activity of Borago officinalis seed extracts. Ind Crops Prod 31:2004–2007
Mirhosseini F, Rahimmalek M, Pirbalouti AG, Taghipoor M (2015) Effect of different drying treatments on essential oil yield, composition and color characteristics of Kelussia odoratissima Mozaff. J Essent Oil Res 27:204–211
Mohammed HA, Al-Omar MS, Mohammed SAA, Aly MSA, Alsuqub ANA, Khan RA (2020) Drying induced impact on composition and oil quality of rosemary herb, Rosmarinus officinalis Linn. Molecules 25:1–12
Morshedloo MR, Ebadi A, Fatahi Moghaddam MR, Yazdani D (2012) Essential oil composition, total phenol compounds and antioxidant activity of Hypericum perforatum L. extract collected from north of Iran. J Med Plants 11:218–226
Morshedloo MR, Amani Machiani M, Mohammadi A, Maggi F, Aghdam MS, Mumivand H, Javanmard A (2021) Comparison of drying methods for the extraction of essential oil from dragonhead (Dracocephalum moldavica L., Lamiaceae). J Essent Oil Res 33:162–170
Nieto G, Ros G, Castillo J (2018) Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis, L.): a review. Medicines 5:1–13
Nikkhah F, Sefidkon F, Sharifi Ashooorabadi E (2009) The effect of distillation methods and plant growth stages on the essential oil content and composition of Thymus vulgaris L. Iran J Med Aromat Plants 25:309–320
Omidbaigi R, Kazemi S, Daneshfar E (2008) Harvest time effecting on the essential oil content and compositions of Thymus vulgaris. J Essent Oil-Bear Plants 11:162–167
Özcan MM, Chalchat JC (2008) Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.) oil from Turkey. Int J Food Sci Nutr 59:691–698
Özcan MM, Al Juhaimi F, Ahmed IAM, Uslu N, Babiker EE, Ghafoor K (2020) Effect of microwave and oven drying processes on antioxidant activity, total phenol and phenolic compounds of kiwi and pepino fruits. J Food Sci Technol 57:233–242
Ozdemir N, Ozgen Y, Kiralan M, Bayrak A, Arslan N, Ramadan MF (2018) Effect of different drying methods on the essential oil yield, composition and antioxidant activity of Origanum vulgare L. and Origanum onites L. J Food Meas Charact 12:820–825
Ozkan G, Baydar H, Erbas S (2010) The influence of harvest time on essential oil composition, phenolic constituents and antioxidant properties of Turkish oregano (Origanum onites L.). J Sci Food Agric 90:205–209
Petiwala SM, Puthenveetil AG, Johnson JJ (2013) Polyphenols from the Mediterranean herb rosemary (Rosmarinus officinalis) for prostate cancer. Front Pharmacol 4:1–4
Pirbalouti AG, Mahdad E, Craker L (2013) Effects of drying methods on qualitative and quantitative properties of essential oil of two basil landraces. Food Chem 141:2440–2449
Pirbalouti AG, Salehi S, Craker L (2017) Effect of drying methods on qualitative and quantitative properties of essential oil from the aerial parts of coriander. J Appl Res Med Aromat Plants 4:35–40
Pouryousef M (2015) Effects of terminal drought stress and harvesting time on seed yield and essential oil content of fennel (Foeniculum vulgare Mill.). Iran J Med Aromat Plants 30:889–897
Prakasa Rao EVS, Ganesha Rao RS, Ramesh S (1995) Seasonal variation in oil content and its composition in two chemotypes of scented geranium (Pelargonium sp.). J Essent Oil Res 7:159–163
Prior RL, Cao G (2000) Antioxidant phytochemicals in fruits and vegetables: diet and health implications. HortScience 35:588–592
Prusinowska R, Śmigielski K (2015) Losses of essential oils and antioxidants during the drying of herbs and spices. Rev Eng Sci Technol 2:52–62
Rakić S, Petrović S, Kukić J, Jadranin M, Tešević V, Povrenović D, Šiler-Marinković S (2007) Influence of thermal treatment on phenolic compounds and antioxidant properties of oak acorns from Serbia. Food Chem 104:830–834
Rašković A, Milanović I, Pavlović N, Ćebović T, Vukmirović S, Mikov M (2014) Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement Altern Med 14:1–9
Risner D, Marco ML, Pace SA, Spang S (2020) The potential production of the bioactive compound pinene using whey permeate. Processes 8:1–16
Rustaiee AR, Khorshidi J, Tabatabaei MF, Omidbaigi R, Sefidkon F (2010) Essential oil composition of Thymus daenensis Celak. during its phenological cycle. J Essent Oil-Bear Plants 13:556–560
Saeidi K, Ghafari Z, Rostami S (2016) Effect of drying methods on essential oil content and composition of Mentha longifolia (L.) Hudson. J Essent Oil-Bear Plants 19:391–396
Santos CPD, Pinto JAO, dos Santos CA, Cruz EMO, Arrigoni-Blank MDF, Andrade TM, Santos DDA, Alves PB, Blank AF (2016) Harvest time and geographical origin affect the essential oil of Lippia gracilis Schauer. Ind Crops Prod 79:205–210
Sathishkumar R, Lakshmi PTV, Annamalai A (2009) Effect of drying treatment on the content of antioxidants in Enicostemma littorale Blume. Res J Med Plants 3:93–101
Sellami IH, Wannes WA, Bettaieb I, Berrima S, Chahed T, Marzouk B, Limam F (2011) Qualitative and quantitative changes in the essential oil of Laurus nobilis L. leaves as affected by different drying methods. Food Chem 126:691–697
Sellami IH, Rahali FZ, Rebey IB, Bourgou S, Limam F, Marzouk B (2013) Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Technol 6:806–817
Souhila T, Fatma Zohra B, Tahar HS (2019) Identification and quantification of phenolic compounds of Artemisia herba-alba at three harvest time by HPLC–ESI–Q-TOF–MS. Int J Food Prop 22:843–852
Soysal Y (2004) Microwave drying characteristics of parsley. Biosyst Eng 89:167–173
Soysal Y, Öztekin S (2001) Sorption isosteric heat for some medicinal and aromatic plants. J Agric Eng Res 78:159–166
Szumny A, Figiel A, Gutiérrez-Ortíz A, Carbonell-Barrachina ÁA (2010) Composition of rosemary essential oil (Rosmarinus officinalis) as affected by drying method. J Food Eng 97:253–260
Tai J, Cheung S, Wu M, Hasman D (2012) Antiproliferation effect of rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine 19:436–443
Takayama C, de Faria FM, de Almeida ACA, Dunder RJ, Manzo LP, Socca EAR, Batista LM, Salvador MJ, Souza-Brito ARM, Luiz-Ferreira A (2016) Chemical composition of Rosmarinus officinalis essential oil and antioxidant action against gastric damage induced by absolute ethanol in the rat. Asian Pac J Trop Biomed 6:677–681
Van Der Werf MJ, Boot AM (2000) Metabolism of carveol and dihydrocarveol in Rhodococcus erythropolis DCL14. Microbiology 146:1129–1141
Verma RS, Rahman L, Verma RK, Chanotiya CS, Chauhan A, Yadav A, Yadav AK, Singh A (2010) Changes in the essential oil content and composition of Origanum vulgare L. during annual growth from Kumaon Himalaya. Curr Sci 98:1010–1012
Verma RS, Padalia RC, Arya V, Chauhan A (2012) Aroma profiles of the curry leaf, Murraya koenigii (L.) Spreng. chemotypes: variability in north India during the year. Ind Crops Prod 36:343–348
Wang W, Li N, Luo M, Zu Y, Efferth T (2012) Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules 17:2704–2713
Wojdylo A, Oszmianski J, Czemerys R (2007) Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 105:940–949
Xing Y, Lei H, Wang J, Wang Y, Wang J, Xu H (2017) Effects of different drying methods on the total phenolic, rosmarinic acid and essential oil of purple perilla leaves. J Essent Oil-Bear Plants 20:1594–1606
Yang P, Seib PA (2000) Low-input wet-milling of grain-sorghum for readily accessible starch and animal feed. Cereal Chem 72:498–503
Yao L, Caffin N, D’Arcy B, Jiang Y, Shi J, Singanusong R, Liu X, Datta N, Kakuda Y, Xu Y (2005) Seasonal variations of phenolic compounds in Australia-grown tea (Camellia sinensis). J Agric Food Chem 53:6477–6483
Zhang T, Yang H, Wen S, Qiu F, Liu X (2019) Effects of harvest season and storage time on the essential oil of the linalool chemotype of Cinnamomum camphora. J Essent Oil-Bear Plants 22:1379–1385
Acknowledgements
The authors are grateful to the University of Kurdistan for their supports.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SFKD did field and laboratory works. JK done investigation, methodology, validation and writing. MRM contributed to laboratory analysis. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khademi Doozakhdarreh, S.F., Khorshidi, J. & Morshedloo, M.R. Essential oil content and components, antioxidant activity and total phenol content of rosemary (Rosmarinus officinalis L.) as affected by harvesting time and drying method. Bull Natl Res Cent 46, 199 (2022). https://doi.org/10.1186/s42269-022-00902-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00902-0