Abstract
Background
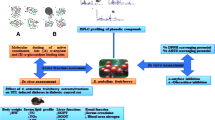
A perusal of the literature suggested that Manilkara zapota (L.) P. Royen stem bark (MZSB) is enriched with several bioactive phytoconstituents but had not been yet screened for its in vitro and in vivo antidiabetic potentials. Thus, the present study aimed to investigate the effects of 70% ethanolic extract of Manilkara zapota (L) P. Royen stem bark (EMZSB) in DPPH- and H2O2-scavenging assay, in vitro α-glucosidase inhibition assay, ameliorating diabetes and its complications in alloxan-induced diabetes in Wistar rats.
Results
With a maximum extractive yield of 9.16% w/w, EMZSB has shown the presence of various phytochemicals like flavonoids, phenolic compounds, tannins, anthraquinone glycosides, steroids, terpenoids, and alkaloids. EMZSB has elucidated a considerable in vitro free radical scavenging potential by DPPH and H2O2 assays when compared with absolute ethanolic extract of Manilkara zapota (L) P. Royen stem bark (AEMZSB), while ascorbic acid was taken as the standard. Further, EMZSB demonstrated high in vitro α-glucosidase enzyme inhibition potential (IC50 = 119.79 ± 1.52 µg/mL) than AEMZSB (IC50 = 129.92 ± 2.29 µg/mL) with a significant difference (p < 0.01), when acarbose was taken as reference inhibitor (IC50 = 86.43 ± 1.26 µg/mL). During acute toxicity studies EMZSB was safe up to 2000 mg kg−1 doses while, found causing moribund status followed by mortality in mice at 3000 mg kg−1 and above doses. A preliminary antidiabetic study with EMZSB-250 mg kg−1 in normal rats showed no sign of hypoglycemia; however, a dose-dependent antihyperglycemic effects were observed in oral glucose tolerance test in glucose-loaded rats. In vivo assessment with EMZSB-250 mg kg−1 in alloxan-induced rats demonstrated significant blood glucose-lowering effects with perfection in serum lipid profile, body weight enhancement, cardiovascular risk indices, nephroprotective effects, augmentation in liver glycogen content, and histopathological evidence of normal architecture of kidneys with no marks for nephritis.
Conclusions
EMZSB-250 showed significant antidiabetic effects and ameliorated diabetic complications by improving glycemic control and accompanying biochemical alteration.
Similar content being viewed by others
Background
Type 2 diabetes (T2D) is more prevalent than type 1 diabetes, with over 90% of total diabetes cases. T2D is a disease characterized by advances in insulin resistance and β-cell dysfunction leading to hyperglycemia (Kishore et al. 2017). International Diabetes Federation (IDF) predicted that by the year 2030 about 552 million people in the world may be manifested as diabetic (IDF 2011). Controlling hyperglycemia is the first line of treatment in the management of diabetes mellitus (DM) in order to control the progression of further diabetic-related complications. The main line of therapy in diabetes emphasizes drugs that promotes insulin secretions and sensitizations. Noninvasive management of hyperglycemia is also done by carbohydrate hydrolyzing enzyme inhibitors like α-glucosidase inhibitors—acarbose and miglitol which help to control postprandial hyperglycemia (Ibrahim and Islam 2014). Postprandial metabolic overload induces oxidative stress, which could disturb normal cellular and metabolic functions by damaging biomolecules. Such long-term disturbances further evolve toward the development of multifaceted chronic disease like DM (Kumar et al. 2015). The prolonged and uncontrolled DM may accomplish fatal complications like nephropathy, cardiomyopathy, and retinopathy (Musabayane 2012).
At present, DM is managed by three groups of drug interventions (Chinsembu 2019). Group one drugs that enhance the availability of insulin are sulfonylureas, while sulfonylureas often fail in the perfection of glycemic index and have some side effects like osteoporosis, obesity, and inhibition of hepatic regeneration (Iwaki et al. 2003; Meier et al. 2016). The second group of drugs enhances the sensitivity of insulin–thiazolidinedione and biguanide. Pioglitazone like thiazolidinedione though has the capability to reduce the risk of IGT conversion to T2D, its prolonged use is associated with edema and conspicuous weight gain, which further may increase cardiovascular threats (Kole et al. 2016). The third group of drugs is carbohydrate hydrolyzing enzyme inhibitors like α-glucosidase and α-amylase inhibitors. However, the carbohydrate hydrolyzing enzyme inhibitors are reported to have some gastrointestinal-related side effects like abdominal cramps, flatulence, bloating, intestinal disturbances, and diarrhea (Fujisawa et al. 2005). Due to such curbing of these existing drug interventions, the prospects of ascertaining new antidiabetic principles from the herbal origin are courtesy of diabetes management and/or prevention of its complications.
Manilkara zapota (L.) P. Royen (family: Sapotaceae) is an evergreen tree in all parts of the Indian subcontinent. The several plant parts of Manilkara zapota (L.) are being used conventionally for various diseases and alignments; leaves are used to treat oral inflammatory diseases, a decoction of leaves is used as a gargle in cough, cold, and diarrhea; gummy latex of tree is used for making chewing gum; fruit is consumed for relief in pulmonary diseases; stem bark was being used for making chewing gum in the eighteenth century, in treatment of gastrointestinal disorders and pain, while for treating fever, dysentery, paludism, and diarrhea the decoction of the bark has been used, also as an astringent and febrifuge (Mohiddin et al. 1992; Ma et al. 2003; Kaneria et al. 2009), ethanolic extract of brown bark was proven to possesses an antibacterial effect against S. epidermidis and K. pneumonia (Hilma et al. 2018); seeds as a diuretic, aperients, and febrifuge (Ghani 1998). MZSB is enriched with several flavonoids and phenolic compounds which plays a significant role in free radical scavenging (Kumar and Sahoo 2020). Thus, the utilization of Manilkara zapota (L.) plant parts being as an alternative herbal treatment in the management of various diseases is accounted for by the community. A perusal of the literature suggested that MZSB had not been yet screened for its in vitro and in vivo antidiabetic potentials. Thus, the present investigation was done to evaluate and substantiate the antidiabetic potentials of EMZSB.
Methods
Chemicals
Analytical grade chemicals were used in this investigation. Ethanol, methanol, pet ether, formalin, and xylene were obtained from Merck Life. Sci. Pvt. Ltd., India. Baker’s yeast α-glucosidase enzyme extract, p-Nitrophenyl-α-d-glucopyranoside, and acarbose were obtained from Sigma-Aldrich Pvt. Ltd., India. Glucose GOD/POD kit was obtained from Reckon diagnostic, Pvt. Ltd., India, and alloxan monohydrate was obtained from Fine chem., Pvt. Ltd., Mumbai, India. Hematoxylin and eosin stains, urethane, trichloroacetic acid, and anthrone reagent were obtained from Sigma-Aldrich Pvt. Ltd., India.
Collection and authentication of plant sample
MZSB was collected from the local farm area of Nanded, India (latitude 19.130 N and longitude 77.320 E). For taxonomic identity, authentication (BSI/WRC/100-1/TECH./2019/68), a voucher specimen of bark sample was deposited at the herbarium of the Botanical Survey of India, Pune, India.
Sample preparation and extraction
The collected MZSB sample was thoroughly cleaned and shed dried, powdered in a pulverizer at room temperature, and stored in an airtight container with wrapping in aluminum foil. 1000 ml of absolute ethanol was added to 100 g of dried pet ether defatted MZSB powder and shaken on an orbital incubator shaker (Remi RIS 24+) at 150 rpm, at 28 °C for 05 h. Cooled to room temperature keeping in an ice bath and centrifuged (Remi Centrifuge, Mumbai, India) for 15 min at 2500 rpm. Filtered by Whatman filter paper No 1, the filtrate was evaporated for the next 3 days and kept at 04 °C in airtight dark amber color bottles before further analysis (Osman et al. 2011; Karle et al. 2021). The same procedure was followed for extraction with 70% ethanolic solvent. The extractive yield was calculated as;
Preliminary phytochemical evaluation
EMZSB was evaluated for the preliminary phytochemicals with the standard procedures described (Khandelwal 2008).
Evaluation of in vitro antioxidant activity
DPPH assay
DPPH assay was performed to evaluate the antioxidant potentials of AEMZSB and EMZSB extract. Equal volumes of DPPH in methanol were kept as controls, 5 ml methanol as blank and ascorbic acid as a reference standard. The extract samples were dissolved in methanol and test samples were prepared in concentrations of 0.1 to 1 mg ml−1. Every 1 ml of prepared concentrations of test extracts was mixed with each 3 ml DPPH solution prepared in methanol (0.2 mM). These properly mixed samples were then incubated in dark for 40 min at room temperature, and absorbance was measured at 517 nm (Brand-Williams et al. 1995; Alam et al. 2013; Jing et al. 2015). The mean values ± SEM of the assay carried out in triplicate were presented. The percentage inhibition was estimated as;
where Acontrol is an absorbance by DPPH solution; Asample absorbance by DPPH solution with the sample extracts.
H2O2-scavenging assay
To every 0.6 ml of H2O2 solutions (43 mM), AEMZSB and EMZSB extract samples were added by preparing its various concentrations (10–50 µg ml−1) dissolved in 4 ml of 0.1 M phosphate buffer pH 7.4. Further kept for 30 min to react, afterward, absorbance was recorded at 230 nm, and then followed at every 10 min. For blank, phosphate buffer (pH 7.4) and as a reference standard, ascorbic acid was used (Ruch et al. 1989; Boligon et al. 2014). The % scavenging activity was calculated as;
where Acontrol is an absorbance by H2O2 solution; Asample absorbance by H2O2 solution with the sample extracts.
Evaluation of in vitro antidiabetic activity
In vitro α-glucosidase enzyme inhibition assay
In vitro α-glucosidase enzyme inhibition assay was performed to assess the antidiabetic potentials of AEMZSB and EMZSB extracts. Initially, 0.1 M phosphate buffer (pH 6.8), substrate p-Nitrophenyl-α-D-glucopyranoside 15 mg/10 ml, and extracts samples ranging from 25 to 150 μg ml−1 concentrations were prepared. The mixture of each of 200 μl phosphate buffer, baker’s yeast α-glucosidase enzyme extract (0.5 U ml−1), and MZSB extract samples was formed and pre-incubated at 40 °C for 3 min. The reaction was started with the addition of 200 μl of a substrate to the mixture and for the next 25 min incubated at 40 °C. To this was added 800 μl of 0.2 M sodium hydroxide to terminate the reaction. α-glucosidase enzyme inhibition activity of sample extracts was assessed by recording absorbance at 405 nm by released p-nitrophenol from p-nitrophenyl-α-d-glucopyranoside. The reference standard used was acarbose (Wan et al. 2013; Savikin et al. 2018). The % inhibition was estimated by the formula;
Acute oral toxicity study
The OECD guidelines 423 were followed to estimate any acute oral toxicity in mice by EMZSB. Female mice were treated in two individual stages; for the first stage, three groups (n = 03) of mice were selected and administered orally at doses of 05, 50, 300 and, 2000 mg kg−1 of EMZSB. Then, the mice were observed for the next 03 h and 24 h for any toxicity signs, moribund status, and/or mortality. The results from the first stage suggested the doses for the second stage, where 3000 and 5000 mg kg−1 doses of test extracts were orally administered to another three groups (n = 03) of mice and any signs of toxicity moribund status and/or mortality were noted in next 72 h. The mice were observed initially after dosing at least once during the first 30 min, after 03 h, and periodically during the first 24 h and, 72 h in the second stage.
Experimental animals
Adult Wistar albino rats of either sex, weighing 180–200 g, were selected for this study and kept on a standard pellet diet and water ad libitum throughout the study period. For the collection of blood samples, the lateral tail vein method was followed after restraining the rats (Lee and Goosens 2015). Rats were anesthetized by inhaling vapors of light ether in a closed glass chamber (Shah and Jhade 2018). All the carcasses of rats were burned inside a fired incinerator, and ash was encapsulated in a high-density polyethylene and sent to a landfill (Smith 2013).
Preliminary antidiabetic study
Experimental design
The overnight fasted Wistar rats were divided into five groups (n = 6). Group I (control) received 1% gum acacia (1 ml kg−1, p.o.). Group II (Glimepiride) received (0.09 mg kg−1, p.o.). Group III to V were received EMZSB 150, 200, and 250 mg kg−1, p.o. (Bhat et al. 2005).
Hypoglycemic study in non-diabetic rats
Non-diabetic rats were treated with EMZSB to find out any hypoglycemic effect. The effects of administered extracts were compared with the effects of standard drug glimepiride. To estimate the blood glucose level, blood samples were collected at 0th, 60th, 120th, and 180th min after administration of extracts/drug. Reckon diagnostic’s GOD/POD kit was used for the estimation of blood glucose levels (Maniyar and Bhixavatimath 2012).
(1) Antihyperglycemic study in non-diabetic rats
Oral glucose tolerance test was performed in normoglycemic Wistar albino rats were used to evaluate the antihyperglycemic effects of EMZSB. All the animals were administered with the above-selected dose of extract/drug. After 30 min, rats were received 2 g kg−1, p.o. glucose solution and post-glucose load blood samples were collected at 0th, 30th, 90th, and 150th min (Shirwaikar et al. 2006).
Evaluation of in vivo antidiabetic potential of EMZSB extract
Experimental induction of diabetes in Wistar rats
To the overnight fasted rats, a single i.p. dose (1 ml kg−1) of freshly prepared alloxan monohydrate (150 mg kg−1) dissolved in 0.9% saline was inoculated after light ether anesthesia. To avoid severe acute hypoglycemia, normal water fed was substituted with 5% glucose water for up to the next 48 h (Rees and Alcolado 2005; Ramya et al. 2020; Ighodaro et al. 2017). Fasting blood glucose levels were recorded after 72 h in all the alloxan monohydrate inoculated rats and rats with blood glucose levels ≥ 200 mg/dl were well-thought-out as diabetic (Chika and Bell 2010).
Experimental design
Alloxan-induced diabetic rats of either sex were divided into various groups (n = 6). Extracts/standard reference drugs were administered p.o. in 1 ml kg−1 b.w. suspension of 1% gum acacia. Normal control group and diabetic control group (diabetic untreated rats) received vehicle only, glimepiride-treated diabetic rat’s group received glimepiride 0.09 mg kg−1 b.w., EMZSB 150-treated diabetic rat’s group received EMZSB 150 mg kg−1 b.w., EMZSB 200-treated diabetic rat’s group received EMZSB 200 mg kg−1 b.w., and EMZSB 250-treated diabetic rat’s group received EMZSB 250 mg kg−1 b.w. (Nagarajan et al. 2005; Dinesh Kumar et al. 2011; Muhtadi Primarianti and Sujono 2015).
Antihyperglycemic effect of EMZSB extract
On the days 1st, 7th, 14th, and 21st, blood glucose levels in all the grouped rats were estimated by using standard GOD/POD kits from Reckon diagnostics, Pvt., ltd., India (Maniyar and Bhixavatimath 2012).
Body weight
During treatment on the day 7th, 14th, and 21st, diabetic rats were assessed for any changes in body weight (Akhtar et al. 2018).
Estimation of serum levels of HDL, LDL, total cholesterol, and triglyceride
Post-treatment on the day 21st, serum levels of HDL, LDL, total cholesterol, and triglyceride were assessed using standard diagnostic kits from Reckon diagnostics, Pvt., ltd., India (Ajiboye et al. 2018).
Assessment of the nephroprotective effect
Kidney parameters
Post-treatment on the day 21st, any possible nephroprotective effects of EMZSB on diabetic renal conditions in all the treated rats were estimated by assessing serum levels of creatinine, albumin, and total protein using standard diagnostic kits from Reckon diagnostics, Pvt., ltd., India (Elshemy 2018).
Histological study
Post-treatment, on the day 21st kidneys were isolated from rats killed by urethane-overdose and preserved in 10% formalin solution until further histological finding procedures. Paraffin wax embedded kidneys were sliced into 5 μm thickness using a rotary microtome. Deparaffinization was done with xylene and ethanol. Further, hematoxylin and eosin-stained sections were microscopically examined for appraisal of histoarchitecture, glomerulosclerosis, and hyalinization (Soussi et al. 2018).
Evaluation of liver glycogen content
Post-treatment on the 21st day, livers were isolated from killed rats, cleaned, and kept in a homogenizer vessel with 40 ml of 5% trichloroacetic acid for 5 min. Homogenate was centrifuged at 3500 rpm (10 min), and the supernatant collected was filtered by filter paper. The same procedure was repeated with the residue to extract 98% of liver glycogen. The filtrate was precipitated overnight at 1:5 ratios with 95% ethanol in a test tube, precipitated content was centrifuged for 15 min at 3000 rpm, and supernatant clear content was decanted. The dry pellet was collected by inverting the test tube for 15 min, and to it 10 ml of anthrone reagent and 2 ml of distilled water were added and shaken vigorously for 5 min. This tube was then kept in boiling water for 15 min later on and cooled to room temperature, and optical density was colorimetrically determined at 620 nm. For standard, a solution of 2 ml standard glucose (0.1 mg) solution and anthrone reagent (10 mL) was used, while 2 ml water with 10 ml anthrone reagent was used as a blank solution (Carroll et al. 1956; Ajiboye et al. 2018). Liver glycogen content was estimated by the formula;
where DU is the optical density of unknown; DS is the optical density of standard.
Assessment of cardiovascular risk indices (Kang et al. 2004)
Various cardiovascular risk indices were calculated as;
\({\text{Coronary artery index}} = {\text{LDL cholesterol/HDL cholesterol}}\) \({\text{Cardiac index}} = {\text{Total cholesterol/HDL cholesterol}}\) \({\text{Atherogenic index}} = ({\text{Total cholesterol/HDL cholesterol}}) - {\text{HDL cholesterol}}{.}\)
Assessment of neuropathic pain
The hot plate apparatus method (Boyce-Rustay and Jarvis 2009) was used to assess the neuropathic pain sensation ability of EMZSB treated diabetic rats. On the 21st day, paw-licking responses by the diabetic rats were noted down with a 10 s of cutoff time to prevent any tissue damage. Response latency for ipsilateral (uninjured) paw licking was measured from two repetitions, with 15 min of time intervals.
Statistical analysis
Linear regression analysis in program Excel 2010 was done to interpret IC50 values for in vitro antioxidant assays with mean ± SEM of three replicates. For assessing in vitro α-glucosidase enzyme inhibition potentials, mean ± SD values were calculated from three replicates and IC50 values were estimated by linear regression analysis from the mean inhibitory values of plots of percent inhibition versus log inhibitor. The difference among inhibition by acarbose, AEMZSB, and EMZSB was considered statistically significant using one-way ANOVA followed by Tukey's multiple comparisons test at 95% confidence interval (p < 0.05) by GraphPad Prism software, version 9.2.0 (Table 1).
Results
Preliminary phytochemical evaluation
Evaluation of antioxidant activity
DPPH radical-scavenging activity
Figure 1 depicts the DPPH-scavenging activity of AEMZSB and EMZSB at 0.1 to 1 mg ml−1. Reference standard ascorbic acid exhibited 96.82% inhibition; however, AEMZSB and EMZSB elucidated 78.27% and 82.72% inhibition at 1 mg ml−1, respectively, whereas IC50 values for AEMZSB, EMZSB, and ascorbic acid were 0.48, 0.40, and 0.24 mg ml−1, respectively (Table 2).
H2O2-scavenging assay
Figure 2 depicts the H2O2-scavenging capacity of AEMZSB and EMZSB at 10 to 50 µg ml−1. The reference standard ascorbic acid revealed 75.98% inhibition, whereas AEMZSB and EMZSB interpreted 54.82 and 59.54% at 50 µg ml−1. However, IC50 values of AEMZSB, EMZSB, and ascorbic acid were 42.12, 37.54, and 26.65 µg ml−1, respectively (Table 2).
Evaluation of in vitro antidiabetic activity
In vitro α-glucosidase enzyme inhibition assay
Results (Fig. 3) depicted the percent inhibition and IC50 values for in vitro α-glucosidase enzyme inhibition activity by AEMZSB and EMZSB extracts at varying concentrations. Reference standard acarbose inhibited 95.24%, whereas AEMZSB and EMZSB inhibited 59.67% and 63.58% of α-glucosidase at 150 μg mL−1, respectively. Acarbose showed the lowest IC50 value of 86.43 ± 1.26 µg mL−1; however, AEMZSB and EMZSB elucidated 129.92 ± 2.29 and 119.79 ± 1.52 µg mL−1 of IC50 values (Table 3). Though the acarbose was more potent in α-glucosidase enzyme inhibition, the EMZSB exhibited a more significant inhibition activity when statistically compared with inhibition by AEMZSB. (Fig. 4).
In vitro evaluation for α-glucosidase enzyme inhibition by MZSB extracts. Values were presented as mean ± SD of three replicates. EMZSB showed a lower IC50 value of 119.79 ± 1.52 µg ml−1 than AEMZSB. However, acarbose was used as reference standard. Blue line acarbose, orange line EMZSB, gray line AEMZSB
Comparison of IC50 values of in vitro evaluation for α-glucosidase enzyme inhibition by MZSB extracts. IC50 values were presented as mean ± SD, p < 0.05. EMZSB showed a lower IC50 value of 119.79 ± 1.52 µg/ml than AEMZSB (129.92 ± 2.29 µg/m). However, acarbose (86.43 ± 1.26 µg/mL) was used as a reference standard. Green bar EMZSB, yellow bar AEMZSB, gray bar Acarbose
Acute oral toxicity study
During acute toxicity studies, in all the cases, mice when treated up to 2000 mg kg−1 doses of EMZSB behaved normally and no death was observed up to this dose. Thus, the therapeutic dose preferred was the 1/10th (200 mg kg−1) of the safe dose (2000 mg kg−1). While, in the second stage, when mice were treated with 3000, and 5000 mg kg−1 extracts, caused moribund status followed by mortality in mice.
Preliminary antidiabetic study
Hypoglycemic effect of EMZSB extract
Glimepiride-treated group and groups administered with extracts did not elucidate any notable variance in blood glucose levels when compared with the normal control group (Fig. 5). However, at 120 min a slight reduction in plasma glucose level was noted with a dose of EMZSB 250 mg kg−1, p.o., which was disappeared at 180 min. Except for this, no sign of hypoglycemia was observed with other treated groups (Table 4).
Preliminary hypoglycemic effects of EMZSB extract in non-diabetic rats. Values were presented as mean ± SEM (n = 6), two-way ANOVA followed by Tukey’s test. Insignificant hypoglycemia was elucidated by rats treated with EMZSB 200 and 250 mg kg−1, when compared with normal control rats. Black bar normal control, green bar glimepiride, yellow bar EMZSB 150, gray bar EMZSB 200, blue bar EMZSB 250
Antihyperglycemic effect of EMZSB extract
EMZSB extract-treated rats elucidated a noteworthy dose-dependent decrease in blood glucose level (Fig. 6). The rats treated with EMZSB 250 mg kg−1 revealed a significant (p < 0.05) blood-glucose-lowering effect at 150 min (Table 5).
Evaluation of oral glucose tolerance test in glucose-loaded rats when treated with EMZSB extract. Values were presented as mean ± SEM (n = 6), two-way ANOVA followed by Tukey’s test. A significant antihyperglycemic effect was elucidated by rats treated with EMZSB 250 mg kg−1, when compared with normal control rats. Black bar normal control, gray bar glimepiride, blue bar EMZSB 150, yellow bar EMZSB 200, green bar EMZSB 250
Evaluation of in vivo antidiabetic potential of EMZSB extract
Effect of EMZSB on blood glucose
As predicted, alloxan-induced diabetes in untreated diabetic control group rats was in its progression with continued hyperglycemia throughout the 21 days of study, whereas, during 21 days of treatment, EMZSB 250 mg kg−1 elucidated a significant (p < 0.01) blood-glucose-lowering effect on the day 21st, while with EMZSB 200 mg kg−1 it was significant at p < 0.05. However, rats when treated with the standard drug glimepiride exhibited a reduction in blood glucose levels from the day 14th to 21st day (p < 0.01 to p < 0.001, respectively) (Table 6).
Body weight changes
As expected, during 21 days of study, persistent bodyweight loss was observed in untreated diabetic rats. However, treatment with EMZSB 200 and 250 mg kg−1 elucidated slowly but progressive weight gain during the study (Table 7). On day 21st, the weight of rats from the group treated with EMZSB 250 mg kg−1 exhibited a significant (p < 0.01) body weight (6.93 ± 4.83) gain.
Estimation of serum levels of HDL, LDL, total cholesterol, and triglyceride
Treatment of diabetic rats with EMZSB elucidated slight protective effects on serum lipid profile. Diabetic rats when treated with EMZSB 250 mg kg−1 for 21 days exhibited a significant (p < 0.05) reduction in serum LDL, cholesterol, and triglyceride, while an appreciable rise in serum HDL (p < 0.05), when compared with the diabetic control group rats (Table 8).
Assessment of the nephroprotective effect
Kidney parameters
As expected, diabetic control rats showed a noticeable rise in serum creatinine and a fall in serum albumin, and total protein. However, diabetic rats when treated with EMZSB 250 mg kg−1 elucidated a significant (p < 0.05) increase in serum albumin, and total protein, but showed a significant (p < 0.05) protective effect in kidney function by perfection in serum creatinine (Table 9).
Histopathological findings from kidneys
Persistent DM may promote sever pathological changes in kidney leading to glomerulosclerosis, hyalinization, and alterations in tubule segment and mesangial matrix. In case of untreated diabetic rats, significant interferences were observed in tubule segments along with dilation of bowman capsular space with tubular necrosis. However, diabetic rats treated with EMZSB 250 depicted the normal architecture of kidneys with no histopathological marks for nephritis (Fig. 7).
Photomicrographs (100 ×) presenting 21 days post-treatment effects on kidneys of alloxan-induced diabetic rats when treated with EMZSB. A Control group: depicted normal histoarchitecture of the kidney. B Diabetic control group: abnormal kidney histoarchitecture. C Diabetic + glimepiride group: no signs of glomerulosclerosis and hyalinization. D Diabetic + EMZSB-150 and E Diabetic + EMZSB-200 group showed few signs of tubular necrosis and glomerulosclerosis, respectively. F Diabetic + EMZSB-250 group appears to be normal histoarchitecture of kidney with no scripts of glomerulosclerosis
Evaluation of liver glycogen content
As expected, liver glycogen content was lower down (p < 0.01) in untreated diabetic rats when compared to normal control rats. However, in rats treated with EMZSB 250, the liver glycogen content was significantly (p < 0.05) increased as compared to diabetic control rats (Table 10).
Assessment of cardiovascular risk indices
Noteworthy cardiovascular protective effects were exhibited by the diabetic rats when treated for 21 days with EMZSB. A significant (p < 0.05) decrease in cardiovascular risk indices, viz. coronary artery, cardiac and atherogenic indices, was revealed by EMZSB 250 and standard reference glimepiride when matched with diabetic control rats. However, the effects of EMZSB were lower than that of glimepiride (Table 11).
Assessment of EMZSB on hot plate-induced neuropathic pain in diabetic rats
As expected, untreated diabetic rats were observed with decreased thermal stimuli sensitivity by the reduction in latency to foot licking when compared with normal rats, whereas rats treated with reference standard glimepiride were shown improvement (p < 0.05) in pain sensitivity by licking the foot at an average time of 6.9 s, while EMZSB-treated rats do not show any significant improvement in the same when compared with the diabetic control group rats (Table 12).
Discussion
Available data from the literature revealed that materials from plant sources are being widely used in the treatment and management of diabetes. Therefore, plant source materials are considered for new leads for antidiabetic agents. A perusal of the literature put forward the presence of various phytoconstituents in the MZSB like spinasterol, 6-hydroxyflavanone, (+)-dihydrokaempferol, 3, 4-dihydroxybenzoic acid, taraxerol, taraxerone, while lupeol acetate was reported to have antityrosinase activity (Chunhakant and Chaicharoenpong 2019). Flavonoids, terpenoids, glycosides, and phenolic compounds identified in MZSB proved to play a significant role in the healing of ulcerative colitis probably by their antioxidant and free radical scavenging property (Kumar and Sahoo 2020). Chondrillasterol, stigmasterol, β-sitosterol, lupeol, lupenone, glut-5(6)-en-3β-acetate, and olean-12-en-3β-acetyl-11-one were isolated from MZSB and studied for antimicrobial, thrombolytic, and cytotoxic activities (Noor et al. 2014). Duong et al. isolated manilkzapotane, lupeol acetate, lupeol, arjunolic acid, ergosterol peroxide, taraxerol, hederagonic acid, and glochidiol from MZSB (Duong et al. 2020), while Toze et al. isolated two new pentacyclic triterpenoids, 3acetyltaraxer-14-en-12-one and 3-hydroxy-7oxolup-20(29)-en-28-oic acid from methanolic extract of MZSB (Toze et al. 2015).
In the present study, the extract of Manilkara zapota (L) P. Royen stem bark was subjected to evaluate its in vitro and in vivo antidiabetic potentials. After extraction, the extractive yield obtained with AEMZSB and EMZSB was 8.68% and 9.16% w/w, respectively. Preliminary phytochemical investigation of EMZSB revealed the presence of various phytochemicals like flavonoids, phenolic compounds, tannins, anthraquinone glycosides, steroids, terpenoids, and alkaloids (Table 1).
Interaction of DPPH with proton donor phytoconstituents (antioxidants) in extract causes a color change in the product diphenylpicryl hydrazine from purple to yellow. The amount of DPPH radical scavenged is indicated by the decrease in intensity of the purple color, measured by a decrease in absorbance at 517 nm and determined in the form of IC50 values (Gomathy et al. 2013). Though the DPPH-scavenging effect of test extracts was lower than the reference standard ascorbic acid (0.24 mg ml−1), EMZSB exhibited a least IC50 value of 0.40 mg ml−1 than the IC50 value from AEMZSB (0.48 mg ml−1), showing its potential to scavenge DPPH radicals. Enriched flavonoids and phenolic compounds have a linear correlation with free radical scavenging potentials and metal-ion chelation (Withouck et al. 2019). Bioactive phenolics exert their antioxidant effects by directly countering the free radicals and thus generating a less reactive radical species or dismissing the free radical chain reaction (Venkatesan et al. 2019).
Electron transport chain reaction in human cellular mitochondrion produces hydrogen peroxide subsequently from superoxide anion radicals. In the cellular environment, the decomposition product of hydrogen peroxide—a highly reactive hydroxyl radical, on interaction with the transition metal iron ions or on exposure to UV becomes hazardous to and damages tissues. Thus, cellular prerequisite is to scavenge residing hydrogen peroxide (Halliwell et al. 2000). During the H2O2 assay, EMZSB exhibited a lower IC50 value of 37.54 µg ml−1 than AEMZSB (IC50 = 42.12 µg ml−1), when ascorbic acid was used as a reference standard (IC50 = 26.65 µg ml−1). The DPPH- and H2O2-scavenging potentials (Figs. 1, 2) of EMZSB are most likely due to varied amount of bioactive phytoconstituents like β-sitosterol which was reported to decrease ROS and increases natural antioxidant synthesis (Babu and Jayaraman 2020), and arjunolic acid can exhibit antioxidant properties through the scavenging of oxygen-derived free radicals, the action of hydrogen donation, the resonance stabilization of carboxyl radical and metal-ion chelation (Ghosh and Sil 2013), (+)-dihydrokaempferol (Lee et al. 2011), 3, 4-dihydroxybenzoic acid (Dra et al. 2019), and lupeol acetate (Gurupriya et al. 2018).
Modulation of postprandial hyperglycemia in T2DM is therapeutically achieved by targeting intestinal α-glucosidase enzyme. Inhibition of this enzyme aids to reduce the rate of breakdown of carbohydrates into simple sugars, which can be assimilated at the intestine and a consequential delay in postprandial hyperglycemia with fortunate influence on insulin conflict and glycemic index regulation (Arun et al. 2017). Unfortunately, clinically used synthetic α-glucosidase inhibitors such as acarbose and miglitol have certain adverse side effects like diarrhea, hepatotoxicity, and flatulence (Nathan et al. 2009). Investigation for the α-glucosidase inhibitory activity by MZSB extracts showed that α-glucosidase inhibition followed a linear relationship with the extract concentration (Fig. 3). The EMZSB has exhibited significant (p < 0.01) α-glucosidase inhibition with an IC50 value of 119.79 ± 1.52 µg mL−1 as compared to AEMZSB, when acarbose was the reference standard. These findings of moderate α-glucosidase inhibitory effects by EMZSB inclined us to rely on the provenance of a profusion of the varied amount of phytochemicals in this bark like spinasterol (Nkobole et al. 2021), (+)-dihydrokaempferol (Saltos et al. 2015), lupeol acetate (Srisurichan and Pornpakakul 2015), stigmasterol (Kumar et al. 2013), ergosterol peroxide (Eawsakul et al. 2021), and hederagonic acid (Xiao-An et al. 2010) which were earlier reported for their α-glucosidase inhibition capabilities.
Fasting blood glucose level is a vital indicator of diabetes status. The outcome of this investigation showed a dose-dependent reduction in blood glucose levels in rats treated with EMZSB for 21 days. A significant (p < 0.01) reduction in blood glucose was observed on the 21st day in rats treated with EMZSB 250 mg kg−1 (Fig. 8). These antihyperglycemic effects were likely to be the contribution of MZSB phytochemicals such as β-sitosterol which was reported to cause a decrease in fasting blood glucose by decreasing insulin resistance and regulating insulin signaling (Babu and Jayaraman 2020). However, β-sitosterol with stigmasterol was also reported to decrease glycogen phosphorylase activity in diabetic rats by increasing levels of glycogen synthase and hepatic glycogen, further by hepatic glucose utilization through remnant β-cells and enhanced glycolytic activity (Ramu et al. 2016). Previous study findings with phytoconstituents like lupenone, lupeol, arjunolic acid, and (+)-dihydrokaempferol were reported to have blood glucose-lowering effects (Ghosh and Sil 2013; Xu et al. 2014; Gupta et al. 2012; Wang et al. 2013). Taraxerol conveyed with alleviated fasting blood glucose level by insulin sensitization mediated by stimulation of signaling pathways like AKT/GSK3b/PI3K/AMPK/IRS1/GLUT4 (Khanra et al. 2017). For a long time, hederagonic acid, a natural pentacyclic triterpenes and a moderate inhibitor of glycogen phosphorylase, is known for its hypoglycemic effects by inhibiting α-glucosidase, PTP1B, and ACAT-1/ACAT-2 (Xiao-An et al. 2010). Hence, antihyperglycemic effects by EMZSB could be anticipated for these phytoconstituents.
Effect of EMZSB on blood glucose level in alloxan-induced diabetic rats. Values were presented as mean ± SEM (n = 6); statistics employed was two-way ANOVA followed by Tukey’s multiple comparison test. Diabetic animals when treated with EMZSB 250 showed a significant (p < 0.01) reduction in the blood glucose levels on 21st day. $p < 0.001 when compared with normal control; *p < 0.05, and **p < 0.01 when compared with diabetic control. Gray bar normal control, red bar diabetic control, brown bar diabetic + glimepiride, blue bar diabetic + EMZSB 150, yellow bar diabetic + EMZSB 200, green bar diabetic + EMZSB 250
As the study went on, the effects of extracts on body weight and gain in the weight have started to appear (Fig. 9). The body weights of rats in EMZSB 250-treated groups and the glimepiride-treated group were progressively increased in the following weeks. After 3 weeks of treatment, EMZSB 250 mg kg−1 treated group rats revealed a significant body weight gain compared to those of the diabetic control group, which was probably due to perfection in the synthesis of a structural protein and glycemic control (Eliza et al. 2009).
Body weights changes in alloxan-induced diabetic rats when treated with EMZSB during 21 days of treatment. Values were presented as mean ± SEM (n = 6); statistics employed was two-way ANOVA followed by Tukey's multiple comparison test. $p < 0.05 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control. Diabetic rats treated with EMZSB 250 mg kg−1 showed a significant bodyweight gain on the day 21st. Gray bar normal control, red bar diabetic control, black bar diabetic + glimepiride, blue bar diabetic + EMZSB 150, yellow bar diabetic + EMZSB 200, green bar diabetic + EMZSB 250
In DM, the rise in lipid peroxidation is an outcome of a raised level of free radicals (Quine and Raghu 2005). The observed perfection in serum lipid profile (Fig. 10) and general improved antioxidant prominence by EMZSB 250 mg kg−1 treated diabetic rats highlights its healthy perspective against oxidative stress and lipid peroxidation. Hypolipidemic effects of EMZSB extract might be attribution of previously reported effects by phytoconstituents; for example, Stigmasterol has antihyperglycemic effects and improving blood lipid indexes like triglyceride and cholesterol with the possible mechanism of targeting GLUT4 glucose transporter encompassing increase in GLUT4 expression and translocation (Wang et al. 2017). β-sitosterol has a positive impact on serum triglyceride, LDL, cholesterol, and an increase in HDL by its antihyperglycemic effects through decreasing insulin resistance and regulating insulin signaling (Babu and Jayaraman 2020). Arjunolic acid proved in preventing the modification in serum lipid profile by preventing intracellular reactive intermediates accountable for finding hyperglycemia, variation in the level of oxidative stress-related biomarkers, and thus signifying its efficacy in perfecting the lipid profile (Ghosh and Sil 2013). A natural pentacyclic triterpenes hederagonic acid is a moderate inhibitor of glycogen phosphorylase quoted to have inhibitory effects on PTP1B, α-glucosidase, ACAT-1/ACAT-2 thereby exerting its hypoglycemic and hypolipidemic effects (Xiao-An et al. 2010). Along with the above effects from various phytoconstituents of MZSB, the overall hypolipidemic effects might be a contribution of hydroxyl radical scavenging action leading to antiperoxidative and antioxidant effects may accompany an enriched variety of phytoconstituents in it.
Serum lipid profile in alloxan-induced diabetic rats when treated with EMZSB for 21 days. Values were presented as mean ± SEM (n = 6), statistics employed was two-way ANOVA followed by Tukey's multiple comparison test. $p < 0.01, and #p < 0.001 when compared with normal control; *p < 0.05, **p < 0.01, and ***p < 0.001 when compared with diabetic control. Diabetic rats treated with EMZSB 250 mg kg−1 exhibited a significant reduction in serum LDL, cholesterol, and triglyceride, while there is a substantial rise in serum HDL. Gray bar normal control, red bar diabetic control, brown bar diabetic + glimepiride, blue bar diabetic + EMZSB 150, yellow bar diabetic + EMZSB 200, green bar diabetic + EMZSB 250
In diabetic nephropathy, progression in renal dysfunction with glomerulosclerosis is an outcome of progressive proteinuria, an increment in uric acid, and accumulation of urea nitrogen (Latha and Daisy 2011). However, treatment of diabetic rats with EMZSB 250 mg kg−1 could significantly escalate serum albumin, total protein, and arrest in creatinine (Fig. 11), whereas the histological assessment showed evidence of a significant improvement toward normal histoarchitecture with visualized no marks for nephritis. This significant reinstate in nephritic parameters might have a prophylactic effect by varying amounts of phytoconstituents of MZSB like flavonoids, polyphenols, and taraxerol (Khanra et al. 2017).
Assessment of kidney parameters in alloxan-induced diabetic rats after 21 days treatment of EMZSB. Values were presented as mean ± SEM (n = 6), statistics employed was two-way ANOVA followed by Tukey's multiple comparison test. $p < 0.05, #p < 0.01 when compared with normal control; *p < 0.05 when compared with diabetic control. Treatment of diabetic rats with EMZSB 250 mg kg−1 showed a significant increase in serum albumin, total protein, and perfection in serum creatinine. Gray bar normal control, red bar diabetic control, brown bar diabetic + glimepiride, blue bar diabetic + EMZSB 150, yellow bar diabetic + EMZSB 200, green bar diabetic + EMZSB 250
In a diabetic state, depletion in overall glycogen content is followed by imperfections in the activation of enzymes like synthase and phosphatase (Grover et al. 2002). Post-treatment a marked increase (Fig. 12) in liver glycogen content by EMZSB 250 mg kg−1 might be the provenance of augmentation in insulin response encouraging glycogenesis and/or decline in hepatic glycogenolysis with further accomplishment toward the activation of enzymes like synthase and phosphatase (Ramkumar et al. 2011).
Assessment of liver glycogen content in alloxan-induced diabetic rats when treated with EMZSB. Values were presented as mean ± SEM (n = 6), statistics employed was one-way ANOVA followed by Tukey’s multiple comparison test. $p < 0.01 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control. Treatment of diabetic rats with EMZSB 250 mg kg−1 elucidated a significant increment in liver glycogen content as compared to diabetic control rats. Gray bar normal control, red diabetic control, brown bar diabetic + glimepiride, blue bar diabetic + EMZSB 150, yellow bar diabetic + EMZSB 200, green bar diabetic + EMZSB 250
Persistent hyperglycemia with a negative shift in the level of lipid profile is one of the etiologies for cardiovascular disease in DM. Hyperglycemia-induced cardiovascular complications underlie multifactorial mechanisms. Oxidative stress plays one of the foremost roles in the development and progression of cardiovascular complications in DM (Crespo et al. 2008). Although the clear-cut causes of reactive oxygen species in the DM pathophysiology have not been well understood until now, auto-oxidation of glucose, advanced glycation end products generating processes, mitochondrial dysfunction, and several others have been stated as the conceivable causes (Manna and Sil 2012). Notable cardiovascular protective effects (Fig. 13) by EMZSB were possibly due to perfection of hyperglycemia and hyperlipidemia by an enriched variety of phytocontents in MZSB like; Lupeol, (+)-dihydrokaempferol, stigmasterol, β-sitosterol, arjunolic acid, hederagonic acid which reported to have antihyperglycemic and antihyperlipidemic effects by attenuating insulin resistance, signaling, and/or regeneration of the β-cells (Munhoz and Frode 2018).
Assessment of cardio-vascular risk indices in alloxan-induced diabetic rats when treated with EMZSB. Values were presented as mean ± SEM (n = 6), statistics employed was two-way ANOVA followed by Tukey's multiple comparison test. $p < 0.05, #p < 0.01 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control. Diabetic rats treated with EMZSB 250 mg kg−1 showed a significant decrease in cardiovascular risk indices. Gray bar normal control, red bar diabetic control, brown bar diabetic + glimepiride, blue bar diabetic + EMZSB 150, yellow bar diabetic + EMZSB 200, green bar diabetic + EMZSB 250
Diabetic neuropathic complications like numbness and tingling, pain insensitivity or sharp pains, loss of sense of vibration, motor incoordination, etc., are responsible for morbidity in the diabetic population. Pathophysiologically manifested by degeneration of demyelinated and myelinated sensory nerve fibers. While concerning mechanisms in its pathogenesis are decreased anti-oxidant defense, glucose oxidation convinced oxidative stress followed by the formation of advanced glycation end (AGE) products, altered glucose metabolism, and polyol pathway disruption (Tawakoli et al. 2012). At present, not any conclusive treatment for diabetic neuropathy is available except that it is managed by opioids and topical capsaicin, SNRIs, tricyclic antidepressants, and anticonvulsants despite its cost and side effects (Nadig et al. 2012). Though the assessment of EMZSB had not shown any significant improvement in pain sensitivity (Fig. 14), further investigations with isolated compounds encompassing nerve conduction studies would be more confirmatory.
Assessment of EMZSB on hot plate-induced neuropathic pain in diabetic rats. Values were presented as mean ± SEM of two readings (n = 6), statistics employed was one-way ANOVA followed by Tukey’s test. $p < 0.001 when compared with normal control; *p < 0.05, ns (non-significant) when compared with diabetic control. Diabetic rats treated with EMZSB do not exhibit any significant improvement in the pain sensitivity by licking the foot. Gray bar normal control, red bar diabetic control, brown bar diabetic + glimepiride, blue bar diabetic + EMZSB 150, yellow bar diabetic + EMZSB 200, green bar diabetic + EMZSB 250
Conclusions
Data of our study exhibited that EMZSB exerts in vitro antioxidant, α-glucosidase enzyme inhibition, and in vivo antidiabetic properties with amelioration of diabetes-associated complications. Significant blood glucose-lowering effects with perfection in serum lipids, liver glycogen content, cardiovascular risk indices, and improvement in body weight and nephroprotective effects were observed, while a supportive histopathological mark of the normal architecture of kidneys was also documented. It can be concluded from these study results that bioactive phytocontents of MZSB, including flavonoids, terpenoids, glycosides, phenolic compounds, and β-sitosterol, stigmasterol, arjunolic acid, (+)-dihydrokaempferol, 3,4-dihydroxybenzoic acid, lupeol acetate, spinasterol, ergosterol peroxide, hederagonic acid, lupenone, and lupeol that are recognized to have several pharmacological benefits, might be actively and or synergistically responsible for these observed effects.
These preliminary study results suggest that Manilkara zapota stem bark extract could serve as a potential herbal alternative for the management of DM and likely prevention of its complications. Thus, ascribing a definite mechanism of action for antidiabetic effects could be more fruitful when further investigations with isolated compounds from this bark support these results. Studies are in progress in our laboratory to isolate its active phytoconstituents and explicate the likely mechanisms of action through which this bark seems to act.
Highlights from study
-
(a)
This is the first study exploring the antidiabetic effects of Manilkara Zapota (L.) P. Royen stem bark 70% ethanolic extract.
-
(b)
EMZSB-250 exerts potential antihyperglycemic and hypolipidemic effects with significant improvement in nephroprotective parameters, cardiovascular risk indices, and evidence of reversal of normal histoarchitecture of the kidneys.
-
(c)
Studied EMZSB has a strong future prospective for isolation of active antidiabetic principles and elucidation of its mechanism of action through which it seems to act.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACAT-1:
-
Acetyl-CoA Acetyltransferase 1 Protein Coding gene
- ACAT-2:
-
Acetyl-CoA Acetyltransferase 2 Protein Coding gene
- AKT pathway:
-
Protein kinase B signal transduction pathway
- AMPK:
-
AMP-activated protein kinase
- ANOVA:
-
Analysis of variance
- DPPH:
-
2, 2-Diphenyl-1-picrylhydrazyl
- GLUT4:
-
Glucose transporter type 4
- GOD-POD:
-
Glucose oxidase–peroxidase
- GSK3b:
-
Glycogen synthase kinase-3
- HDL:
-
High-density lipoprotein
- H2O2 :
-
Hydrogen peroxide
- IC50 :
-
The concentration of drug required for 50% inhibition
- IGT:
-
Impaired glucose tolerance
- IRS1:
-
Insulin receptor substrate 1
- LDL:
-
Low density lipoprotein
- K. pneumoniae :
-
Klebsiella pneumoniae
- OECD:
-
The Organization for Economic Co-operation and Development
- PI3K:
-
Phosphatidylinositol 3-kinase
- PTP1B:
-
Protein tyrosine phosphatase 1B
- S. epidermidis :
-
Staphylococcus epidermidis
- SEM:
-
Standard error mean
- SD:
-
Standard deviation
- SNRIs:
-
Serotonin and norepinephrine reuptake inhibitors
References
Ajiboye BO, Oloyede HO, Salawu MO (2018) Antihyperglycemic and antidyslipidemic activity of Musa paradisiaca-based diet in alloxan-induced diabetic rats. Food Sci Nutr 6(1):137–145
Akhtar N, Akram M, Daniyal M, Ahmad S (2018) Evaluation of antidiabetic activity of Ipomoea batatas L. extract in alloxan-induced diabetic rats. Int J Immunopathol Pharmacol 32:1–6. https://doi.org/10.1177/2058738418814678
Alam MN, Bristi NJ, Rafiquzzaman M (2013) Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 21(2):143–152. https://doi.org/10.1016/j.jsps.2012.05.002
Arun KB, Jayamurthy P, Anusha CV, Mahesh SK, Nisha P (2017) Studies on activity guided fractionation of pomegranate peel extracts and its effect on antidiabetic and cardiovascular protection properties. J Food Process Preserv 41(1):e13108. https://doi.org/10.1111/jfpp.13108
Babu S, Jayaraman S (2020) An update on β-sitosterol: a potential herbal nutraceutical for diabetic management. Biomed Pharmacother 131:110702. https://doi.org/10.1016/j.biopha.2020.110702
Bhat ZA, Ansari SH, Mukhtar HM, Naved T, Khan NA, Chashoo IA (2005) Anti-hyperglycemic activity of the alcoholic extracts of Aralia cachemirica Decneroota. J Nat Rem 5:160–164
Boligon AA, Machado MM, Athayde ML (2014) Technical evaluation of antioxidant activity. Med Chem 4(7):517–522. https://doi.org/10.22377/ijgp.v12i03.2025
Boyce-Rustay JM, Jarvis MF (2009) Neuropathic pain: models and mechanisms. Curr Pharm Des 15(15):1711–1716
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28(1):25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Carroll NV, Longley RW, Roe JH (1956) The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem 220(2):583–593
Chika A, Bello SO (2010) Antihyperglycemic activity of aqueous leaf extract of Combretum micranthum (Combretaceae) in normal and alloxan-induced diabetic rats. J Ethnopharmacol 129(1):34–37. https://doi.org/10.1016/j.jep.2010.02.008
Chinsembu KC (2019) Diabetes mellitus and nature’s pharmacy of putative antidiabetic plants. J Herb Med 15:100230
Chunhakant S, Chaicharoenpong C (2019) Antityrosinase, antioxidant, and cytotoxic activities of phytochemical constituents from Manilkara zapota L. Bark. Molecules 24(15):2798
Crespo MJ, Zalacaín J, Dunbar DC, Cruz N, Arocho L (2008) Cardiac oxidative stress is elevated at the onset of dilated cardiomyopathy in streptozotocin-diabetic rats. J Cardiovasc Pharmacol Ther 13(1):64–71
Dra LA, Sellami S, Rais H, Aziz F, Aghraz A, Bekkouche K, Larhsini M (2019) Antidiabetic potential of Caralluma europaea against alloxan-induced diabetes in mice. Saudi J Biol Sci 26(6):1171–1178
Duong TH, Trung NT, Phan CTD, Nguyen VK, Musa V, Ruksilp T, Sichaem J (2020) Manilkzapotane, a novel dimeric alkylresorcinol derivative from the stem bark of Manilkara zapota. J Asian Nat Prod Res 23:1–7
Eawsakul K, Panichayupakaranant P, Ongtanasup T, Warinhomhoun S, Noonong K, Bunluepuech K (2021) Computational study and in vitro alpha-glucosidase inhibitory effects of medicinal plants from a Thai folk remedy. Heliyon 7(9):e08078. https://doi.org/10.1016/j.heliyon.2021.e08078
Eliza J, Daisy P, Ignacimuthu S, Duraipandiyan V (2009) Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.) Sm., in STZ-induced diabetic rats. Chem Biol Interact 182(1):67–72. https://doi.org/10.1016/j.cbi.2009.08.012
Elshemy MA (2018) Antidiabetic and anti-hyperlipidemic effects of virgin coconut oil in rats. Egypt J Vet Sci 49(2):111–117. https://doi.org/10.21608/ejvs.2018.3548.1036
Fujisawa T, Ikegami H, Inoue K, Kawabata Y, Ogihara T (2005) Effect of two αglucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 54:387–390
Ghani A (1998) Medicinal plants of Bangladesh: chemical constituents and uses. Asiatic Society of Bangladesh, Dhaka
Ghosh J, Sil PC (2013) Arjunolic acid: a new multifunctional therapeutic promise of alternative medicine. Biochimie 95(6):1098–1109. https://doi.org/10.1016/j.biochi.2013.01.016
Gomathy K, Baskar R, Kumaresan K (2013) Comparison of antioxidant potential in pulp and peel extracts of Manilkara zapota (L.) P. Royen. Afr J Biotech 12(31):4936–4943. https://doi.org/10.5897/AJB2012.2961
Grover JK, Vats V, Yadav S (2002) Effect of feeding aqueous extract of Pterocarpus marsupium on glycogen content of tissues and the key enzymes of carbohydrate metabolism. Mol Cell Biochem 241(1):53–59
Gupta R, Sharma AK, Sharma MC, Dobhal MP, Gupta RS (2012) Evaluation of antidiabetic and antioxidant potential of lupeol in experimental hyperglycaemia. Nat Prod Res 26(12):1125–1129
Gurupriya S, Cathrine L, Ramesh J (2018) In vitro antidiabetic and antioxidant activities of lupeol isolated from the methanolic extract of Andrographis echioides leaves. J Pharmacogn Phytochem 7(4):768–775
Halliwell B, Clement MV, Long LH (2000) Hydrogen peroxide in the human body. FEBS Lett 486(1):10–13. https://doi.org/10.1016/S0014-5793(00)02197-9
Hilma R, Herliani H, Almurdani M (2018) Determination of total phenolic, flavonoid content and free radical scavenging activity of ethanol extract Sawo stem bark (Manilkara Zapota (L.)). Pros CELSciTech 3:62–68
Ibrahim MA, Islam MS (2014) Anti-diabetic effects of the acetone fraction of Senna singueana stem bark in a type 2 diabetes rat model. J Ethnopharmacol 153(2):392–399
International Diabetes Federation (IDF) (2011) Diabetes Atlas, 5th edn. International Diabetes Federation, Brussels, Belgium
Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I (2003) Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52(7):1655–1663
Jing L, Ma H, Fan P, Gao R, Jia Z (2015) Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement Altern Med 15(1):1–12. https://doi.org/10.1186/s12906-015-0820-3
Kaneria M, Baravalia Y, Vaghasiya Y, Chanda S (2009) Determination of antibacterial and antioxidant potential of some medicinal plants from Saurashtra region, India. Indian J Pharm Sci 2009(71):406
Kang MJ, Lee EK, Lee SS (2004) Effects of two P/S ratios with same peroxidizability index value and antioxidants supplementation on serum lipid concentration and hepatic enzyme activities of rats. Clin Chim Acta 350(1–2):79–87. https://doi.org/10.1016/j.cccn.2004.07.005
Karle PP, Dhawale SC, Navghare VV, Shivpuje SS (2021) Optimization of extraction conditions and evaluation of Manilkara zapota (L.) P. Royen fruit peel extract for in vitro α-glucosidase enzyme inhibition and free radical scavenging potential. Future J Pharm Sci 7(1):1–10
Khandelwal K (2008) Practical pharmacognosy. Pragati Books Pvt Ltd, Pune
Khanra R, Bhattacharjee N, Dua TK, Nandy A, Saha A, Kalita J, Dewanjee S (2017) Taraxerol, a pentacyclic triterpenoid, from Abroma augusta leaf attenuates diabetic nephropathy in type 2 diabetic rats. Biomed Pharmacother 94:726–741. https://doi.org/10.1016/j.biopha.2017.07.112
Kishore L, Kajal A, Kaur N (2017) Role of nicotinamide in streptozotocin induced diabetes in animal models. J Endocrinol Thyroid Res 2(1):555–577. https://doi.org/10.19080/JETR.2017.02.555577
Kole L, Sarkar M, Deb A, Giri B (2016) Pioglitazone, an anti-diabetic drug requires sustained MAPK activation for its anti-tumor activity in MCF7 breast cancer cells, independent of PPAR-γ pathway. Pharmacol Rep 68(1):144–154
Kumar A, Sahoo HB (2020a) Preliminary pharmacological evaluation of stems bark extract for Manilkara zapota ulcerative colitis in rats. Adv Pharm J 5(4):144–148
Kumar D, Kumar S, Kohli S, Arya R, Gupta J (2011) Antidiabetic activity of methanolic bark extract of Albizia odoratissima Benth. in alloxan induced diabetic albino mice. Asian Pac J Trop Med 4(11):900–903
Kumar S, Kumar V, Prakash O (2013) Enzymes inhibition and antidiabetic effect of isolated constituents from Dillenia indica. BioMed Res Int 2013:1–7
Kumar DA, Anusha SV, Oruganti S, Deshpande M, Zehra A, Tiwari AK (2015) Raw versus cooked vegetable juice. Nutrafoods 14(1):27–38
Latha RC, Daisy P (2011) Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chem Biol Interact 189(1–2):112–118. https://doi.org/10.1016/j.cbi.2010.11.005
Lee G, Goosens KA (2015) Sampling blood from the lateral tail vein of the rat. JoVE (j vis Exp) 99:e52766. https://doi.org/10.3791/52766
Lee YJ, Kim EO, Choi SW (2011) Isolation and identification of antioxidant polyphenolic compounds in mulberry (Morus alba L.) seeds. J Korean Soc Food Sci Nutr 40(4):517–524
Ma J, Luo XD, Protiva P, Yang H, Ma C, Basile MJ, Kennelly EJ (2003) Bioactive novel polyphenols from the fruit of Manilkara zapota (Sapodilla). J Nat Prod 66(7):983–986. https://doi.org/10.1021/np020576x
Macdonald Ighodaro O, Mohammed Adeosun A, Adeboye Akinloye O (2017) Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 53(6):365–374. https://doi.org/10.1016/j.medici.2018.02.001
Maniyar Y, Bhixavatimath P (2012) Antihyperglycemic and hypolipidemic activities of aqueous extract of Carica papaya Linn. leaves in alloxan-induced diabetic rats. J Ayurveda Integr Med 3(2):70. https://doi.org/10.4103/0975-9476.96519
Manna P, Sil PC (2012) Impaired redox signaling and mitochondrial uncoupling contributes vascular inflammation and cardiac dysfunction in type 1 diabetes: protective role of arjunolic acid. Biochimie 94(3):786–797
Meier C, Schwartz AV, Egger A, Lecka-Czernik B (2016) Effects of diabetes drugs on the skeleton. Bone 82:93–100
Mohiddin YBH, Chin W, Worth DH (1992) Traditional medicinal plants of Brunei Darussalam part III. Sengkurong. Int J Pharmacogn 30(2):105–108. https://doi.org/10.3109/13880209209053967
Muhtadi Primarianti AU, Sujono TA (2015) Antidiabetic activity of durian (Durio zibethinus Murr.) and rambutan (Nephelium lappaceum L.) fruit peels in alloxan diabetic rats. Procedia Food Sci 3:255–261
Munhoz A, Frode TS (2018) Isolated compounds from natural products with potential antidiabetic activity-a systematic review. Curr Diabetes Rev 14(1):36–106
Musabayane CT (2012) The effects of medicinal plants on renal function and blood pressure in diabetes mellitus. Cardiovasc J S Afr 23:462–468
Nadig PD, Revankar RR, Dethe SM, Narayanswamy SB, Aliyar MA (2012) Effect of Tinospora cordifolia on experimental diabetic neuropathy. Indian J Pharmacol 44:580
Nagarajan NS, Murugesh N, Kumaresan PT, Radha N, Murali A (2005) Antidiabetic and antihyperlipemic effects of Clemeo felina. Fitoterapia 76(34):310–315. https://doi.org/10.1016/j.fitote.2005.03.020
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B (2009) American Diabetes Association, European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32:193–203
Nkobole N, Bodede O, Hussein AA, Prinsloo G (2021) In vitro α-glucosidase and α-amylase activities of wild and cultivated Amaranthus spp. and isolated compounds. Pharmacogn J 13(6):1614–1620
Noor S, Prodhan A, Zohora FT, Tareq FS, Ahsan M, Hasan CM, Islam SN (2014) Phytochemical, antioxidant, antimicrobial, thrombolytic as well as cytotoxic studies on the stem bark of Manilkara zapota (sapotaceae). Asian J Chem 26(18):6138
Osman MA, Rashid MM, Aziz MA, Habib MR (2011) Inhibition of Ehrlich ascites carcinoma by Manilkara zapota L. stem bark in Swiss albino mice. Asian Pac J Trop Biomed 1(6):448–451
Quine SD, Raghu PS (2005) Effects of (-)-epicatechin, a flavonoid on lipid peroxidation and antioxidants in streptozotocin-induced diabetic liver, kidney and heart. Pharmacol Rep 57(5):610–615
Ramkumar KM, Vanitha P, Uma C, Suganya N, Bhakkiyalakshmi E, Sujatha J (2011) Antidiabetic activity of alcoholic stem extract of Gymnema montanum in streptozotocin-induced diabetic rats. Food Chem Toxicol 49(12):3390–3394. https://doi.org/10.1016/j.fct.2011.09.027
Ramu R, Shirahatti PS, Nayakavadi S, Vadivelan R, Zameer F, Dhananjaya BL, Prasad N (2016) The effect of a plant extract enriched in stigmasterol and β-sitosterol on glycaemic status and glucose metabolism in alloxan-induced diabetic rats. Food Funct 7(9):3999–4011
Ramya S, Narayanan V, Ponnerulan B, Saminathan E, Veeranan U (2020) Potential of peel extracts of Punica granatum and Citrus aurantifolia on alloxan-induced diabetic rats. Beni-Suef Univ J Basic Appl Sci 9(1):1–11
Rees DA, Alcolado JC (2005) Animal models of diabetes mellitus. Diabet Med 22(4):359–370
Ruch RJ, Cheng SJ, Klaunig JE (1989) Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10(6):1003–1008. https://doi.org/10.1093/carcin/10.6.1003
Saltos MBV, Puente BFN, Faraone I, Milella L, De Tommasi N, Braca A (2015) Inhibitors of α-amylase and α-glucosidase from Andromachia igniaria Humb. & Bonpl. Phytochem Lett 14:45–50
Savikin K, Zivkovic J, Alimpic A, Zdunic G, Jankovic T, Duletic-Lausevic S, Menkovic N (2018) Activity guided fractionation of pomegranate extract and its antioxidant, antidiabetic and antineurodegenerative properties. Ind Crops Prod 113:142–149
Shah SK, Jhade DN (2018) Evaluation of antifertility potential of Piper betle (Petiole) on female Wistar rats “rising approaches of herbal contraception’’. Biochem Biophys Rep 15:97–102. https://doi.org/10.1016/j.bbrep.2018.08.001
Shirwaikar A, Rajendran K, Barik R (2006) Effect of aqueous bark extract of Garuga pinnata Roxd. in streptozotocin-nicotinamide induced type-II diabetes mellitus. J Ethnopharmacol 107:285–290
Smith DC (2013) Methods of euthanasia and disposal of laboratory animals. Methods Anim Exp 1:167–195
Soussi A, Gargouri M, ElFeki A (2018) Effects of co-exposure to lead and zinc on redox status, kidney variables, and histopathology in adult albino rats. Toxicol Ind Health 34(7):469–480. https://doi.org/10.1177/0748233718770293
Srisurichan S, Pornpakakul S (2015) Triterpenoids from the seedpods of Holarrhena curtisii King and Gamble. Phytochem Lett 12:282–286
Tawakoli M, Mojadidi M, Fadavi H, Malik RA (2012) Pathophysiology and treatment of painful diabetic neuropathy. Curr Pain Headache Rep 3:192–197
Toze FAA, Fomani M, Nouga AB, Chouna JR, Waffo AFK, Wansi JD (2015) Taraxastane and lupane triterpenoids from the bark of Manilkara zapota. Int Res J Pure Appl Chem 7:157–164
Venkatesan T, Choi YW, Kim YK (2019) Impact of different extraction solvents on phenolic content and antioxidant potential of Pinus densiflora bark extract. BioMed Res Int 2019:1–14
Wan LS, Min QX, Wang YL, Yue YD, Chen JC (2013) Xanthone glycoside constituents of Swertia kouitchensis with α-glucosidase inhibitory activity. J Nat Prod 76(7):1248–1253. https://doi.org/10.1021/np400082g
Wang Y, Xiang L, Wang C, Tang C, He X (2013) Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE 8(7):e71144
Wang J, Huang M, Yang J, Ma X, Zheng S, Deng S, Zhao P (2017) Anti-diabetic activity of stigmasterol from soybean oil by targeting the GLUT4 glucose transporter. Food Nutr Res 61(1):1364117
Wen X-A, Liu J, Zhang LY, Ni P-Z, Sun H-B (2010) Synthesis and biological evaluation of arjunolic acid, bayogenin, hederagonic acid and 4-epi-hederagonic acid as glycogen phosphorylase inhibitors. Chin J Nat Med 8(6):441–448. https://doi.org/10.1016/S1875-5364(11)60006-X
Withouck H, Boeykens A, Broucke MV, Moreira MM, Delerue-Matos C, De Cooman L (2019) Evaluation of the impact of pre-treatment and extraction conditions on the polyphenolic profile and antioxidant activity of Belgium apple wood. Eur Food Res Technol 245(11):2565–2578
Xu F, Wu H, Wang X, Yang Y, Wang Y, Qian H, Zhang Y (2014) RP-HPLC characterization of lupenone and β-sitosterol in Rhizoma musae and evaluation of the anti-diabetic activity of lupenone in diabetic Sprague-Dawley rats. Molecules 19(9):14114–14127
Acknowledgements
The authors are thankful to the research center, School of Pharmacy, and RUSA Centre for Herbo Medicinal Studies at S.R.T.M. University, Nanded, India. Sincerely acknowledges S. N. Institute of Pharmacy, Pusad, India, for providing laboratory animal research facilities. The authors are grateful to the Botanical Survey of India, Pune, India, for the identification and authentication of plant material.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
P.P.K. contributed to conceptualization, methodology, investigation, data interpretation, and editing and writing original draft. S.C.D. was involved in methodology supervision. R.J.M. and V.V.N. contributed to formal analysis. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional animal ethics committee (Approval No.: SNIOP/CPCSEA/IAEC/CP-PL/01-2021, Dated: 20-01-2021), and animal care and handling were in accordance with the CPCSEA guidelines for usage of laboratory experimental animals in India.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karle, P.P., Dhawale, S.C., Mandade, R.J. et al. Screening of Manilkara zapota (L) P. Royen stem bark ethanolic extract for in vitro α-glucosidase inhibition, preliminary antidiabetic effects, and improvement of diabetes and its complications in alloxan-induced diabetes in Wistar rats. Bull Natl Res Cent 46, 110 (2022). https://doi.org/10.1186/s42269-022-00783-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00783-3