Abstract
Background
Chrysophyllum albidum is a well-known medicinal plant in Africa and has many medicinal properties. This study investigated the effect of sonication, solvent polarity (acetone and ethanol), and plant matrix (bark and seeds) on the antioxidant property of C. albidum. The bark of C. albidum was subjected to sonication and soaking with acetone to evaluate the effect of sonication on the antioxidant property, and C. albidum bark and seeds were subjected to ultrasonic-assisted extraction of acetone and ethanol to evaluate the effect of solvents and plant matrix on the antioxidant property of C. albidum. The phytochemical composition, total flavonoid content, total antioxidant activity, total phenol content, lipid peroxidation (LPO) inhibition activity, nitric oxide, and 2, 2-diphenyl-1-picrylhydrazyl radicals scavenging activities were evaluated in all extracts.
Results
Sonication increased the percentage yield of extracts compared to maceration. Flavonoids and terpenoids were present, while saponins were absent in all extracts evaluated. Ultrasound-assisted extraction increased C. albidum antioxidant property compared to maceration. Ethanol was the most suitable solvent for C. albidum bark, while acetone was the most suitable solvent for C. albidum seeds. C. albidum bark extracts were most active as free radical scavengers, while the seed extracts were most active as inhibitors of lipid peroxidation.
Conclusions
Extraction technique, extraction solvent, and plant matrix significantly affect the antioxidant properties of C. albidum. This study indicates that the selection of an effective extraction process for medicinal plants depends on the phytochemical compound responsible for the biological activity of interest.
Similar content being viewed by others
Background
Plants are known to possess biologically active compounds with significant folkloric health benefits. However, the identification, isolation, and characterization of these bioactive compounds from medicinal plants matrix require the careful selection of suitable extraction procedures and solvents (Azwanida 2015). Convenient, inexpensive, efficient, and highly applicable extraction technology is desirable in small- and medium-scale phytochemical exploration operations (de Morais Rodrigues et al. 2016; Sousa et al. 2014). Sonication is a highly reproducible and efficient modern extraction technique that accelerates bulk transfer and solvent diffusion through membranes to release cell contents (Toma et al. 2001; Yang et al. 2009). Maceration is a convenient, less costly, and more applicable method of extraction (Dhanani et al. 2017; Vongsak et al. 2013). These extraction techniques require the use of solvents, and a suitable solvent increases phytochemical components of the extract which in turn influences the antioxidant properties (Dhanani et al. 2017; Vongsak et al. 2013). Plant parts have been reported to synthesize and store varieties of phytochemicals responsible for their antioxidant property and diverse biological activities (Iloki-Assanga et al. 2015; Rafat et al. 2010).
C. albidum, G Don-Holl (Sapotaceae) commonly known as African Star Apple, is a tree commonly found in Africa with diverse ethnomedicinal uses (Adebayo et al. 2010; Adebayo and Krettli 2011). C. albidum leaves, fruits, roots, bark, and seeds extracts have been reported to possess various pharmacological properties attributed to their phytochemical contents and antioxidant activities (Adebayo et al. 2010; Adebayo and Krettli 2011; Amusa et al. 2003; Abiodun 2014; Orijajogun et al. 2013). The plant is also known for its contraceptive (Onyeka et al. 2012), anti-plasmodial (Adewoye et al. 2010; Idowu et al. 2006), hypolipidemic, and anti-hyperglycemic (Olorunnisola et al. 2008) properties. Hence, it is important to investigate a suitable extraction technique, extraction solvent, and plant parts for the optimization of its antioxidant activity. This study investigated the effect of extraction technique, solvent polarity, and plant matrix on the antioxidant properties of C. albidum.
Methods
Chemicals
Naphthalene ethylenediamine dihydrochloride, trichloroacetic acid (TCA), ammonium molybdate, thiobarbituric acid (TBA), orthophosphoric acid, sodium carbonate, sodium acetate, chloroform, concentrated sulfuric acid, trisodium chloride, hexane, acetic acid, sodium phosphate monobasic, potassium phosphate dibasic salt, sodium orthophosphate, sodium nitroprusside, sulfanilamide, sodium dodecyl sulfate, DPPH (1,1 diphenyl-2-picrylhydrazyl), ferrous sulfate. All chemicals and reagents used for this study are of analytical grade and are purchased from Merck Millipore and Sigma-Aldrich Inc. St Louis, USA.
Plant material
The bark and fruits of C. albidum were harvested from Owena-Ijesha community farm, Osun State, Nigeria. They were then identified and assigned specimen voucher number FHI 110105 and deposited at the Forest Research Institute Nigeria (FRIN). The bark and seeds of C. albidum were air-dried for 14 days at room temperature and pulverized.
Preparation of Chrysophyllum albidum extracts
The extraction of C. albidum bark and seeds was done with two solvents (99.8% acetone and 99.8% ethanol) using two methods: maceration and sonication. The extracts were prepared as follows: powdered bark (5 g) was macerated in 100 ml (99.8%) of acetone for 30 min with constant shaking, and the solvent was evaporated to dryness to yield the dried extract labeled CABAM. Another set of powdered bark (10 g) was soaked in 200 ml acetone (99.8%) and subjected to sonication (Model: Sonicator (XUB18UK, Grant) for 30 min to yield the extract labeled CABAS. The powdered seeds (10 g) were soaked in 200 ml acetone (99.8%) and subjected to sonication (Model: Sonicator (XUB18UK, Grant) for 30-min labeled CASAS 30. A separate set of powdered bark (10 g) and powdered seeds (10 g) was soaked in ethanol (200 ml) and subjected to sonication (Model: Sonicator (XUB18UK, Grant) for 30 min, respectively, and the extracts were labeled CABES and CASES, respectively.
Phytochemical screening of the extracts
The extracts were tested for the presence of saponins, steroids, and terpenoids as described by Deepti et al. (2012), Edeoga et al. (2005), and Ghagane et al. (Ghagane et al. 2017); tannins, phlobatannins, and cardiac glycosides (Salkowski’s test) according to the method described by Deepti et al. (2012), Ghagane et al. (2017), and Evans and Trease (2002); and flavonoids as described by, Deepti et al. (2012), Ghagane et al. (2017), and Sofowora (1993).
Evaluation of antioxidant property of extracts
Determination of the total phenolic content of extracts
The total phenolic present in each extract was evaluated by a method previously described by Ghagane et al. (Ghagane et al. 2017), Muhammad et al. (2021), and Singleton et al. (Singleton et al. 1999). In brief, 1 mg/ml of crude extract (200 µL) was mixed with Folin-Ciocalteu reagent (0.5 mL), followed by 20% (w/v) sodium carbonate (2 mL) after 3 min. The mixture was kept in the dark for 1 h and the absorbance was read at 650 nm. A calibration curve was prepared for the estimation of the total phenolic content in each sample using gallic acid. The results were expressed as milligram of gallic acid equivalent per gram dry weight of the extract.
Determination of the total flavonoid content of extracts
The aluminum chloride method previously described by Ghagane et al. (2017), Muhammad et al. (2021), and Chang et al. (2002) was used to determine the total flavonoid contents of the extracts. Briefly, 50 µL of crude extract (1 mg/mL) was mixed with methanol to make 1 ml of the mixture. The mixture was further mixed with 4 mL of distilled water and incubated for 5 min. Then, 0.3 mL of AlCl3 (10%) and 0.3 mL of NaNO2 (5%) were added to the mixture and allowed to settle. After 6 min, 1 M NaOH (2 mL) was added to the mixture and incubated for 15 min. The absorbance of each mixture was taken at 510 nm and the total flavonoid content was estimated from a quercetin calibration curve. The result was expressed as milligram quercetin equivalent per gram dry weight of the extract.
Determination of the total antioxidant capacity of the extracts
The total antioxidant activity of each extract was calculated by the method described by Ghagane et al. (2017), Muhammad et al. (2021), and Prieto et al. (1999). 0.1 mL of sample (100 μg) solution was combined with 1 mL of reagent (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). It was then incubated at 95 °C in a water bath for 90 min. The absorbance was read at 695 nm against blank in a spectrophotometer, after cooling to room temperature. An equal volume of solvent used to dissolve the sample was incubated with 1 mL of reagent solution under the same conditions as the samples, which served the blank. The antioxidant capacity of each extract was expressed as equivalents of ascorbic acid.
Evaluation of DPPH radical scavenging activity of the extracts
In this experiment, the DPPH radical scavenging activity was determined according to the method of Ghagane et al. (2017), Muhammad et al. (2021), and Manzocco et al. (1998). 0.0158 g of 1,1-diphenyl-2-picrylhydrazyl (DPPH) was dissolved in 400 ml of methanol. 1 ml of it was added to 1 ml of extracts of various concentrations ranging from 5 µg/ml to 100 µg/ml and allowed to react at room temperature for 30 min. The reaction mixture was vortexed thoroughly and incubated at room temperature in the dark for 30 min. The absorbance of the mixture was read at 517 nm. DPPH radical scavenging activity of extracts and standard was expressed as % scavenging activity. It was calculated using the given formula: % Scavenging activity = ((Ao − A)/Ao) × 100 where Ao is the absorbance of the control and A is the absorbance of the extract. The percentage of DPPH radical scavenging activity was used to acquire the IC50 value of each extract, defined as the concentration of extract necessary to cause 50% inhibition. The smaller the IC50 value, the higher is the antioxidant activity.
Evaluation of nitric oxide scavenging activity of the extracts
In this experiment, the nitric oxide radical scavenging activity was determined according to the method of Marcocci et al. (1994). 2 mL of 10 mM sodium nitroprusside dissolved in 0.5 mL phosphate buffer saline (pH 7.4) was mixed with 0.5 mL of extract at various concentrations (5 µg/ml to 100 µg/ml). The mixture was then incubated at 25 °C. After 150 min of incubation, 0.5 ml of the incubated solution was withdrawn and mixed with 0.5 mL of Griess reagent (2% sulfanilamide in 5% phosphoric acid, and 0.2% naphthyl ethylenediamine dihydrochloride (NEDD)). The mixture was then incubated at room temperature for 30 min and its absorbance was measured at 546 nm. Nitric oxide radical scavenging activity of extracts and standard (ascorbic acid) was expressed as % scavenging activity. It was calculated using the given formula: % Scavenging activity = ((Ao − A)/Ao) × 100 where Ao is the absorbance of the control and A is the absorbance of the extract. The percentage of nitric oxide radical scavenging activity was used to acquire the IC50 value of each extract, defined as the concentration of extract necessary to cause 50% inhibition. The smaller the IC50 value, the higher is the antioxidant activity.
Assay of lipid peroxidation inhibition activity of the extracts
Yolk sample (50 g) was dissolved in 150 ml of chloroform and 1% sulfuric acid in methanol (75 ml). The mixture was mixed in a stoppered tube for 2 h. 50 ml of 5% NaCl solution was added and the required esters were extracted with hexane (3 \(\times\) 30 ml) using Pasteur pipette, and the layer was separated. The hexane aliquot was washed with 2% KHCO3 (40 ml) and dried over anhydrous sodium sulfate. The solution was then filtered to remove the drying agent (Kaźmierska et al. 2005). Lipid peroxidation inhibitory activity of each extract was determined according to the method of Ohkawa et al. (Ohkawa et al. 1979). The tissue (egg yolk lipid extract) was homogenized in 0.1 M buffer pH 7.4 with a Teflon-glass homogenizer. Lipid peroxidation (LPO) in this homogenate was determined by measuring the amounts of malondialdehyde (MDA) produced primarily. Extracts at varying concentrations (5 µg/ml to 100 µg/ml) were added to 100 μl of (15 mM) ferrous sulfate followed by the addition of 3 ml of homogenate. After incubation for 30 min, 0.1 ml of this reaction mixture was mixed with 1.5 ml of 10% TCA and incubated for 10 min. 1.5 ml of 0.67% TBA (in 50% acetic acid) was added and placed in a boiling water bath for 30 min. After centrifugation at 3000 rpm for 10 min, the upper organic layer was taken and its OD (optical density) was measured at 532 nm against an appropriate blank without the sample. The lipid peroxidation inhibitory activity of the extracts and standard (ascorbic acid) was expressed as % inhibition. It was calculated using the following formula: % Inhibition = ((Ao − A)/Ao) × 100 where Ao is the absorbance of the control and A is the absorbance of the extract. The percentage of nitric oxide radical scavenging activity was used to acquire the IC50 value of each extract, defined as the concentration of extract necessary to cause 50% inhibition. The smaller the IC50 value, the higher is the antioxidant activity.
Statistical analysis
All the analyses were run in triplicates. Results were then computed using Microsoft Excel software (Microsoft Corporation, Redmond, WA) and analyzed using appropriate analysis of variance (ANOVA) followed by Duncan’s new multiple range test (DNMRT) and Pearson’s correlation analysis using GraphPad Prism 6.01 (GraphPad Software Inc., CA, USA). The criterion for statistical significance level was set at p < 0.05.
Results
The percentage yield and phytochemical constituents of C. albidum extracts
The percentage yield and phytochemical screening results of C. albidum crude extracts are presented in Table 1. CASES had the highest yield of 13%, while CABAM had the lowest yield of 2%. Sonication (4%) increased the percentage yield compared to maceration (2%). Ethanol gave a higher percentage yield (bark: 12% and seeds: 13%) compared to acetone (bark: 4% and seeds: 5%). The percentage yield of the seeds was high compared to the bark. Terpenoids and flavonoids were present in all extracts, while saponins were absent in all extracts. Bark acetone extract obtained by sonication had cardiac glycosides which were absent in the extract obtained by maceration. Ethanol extracts had more phytochemical constituents compared to acetone extracts, especially the ethanol seeds extract which had steroids, tannins, phlobatannins, and cardiac glycosides that were absent in acetone seed extract. Phlobatannins were present in the bark extracts but absent in seeds extracts.
Total phenolic content, total flavonoid content, and total antioxidant capacity of C. albidum extracts
The total phenolic content and flavonoids content of C. albidum extracts are shown in Table 2. The total phenolic content of extract obtained by sonication (74.40 ± 10.15 mg GAE/100 g) was not significantly different (p < 0.05) from the extract obtained by maceration (55.58 ± 3.49 mg GAE/100 g). Acetone seeds extract (84.65 ± 7.57 mg GAE/100 g) had significantly higher total phenolic content (p < 0.05) compared to ethanol seeds extract (63.72 ± 6.93 mg GAE/100 g). Ethanol and acetone bark extracts was not significantly different from each other. The total phenolic content of bark acetone and ethanol extracts were not significantly different from seeds acetone and ethanol extracts, respectively.
CABAM had the highest total flavonoid content (74.69 ± 1.59 mg QE/100 g), while CASES had the lowest total flavonoid content (27.34 ± 4.76 mg QE/100 g). The total flavonoid content of extract obtained by maceration (74.69 ± 1.59 mg QE/100 g) was significantly (p < 0.05) higher compared to extract obtained by sonication (35.68 ± 0.09 mg QE/100 g). Ethanol bark extract (69.84 ± 4.22 mg QE/100 g) had significantly (p < 0.05) higher total flavonoid content than acetone bark extract (35.68 ± 0.09 mg QE/100 g), while acetone seed extract (50.10 ± 2.49 mg QE/100 g) had significantly (p < 0.05) higher total flavonoid content than ethanol seed extract (27.34 ± 4.76 mg QE/100 g). The total flavonoid content of bark ethanol extract was significantly (p < 0.05) higher compared to seed ethanol extract, while seed acetone extract was significantly (p < 0.05) higher compared to bark acetone extract.
CABES had the highest total antioxidant capacity (2625.34 ± 6.31 mg AAE/100 g), while CABAM had the lowest total antioxidant capacity (914.60 ± 23.37 mg AAE/100 g). Sonication significantly (p < 0.05) increased the total antioxidant capacity (1431.82 ± 96.42 mg AAE/100 g) of bark extract compared to maceration (914.60 ± 23.37 mg AAE/100 g). Ethanol extracts (bark: 2625.34 ± 6.31 mg AAE/100 g; seed: 2014.46 ± 14.61 mg AAE/100 g) had significantly (p < 0.05) higher total antioxidant capacity compared to acetone extracts (bark: 1431.82 ± 96.42 mg AAE/100 g; seed: 975.20 ± 87.65 mg AAE/100 g). The total antioxidant capacity of the bark extracts was significantly (p < 0.05) higher compared to the seed extracts.
Inhibition and free radical scavenging activity of C. albidum extracts
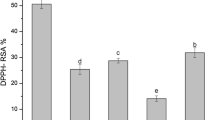
Figure 1 shows the DPPH scavenging activity of C. albidum extracts. All extracts evaluated scavenged DPPH radical in a concentration-dependent manner. The nitric oxide scavenging activity of C. albidum extracts is shown in Fig. 2. Nitric oxide scavenging activity of the studied extracts increased with increasing concentration. Figure 3 shows lipid peroxidation inhibitory activity of C. albidum extracts. All extracts were able to inhibit lipid peroxidation in a concentration-dependent manner. The IC50 values for DPPH scavenging activity, nitric oxide scavenging activity, and lipid peroxidation inhibition activity of C. albidum extracts are presented in Table 3. CASAS had the highest DPPH scavenging activity (IC50 value = 5.34 ± 0.85), while CABAM had the lowest DPPH scavenging activity (IC50 value = 41.52 ± 5.46). There was no significant difference in the DPPH scavenging activity of bark extract obtained by maceration compared to bark extract obtained by sonication. Ethanol bark extract (IC50 value = 18.66 ± 1.42) had significantly (p < 0.05) higher DPPH scavenging activity compared to acetone bark extract (IC50 value = 39.44 ± 4.62), while acetone seed extract (IC50 value = 5.34 ± 0.85) had significantly (p < 0.05) higher DPPH scavenging activity compared to ethanol seed extract (IC50 value = 20.04 ± 1.62). The DPPH scavenging activity of bark ethanol extract was not significantly different from seed ethanol extract, while seed acetone extract had significantly (p < 0.05) higher DPPH scavenging activity compared to bark acetone extract.
DPPH radical scavenging activity of C. albidum extracts. Values represent mean ± standard deviation (n = 3). CABAM C. albidum bark acetone extract obtained by maceration for 30 min, CABAS C. albidum bark acetone extract obtained by sonication for 30 min, CABES C. albidum bark ethanol extract obtained by sonication for 30 min, CASAS C. albidum seeds acetone extract obtained by sonication for 30 min, CASES C. albidum seeds ethanol extract obtained by sonication for 30 min, Asc. Acid ascorbic acid
Nitric oxide radical scavenging activity of C. albidum extracts. Values represent mean ± standard deviation (n = 3). CABAM C. albidum bark acetone extract obtained by maceration for 30 min, CABAS C. albidum bark acetone extract obtained by sonication for 30 min, CABES C. albidum bark ethanol extract obtained by sonication for 30 min, CASAS C. albidum seeds acetone extract obtained by sonication for 30 min, CASES C. albidum seeds ethanol extract obtained by sonication for 30 min, Asc. Acid ascorbic acid
Lipid peroxidation inhibitory activity of C. albidum extracts. Values represent mean ± standard deviation (n = 3). CABAM C. albidum bark acetone extract obtained by maceration for 30 min, CABAS C. albidum bark acetone extract obtained by sonication for 30 min, CABES C. albidum bark ethanol extract obtained by sonication for 30 min, CASAS C. albidum seeds acetone extract obtained by sonication for 30 min, CASES C. albidum seeds ethanol extract obtained by sonication for 30 min, Asc. Acid ascorbic acid
CASAS had the highest nitric oxide activity (IC50 value = 0.02 ± 0.01), while CABAM had the lowest nitric oxide scavenging activity (IC50 value = 4.16 ± 1.11). Sonication (IC50 value = 0.28 ± 0.16) significantly (p < 0.05) increased the nitric oxide scavenging activity of bark acetone extract compared to maceration (IC50 value = 4.16 ± 1.11). The nitric oxide scavenging activity of ethanol bark extract was not significantly different from acetone bark extract, while acetone seed extract (IC50 value = 0.02 ± 0.01) had significantly (p < 0.05) higher nitric oxide scavenging activity compared to ethanol seed extract (IC50 value = 3.15 ± 0.68). Bark ethanol extract (IC50 value = 1.10 ± 0.19) had significantly (p < 0.05) higher nitric oxide scavenging activity than seed ethanol extract (IC50 value = 3.15 ± 0.68), while the nitric oxide scavenging activity of bark acetone extract was not significantly different from seed acetone extract.
CABES had the highest lipid peroxidation inhibition activity (IC50 value = 19.71 ± 1.34), while CABAS had the lowest lipid peroxidation inhibition activity (IC50 value = 38.22 ± 2.11). The lipid peroxidation inhibition activity of extract obtained by maceration (IC50 value = 29.47 ± 1.17) was significantly higher compared to extract obtained by sonication (IC50 value = 38.22 ± 2.11). Ethanol bark extract (IC50 value = 19.71 ± 1.34) had significantly higher lipid peroxidation inhibition activity compared to acetone bark extract (IC50 value = 38.22 ± 2.11), while the lipid peroxidation inhibition activity of ethanol seed extract was not significantly different from acetone seed extract. Bark ethanol extract (IC50 value = 19.71 ± 1.34) had significantly (p < 0.05) higher lipid peroxidation inhibition activity than seed ethanol extract (IC50 value = 34.97 ± 2.52), while seed acetone extract (IC50 value = 30.63 ± 1.23) had significantly (p < 0.05) higher lipid peroxidation inhibition activity compared to bark acetone extract (IC50 value = 38.22 ± 2.11).
Correlation of phytochemical content and antioxidant activity of C. albidum extracts
The Pearson correlation coefficients of C. albidum extracts are shown in Table 4. Total phenol content had a very strong positive relationship with nitric oxide scavenging activity (r = 0.861) and DPPH scavenging activity (r = 0.717), while total flavonoid content (r = 0.745) and total antioxidant capacity (r = 0.552) had a strong positive relationship with lipid peroxidation inhibition.
Discussion
The bioactive phytochemicals are present in accurate concentrations. Hence, the selection of a suitable extraction method and corresponding extraction parameters is very important in the plant-based drug discovery process. Different parts of C. albidum had been reported to possess arrays of phytochemicals with medicinal and physiological benefits. However, this study aimed to investigate the effect of extraction parameters on the antioxidant activity of C. albidum in in vitro models.
In this study, saponins were absent, while terpenoids were present in all extracts examined. Steroids, tannins, terpenoids, flavonoids, cardiac glycosides, and phlobatannins were present in most of the C. albidum extracts evaluated in this present study. The absence of saponins might be attributed to the type of solvents used, and saponins are extracted majorly with solvents of higher polarity like water and methanol (Tiwari et al. 2011). Terpenoids are known to have anti-diarrheal properties. These classes of phytochemical compounds are known to show medicinal activity against several diseases, and it is not surprising that C. albidum extracts are used traditionally by herbalists to cure oxidative stress-related ill-health (Njoku and Obi 2009).
In this present study, ultrasound-assisted extraction (UAE, Sonication) increased the percentage yield of C. albidum extracts compared to maceration. C. albidum extracts obtained by UAE possessed cardiac glycosides which were absent in extracts obtained by maceration. Sonication significantly increased the total antioxidant content and nitric oxide scavenging property of C. albidum bark extract compared to maceration. Maceration significantly increased the total flavonoid content and lipid peroxidation inhibition capacity of C. albidum bark extracts compared to sonication. The extraction time (30 min) used in this study may be responsible for the inability of ultrasound-assisted extraction to significantly optimize the total flavonoids content of C. albidum extracts compared to maceration. Prolonged ultrasound-assisted extraction duration has been reported to lead to decomposition of some bioactive phytochemicals and formation of free radicals once equilibrium of inner and outer concentration is attained (Kaufmann and Christen 2002; Thoo et al. 2010). Ferreira et al. (Ferreira et al. 2019) suggested 60 min to be the best time for ultrasound-assisted extraction of flavonoids.
In this study, ethanol extracts had a higher percentage yield, extracted more phytochemical components, and possessed more total antioxidant content than acetone extracts. Ethanol extract of C. albidum bark possessed significantly higher total flavonoid content, DPPH radical scavenging activities, and lipid peroxidation inhibitory properties compared to an acetone extract of C. albidum bark. Conversely, total phenolic content, total flavonoid content, DPPH radical, and nitric oxide scavenging activities of acetone extracts of C. albidum seed were significantly high compared to ethanol extract of C. albidum seed. The result suggests that ethanol (relative polarity = 0.654; eluent strength = 0.88; dielectric constant = 24.3) is a more suitable solvent for UAE of bark, while acetone (relative polarity = 0.355; eluent strength = 0.56; dielectric constant = 24.3) is a more suitable solvent for UAE of seeds (Reichardt and Welton 2011). The extraction of bioactive compounds from plant material is strongly affected by the physical and chemical properties of the solvent system. The difference in the observed phytochemical composition and antioxidant activities of ethanol and acetone extracts may be due to the variation of their solvent strength and polarity which alters the solubility of bioactive compounds in the plant matrix (Do et al. 2014; Ngo et al. 2017). Moreover, ethanol is alcohol with hydrogen bonding like water, while acetone is a ketone with a keto group which may be responsible for the variation in the dissolution of polyphenolic compounds from C. albidum bark and seeds (Do et al. 2014).
The results of this study show that the percentage yield of C. albidum seed extracts was higher than the bark extracts. C. albidum bark extracts had more phytochemicals compared to C. albidum seed extracts especially phlobatannins which were present in all bark extracts and absent in all seed extracts. The seeds had significantly higher total flavonoid content compared to the bark extracts. The bark extracts had significantly higher total antioxidant capacity compared to the seed extracts. The scavenging properties of the bark and seed extracts were highly dependent on the extraction solvent. Ethanol was the best solvent for bark extraction, while acetone was the best solvent for seeds extraction. The antioxidant properties of C. albidum bark and seed extracts confirm their efficacy in the treatment of infections and inflammatory reactions (Idowu et al. 2006; Adisa 2000). The difference in antioxidant capacities of C. albidum bark and seeds observed in this study confirms that the anatomical distribution of bioactive compounds in a specific plant part dictates its solubility in a specific solvent (Do et al. 2014).
The statistical relationship between each antioxidant parameter of C. albidum extracts was measured by Pearson’s correlation coefficient. Pearson’s value is between + 1 and − 1, where 1 means perfect positive correlation, 0 means no correlation, and − 1 means perfect negative correlation. The very strong positive correlation between the total phenolic content and radical scavenging activity of C. albidum extracts observed in this study shows that the phenolic compounds in the extracts are responsible for their free radical scavenging properties which are supported by previous studies (Thoo et al. 2010). The strong positive correlation between the total flavonoid content and lipid peroxidation inhibition activity of C. albidum extracts observed in this study shows that the flavonoid compounds in the extracts are responsible for their lipid peroxidation inhibitory properties. Previous reports suggest that flavonoid compounds possess lipophilic character and are more thermally stable than phenolic compounds (Njoku and Obi 2009). This suggests that some phytochemical compounds in the extracts apart from phenolic compounds also contribute to their free radical scavenging properties.
Conclusions
This study provides useful insight into the optimum extraction conditions (technique, solvent, and plant matrix) for the antioxidant property of C. albidum. Extraction technique, extraction solvent, and plant matrix significantly influence the C. albidum antioxidant properties. However, the ultrasound-assisted extraction of bioactive compounds from C. albidum can further be optimized using a response surface methodology (RSM) for industrial application. This study indicates that the selection of an effective extraction process for medicinal plants depends on the phytochemical compound responsible for the biological activity of interest.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- UAE:
-
Ultrasound-assisted extraction
- AAE:
-
Ascorbic acid equivalent
- QE:
-
Quercetin equivalent
- GAE:
-
Gallic acid equivalent
- CABAM:
-
Chrysophyllum albidum Bark acetone extract obtained by maceration for 30 min
- CABAS:
-
Chrysophyllum albidum Bark acetone extract obtained by sonication for 30 min
- CABES:
-
Chrysophyllum albidum Bark ethanol extract obtained by sonication for 30 min
- CASAS:
-
Chrysophyllum albidum Seeds acetone extract obtained by sonication for 30 min
- CASES:
-
Chrysophyllum albidum Seeds ethanol extract obtained by sonication for 30 min
- Asc. Acid:
-
Ascorbic acid
References
Abiodun TG (2014) Phytochemical analysis and antimicrobial effect of Chrysophyllum albidum leave extract on gastrointestinal tract pathogenic bacteria and fungi in human. IOSR J Appl Chem 7(1):01–05
Adebayo JO, Krettli AU (2011) Potential antimalarials from Nigerian plants: a review. J Ethnopharmacol 133(2):289–302
Adebayo AH, Abolaji AO, Opata TK, Adegbenro IK (2010a) Effects of ethanolic leaf extract of Chrysophyllum albidum G. on biochemical and haematological parameters of albino Wistar rats. Afr J Biotechnol. 9(14):2145–2150
Adewoye EO, Salami AT, Taiwo VO (2010b) Anti-plasmodial and toxicological effects of methanolic bark extract of Chrysophyllum albidum in albino mice. J Physiol Pathophysiol 1(1):1–9
Adisa SA (2000) Vitamin C, protein and mineral contents of african apple (Chrysophyllum albidum). In: Garba SA, Ijagbone IF, Iyagba AO, Iyamu AO, Kilani AS, Ufaruna N (eds) Proceedings of the 18th annual conference of NIST, pp 141–146
Amusa NA, Ashaye OA, Oladapo MO (2003) Biodeterioration of the African star apple (Chrysophylum albidum) in storage and the effect on its food value. Afr J Biotechnol 2(3):56–59
Azwanida NN (2015) A review on the extraction methods use in medicinal plants, principle, strength, and limitation. Med Aromat Plants 4(196):1–6
Chang C-C, Yang M-H, Wen H-M, Chern J-C (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10(3):178–182
de Morais Rodrigues M, Borges L, Martins F, Mourão RH, da Conceição E (2016) Optimization of ultrasound-assisted extraction of phenolic compounds from Myrcia amazonica DC. (Myrtaceae) leaves. Pharmacogn Mag 12(45):9
Deepti K, Umadevi P, Vijayalakshmi G (2012) Antimicrobial activity and phytochemical analysis of Morinda tinctoria Roxb. leaf extracts. Asian Pac J Trop Biomed 2(3):S1440-1442
Dhanani T, Shah S, Gajbhiye NA, Kumar S (2017) Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab J Chem 10:S1193-1199
Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S et al (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 22(3):296–302
Edeoga HO, Okwu DE, Mbaebie BO (2005) Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 4(7):685–688
Evans WC, Trease GE (2002) Volatile oils and resins. Trease Evans Pharmacogn 66:253–288
Ferreira T, dos Santos JA, Modesto LA, Souza LS, dos Santos MPV, Bezerra DG et al (2019) An eco-friendly method for extraction and quantification of flavonoids in Dysphania ambrosioides. Rev Bras Farmacogn 29:266–270
Ghagane SC, Puranik SI, Kumbar VM, Nerli RB, Jalalpure SS, Hiremath MB et al (2017) In vitro antioxidant and anticancer activity of Leea indica leaf extracts on human prostate cancer cell lines. Integr Med Res 6(1):79–87
Idowu TO, Iwalewa EO, Aderogba MA, Akinpelu BA, Ogundaini AO (2006) Antinociceptive, anti-inflammatory and antioxidant activities of eleagnine: an alkaloid isolated from Chrysophyllum albidum seed cotyledons. J Biol Sci 6(6):1029–1034
Iloki-Assanga SB, Lewis-Luján LM, Lara-Espinoza CL, Gil-Salido AA, Fernandez-Angulo D, Rubio-Pino JL et al (2015) Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res Notes 8(1):1–14
Kaufmann B, Christen P (2002) Recent extraction techniques for natural products: microwave-assisted extraction and pressurised solvent extraction. Phytochem Anal Int J Plant Chem Biochem Tech 13(2):105–113
Kaźmierska M, Jarosz B, Korzeniowska M, Trziszka T, Dobrzański Z (2005) Comparative analysis of fatty acid profile and cholesterol content of egg yolks of different bird species. Pol J Food Nutr Sci 14(Suppl. 1):69–73
Manzocco L, Mastrocola D, Nicoli MC (1998) Chain-breaking and oxygen scavenging properties of wine as affected by some technological procedures. Food Res Int 31(9):673–678
Marcocci L, Maguire JJ, Droylefaix MT, Packer L (1994) The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun 201(2):748–755
Muhammad DRA, Tuenter E, Patria GD, Foubert K, Pieters L, Dewettinck K (2021) Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem 340:127983
Ngo TV, Scarlett CJ, Bowyer MC, Ngo PD, Vuong QV (2017) Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J Food Qual 6:66
Njoku VO, Obi C (2009) Phytochemical constituents of some selected medicinal plants. Afr J Pure Appl Chem 3(11):228–233
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Olorunnisola DS, Amao IS, Ehigie DO, Ajayi ZAF (2008) Antihyperglycemic and hypolipidemic effect of ethanolic extract of Chrysophyllum albidum seed cotyledon in alloxan induced diabetic rats. Res J Appl Sci 3(2):123–127
Onyeka CA, Aligwekwe AU, Olawuyi TS, Nwakanna AA, Oyeyemi AW (2012) Antifertility effects of ethanolic root bark extract of Chrysophyllum albidum in male albino rats. Int J Appl Res Nat Prod 5(1):12–17
Orijajogun OJ, Olajide OO, Fatokun AO, Orishadipe AT, Batari ML (2013) The preliminary chemical constituents and free radical scavenging activities of the exocarp of the fruit extract of african star apple (Chrysophyllum albidum G. Don). Int J Res Pharm Sci 3(3):66
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269(2):337–341
Rafat A, Philip K, Muni S (2010) Antioxidant potential and content of phenolic compounds in ethanolic extracts of selected parts of Andrographis paniculata. J Med Plants Res 4(3):197–202
Reichardt C, Welton T (2011) Solvents and solvent effects in organic chemistry. Wiley
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–178
Sofowora A (1993) Medicinal plants and traditional medicine in Africa. Ibadan. Niger Spectr Books Ltd., pp 191–289
Sousa JN, Pedroso NB, Borges LL, Oliveira GAR, Paula JR, Conceição EC (2014) Optimization of Ultrasound-assisted extraction of polyphenols, tannins, and epigallocatechin gallate from barks of Stryphnodendron adstringens (Mart.) Coville bark extracts. Pharmacogn Mag 10(Suppl 2):S318
Thoo YY, Ho SK, Liang JY, Ho CW, Tan CP (2010) Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem 120(1):290–295
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H (2011) Phytochemical screening and extraction: a review. Int Pharm Sci 1(1):98–106
Toma M, Vinatoru M, Paniwnyk L, Mason TJ (2001) Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason Sonochem 8(2):137–142
Vongsak B, Sithisarn P, Mangmool S, Thongpraditchote S, Wongkrajang Y, Gritsanapan W (2013) Maximizing total phenolics, total flavonoids contents, and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind Crops Prod 44:566–571
Yang B, Liu X, Gao Y (2009) Extraction optimization of bioactive compounds (crocin, geniposide, and total phenolic compounds) from Gardenia (Gardenia jasminoides Ellis) fruits with response surface methodology. Innov Food Sci Emerg Technol 10(4):610–615
Acknowledgements
Not applicable.
Funding
There was no specific grant received from any funding agency within the public, commercial, or not-for-profit sectors for this study.
Author information
Authors and Affiliations
Contributions
ACA MTO conceived and designed the experiments. OEF, AA performed the experiments. ACA, OBO, ZAA, MTO supervised the experiment. OBO ZAA analyzed the data. OBO ACA wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akinmoladun, A.C., Falaiye, O.E., Ojo, O.B. et al. Effect of extraction technique, solvent polarity, and plant matrix on the antioxidant properties of Chrysophyllum albidum G. Don (African Star Apple). Bull Natl Res Cent 46, 40 (2022). https://doi.org/10.1186/s42269-022-00718-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00718-y