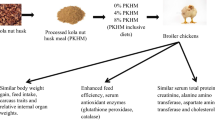
Abstract
Background
In a 42-day feeding trial, the effects of processed cocoa pod husk (PCHM) inclusion in a broiler chicken diet were assessed.
Methods
This experiment was conducted between December 2017 and January 2018. Cocoa pod husk was collected and processed by ash treatment and rumen liquor fermentation to form a processed cocoa pod husk (PCHM). Three experimental diets were formulated at both the starter and finisher phases, in which PCHM was included at 0, 4 and 8% and designated as diets 1, 2 and 3, respectively. One hundred and eighty 1-day-old Arbor Acres broiler chicks were randomly distributed to three dietary treatments (10 birds/replicate; 60 birds/treatment) in a completely randomized design. The growth performance, carcass, relative internal organ weights and haemato-biochemical indices were determined. Histological examination of the liver and heart samples was also determined.
Results
The PCHM inclusion did not affect (P > 0.05) the performance characteristics of the broiler chicks, except for the feed intake that significantly (P < 0.05) increased in birds fed 8% of PCHM-inclusive diet at the starter phase. The carcass traits, relative internal organ weights, haematological indices and serum biochemical indices of the broiler chickens were similar (P > 0.05) across the dietary treatments. The serum glutathione peroxidase and catalase concentration were higher (P < 0.05) in birds fed PCHM-inclusive diets compared to those fed the control diet. Similar histological myocardiac cell appearances were observed among the birds across the various dietary treatments. Sections show the myocardium composed of the cardiac muscle with peripherally placed nucleus separated by a defined interstitium that is free of inflammatory cells and collections. In the birds fed diet 2 and 3, histological variations observed were marked vascular congestion and perivascular inflammatory cells infiltrations in the hepatic tissue and marked infiltration of polymorphonuclear cells around the vessels and activation of hepatic macrophage: Kupffer cells.
Conclusion
Dietary PCHM inclusion up to 8% supports the performance, stability of haemato-biochemical indices and improved antioxidant status of the broiler chickens under heat stress condition. Histological changes were observed in the broiler chicken liver.
Similar content being viewed by others
Background
The problem of low animal protein intake (8–15 g per day) in most African and Pacific countries (Ogunsipe et al. 2017b) has been associated with rise in cost of animal protein beyond the affordable level by the people in these regions (Oloruntola et al. 2016). The increase in price of conventional feed ingredients constitutes the primary cause of the rise in animal feed production cost and the subsequent observed high and unaffordable cost of animal protein (Adeyeye et al. 2017). According to Nworgu et al. (1999), feeding cost covers between 60 to 70% of the total production cost of monogastric animals. Therefore, replacement of one or more of the major conventional feed ingredients with cheap and available non-conventional feed ingredients will have reducing effects on animal feed production cost (Adeyeye et al. 2017). The use of agro-wastes such as cassava peels and cocoa bean shell in monogastric animal production was reported (Egbunike et al. 2009; Ogunsipe et al. 2017a, 2017b; Oloruntola et al. 2018a, 2018b). Oxidative stress was also identified as another major factor that affects the poultry production on a worldwide basis (Akbarian et al. 2016), and heat stress has been reported as one of the most important stressors in the tropical countries (Jimoh et al. 2018). When the environmental variables depict the presence of heat stress, there exist the accumulations of reactive oxygen species in the biological system and subsequent decline in the endogenous enzymatic antioxidant production and total antioxidant activities (Jimoh et al. 2018). Feeding of poultry with phytochemicals (products derived from plant, e.g. dried plant material, essential oil, pure isolated compound, or extract, which contain secondary plant metabolites) has been reported as a reliable means of combating the negative effect of oxidative stress in heat-stressed poultry (Akbarian et al. 2016).
Cocoa pod husk is a typical under-utilized agro-waste from the commercial cocoa farm, which could provide nutritional benefits to monogastric animal production (Adeyeye et al. 2017). Cocoa pod husk forms about 70% (w/w) of the whole mature cocoa fruit; has low crude protein (9.14%), high crude fibre (35.78%) (Eghosa et al. 2010) and anti-nutritional factors such as threobromine (2.64%); and has tannin (0.91%), caffeine (1.14%) and high fibre (Adeyeye et al. 2017).
However, it was also reported that the optimal utilization of agro-waste in monogastric animal production is hampered by the anti-nutritional factors which cause the inactivation of some nutrients, diminution of metabolic utilization of food or the process of food digestion (Gemede and Ratta 2014). Therefore, various treatments such as fermentation (Alemawor et al. 2009; Oloruntola et al. 2015), ash treatment (Adamafio et al. 2004), enzyme supplementation (Oloruntola et al. 2018b, 2018c), soaking and sun-drying (Adebowale 1985; Okeeke et al. 1985) among others have been used to improve the nutritive value of agro-waste. In particular, combination of ash treatment with fermentation was also reported to improve the nutritive values of cocoa pod husk meal and its suitability in monogastric animal production (Adeyeye et al. 2017). Dietary inclusion of processed cocoa pod husk meal up to 150 g/kg was reported to support normal growth performance, carcass traits and relative internal organ weights in the rabbits (Adeyeye et al. 2018). There could be variation in performance response of different species of animals to unconventional feed ingredients, and presently, relatively few works had been reported on the effect of dietary processed cocoa pod husk in broiler chickens nutrition. Therefore, the objective of this study is to determine the effects of dietary inclusion of cocoa pod husk meal that had undergone two subsequent processing methods, i.e. ash treatment and rumen liquor fermentation in broiler chicken.
Methods
Ethical approval and experimental site
This study was carried out after all the animal experimental protocols were approved by the Research and Ethics Committee of the Department of Animal Health and Production Technology, The Federal College of Agriculture, Akure (FCAA), Nigeria. The experiment was conducted at the Avian Experimental Unit of The Teaching and Research Farm, FCAA (Adeyeye et al. 2017). The feeding trial was conducted during the peak of the dry season in the study area (i.e. between December 2017 and January 2018). The average daily temperature-humidity index (THI) of the experimental pen was 29.9 °C ± 1.56. The THI, an indicator of thermal comfort level for enclosed animals was calculated as described by Jimoh et al. (2018) using the following formula: THI = t − [(0.31–0.31 × RHt − 14.4)], where RH = relative humidity/100 and t = ambient temperature.
Cocoa pod husk collection, corn stalk ash extract preparation and collection of layer’s waste, molasses, and bovine rumen liquor
Cocoa pod husk was collected and processed to cocoa pod husk meal as earlier described by Adeyeye et al. (2017). The corn stalks were sun-dried, gathered and burnt to corn stalk ash (CSA). Thereafter, the corn stalk ash extract (CSAE) was prepared as earlier described by Adamafio et al. (2004) and Adeyeye et al. (2017). Droppings of battery cage raised layers wastes (LW) were collected, sun-dried, milled and bagged till used. Molasses (MO) was purchased from a reputable commercial animal feed mill in Akure, Nigeria. Rumen liquor (RL) was squeezed out of the rumen content of freshly slaughtered cattle through a clean muslin cloth at the Akure Central Abattoir, Akure, Nigeria, and used almost immediately.
Processing of cocoa pod husk meal
The two processing methods, i.e. ash treatment and rumen liquor fermentation, adopted for processing cocoa pod husk meal in this study have been earlier described by Adeyeye et al. (2017). The cocoa pod husk meal was thoroughly mixed with CSAE at the rate of 188 g/l in a black plastic container and kept in dark place under anaerobic condition for 7 days. The CSAE-soaked cocoa pod husk meal was thereafter drained, sun-dried for 14 days and labelled as ash-treated cocoa pod husk meal (ACM).
The method of rumen liquor fermentation as earlier described by Oloruntola et al. (2015) was used to further process ACM in this study. The ACM was successively mixed with dried LW at the rate of 100 g/kg and molasses at the rate of 50 ml/kg. Thereafter, the mixture of ACM, LW and MO was sprayed with the freshly collected RL in a black plastic container and allowed to ferment under anaerobic condition for 7 days. After the seventh day, the fermented ACM was sun-dried for 7 days, analysed for proximate composition (AOAC 1995), caffeine (Rade et al. 2008), tannin (Shad et al. 2013), and theobromine (Bisto et al. 2002) and thereafter labelled as processed cocoa pod husk meal (PCHM).
Experimental diets, birds, housing and experimental design
Three experimental diets were formulated to meet the minimum requirements of the birds at both the starter and finisher phase, in which PCHM was included at 0, 4 and 8% and designated as diets 1, 2 and 3 respectively. One hundred and eighty 1-day-old Arbor Acres broiler chicks were randomly distributed to three dietary treatments (10 birds/replicate; 60 birds/treatment) in a completely randomized design (CRD). The birds in each replicate were housed in their respective wood shavings littered 200 × 100 cm pen. The experimental house temperature was maintained within 31 °C ± 2 for the first 7 days and reduces by 2 °C after each consecutive 7 days until the house temperature was 26 °C ± 2. Illumination was provided for 23 h/day. The birds were fed water and mash ad libitum throughout the experimental period.
Chicken growth performance, slaughtering procedure, sample collection and analysis
The performance characteristics of the birds, i.e. the body weight (BW) and the feed intake (FI) were determined on weekly basis. Thereafter, the body weight gain (BWG) and the feed conversion ratio (FCR) were estimated.
On day 42 of the experiment, three birds per replicate were selected, tagged, weighed and sacrificed. After stunning, the jugular veins of the birds were cut with clean, sharp stainless knife. The blood was allowed to flow into plain and EDTA bottles. The blood in the plain bottle was centrifuged; thereafter, its serum was separated and frozen at − 20 °C prior to analysis. The serum enzymes (total protein, cholesterol, alanine aminotransaminase (ALT) and aspartate aminotransferase (AST)) were determined with a Reflectron® Plus 8C79 (Roche Diagnostic, GombH Mannheim, Germany), using kits. The serum glutathione peroxidase (GPx) was determined as described by Rotruck et al. (1973), while the catalase (CAT) activity was determined as described by Aebi (1974). The blood samples collected in EDTA bottle were used for erythrogram (packed cell volume, haemoglobin concentration and red blood cell) determination as described by Lamb (1981). The slaughtered weights and dressed percentage of the birds were estimated after de-feathering, evisceration and dressing. The heart, lung, liver, spleen, pancreas, kidney, gizzard and gall bladder of the birds were excised out, weighted and expressed as percentage of the slaughtered weight. The liver and heart were thereafter fixed in 10% neutral buffered formalin, dehydrated in graded alcohol series (70%, 90%, absolute ethanol), cleared with methyl benzoate and embedded in paraffin wax. Sections of 5 μm were cut and stained for light microscopic examination (Bancroft et al. 1996; Oloruntola et al. 2017). Stained section was examined by light microscope and photographed using digital camera.
Data analysis
The model Xrt = μ + αr + βrt was used in this experiment, where Xrt is any of the response variables, μ is the overall mean, αr is the effect of the rth treatment (r = diets 1, 2 and 3) and βrt is the random error due to experimentation. The data were subjected to one-way analysis of variance using SPSS version 20. The differences among means were determined by Duncan multiple range test of the same package.
Results
Composition of processed cocoa pod husk meal (PCHM) and the effects of PCHM on broiler chicken performance and carcass traits
Table 1 shows the proximate composition and phytochemicals in the PCHM. The dietary inclusion of PCHM did not affect (P > 0.05) the performance characteristics of the broiler chicks at the starter (1 to 21 days), grower (22 to 42 days) and overall (0 to 42 days) phases, except for the feed intake (FI) that significantly (P < 0.05) increased in birds fed 8% of PCHM-inclusive diet at the starter phase (Tables 2, 3, and 4). The carcass traits and relative internal organ weights of the broiler chickens were similar (P > 0.05) across the dietary treatments (Table 5).
The effects of PCHM on broiler erythrogram, serum biochemicals indices and serum antioxidant enzymes
Table 6 shows the effect of PCHM on haemato-biochemical indices and serum antioxidant enzymes. The packed cell volume, haemoglobin concentration and red blood cells of the broiler chickens were stable (P > 0.05) across the dietary treatment. In the same vein, the serum biochemical indices concentration in the experimental birds was not affected (P > 0.05) by the dietary treatment. The serum GPx and CAT concentration were higher (P < 0.05) in birds fed PCHM-inclusive diets compared to those fed the control diet.
Histological studies of heart and liver
Figures 1, 2 and 3 show the histopathological sections of the heart of the broiler chickens fed varying levels of PCHM. Similar histological myocardiac cell appearances were observed among the birds across the various dietary treatments. Sections show the myocardium composed of the cardiac muscle with peripherally placed nucleus (arrowhead) separated by a defined interstitium that is free of inflammatory cells and collections. The cardiac vessels (arrow) appear normal. Figures 4, 5 and 6 show the histopathological sections of the liver of broiler chickens fed varying inclusion levels of PCHM. In the control group, section shows the hepatic tissue composed of sheets of hepatocytes (H) separated by the sinusoids (S). The central veins (arrow) appear unremarkable. However, in the birds fed diet 2 and 3, various histological variations were observed, which include marked vascular congestion (CG) and perivascular inflammatory cell infiltrations (star) in the hepatic tissue and marked infiltration of polymorphonuclear (star) cells around the vessels (arrow) and activation of hepatic macrophage: Kupffer cells (arrow head).
Heart section of broiler chicken fed 0.0% of PCHM-inclusive diet showing normal histological structure of the myocardium which composed of the cardiac muscle with peripherally placed nucleus (arrow head) separated by a defined interstitium that is free from inflammatory cells. The cardiac vessels (arrow) appear normal. (H&E × 400)
Heart section of broiler chicken fed 4.0% of PCHM-inclusive diet showing the myocardium composed of the cardiac muscle with peripherally placed nucleus (arrow head) separated by a defined interstitium that is free of inflammatory cells and collections. The cardiac vessels (arrow) appear normal. (H&E × 400)
Discussion
Total weight gain determination was reported as the most frequent approach of assessing the overall nutritional status or health of broiler chickens (Parvin et al. 2010). The stability of the body weight gain and feed conversion ratio in the experimental birds across the various dietary treatments in this study suggests that PCHM demonstrates similar nutritional quality to the conventional ones and that it supports the normal growth performance in broiler chicken. It also suggests that PCHM could be a suitable replacement for some conventional livestock feed ingredients. This result is in line with the earlier reports of Akinfala et al. (2002) and Afolayan et al. (2012) that conventional feed ingredients such as maize could be replaced in part by cassava and sweet potato meal, respectively, in broiler. Adeyeye et al. (2018) also reported the support of processed cocoa pod husk meal for normal growth of growing rabbits at 15% inclusion level. The larger proportion of broiler chicken growing cycle is represented by the starter period (Gajana et al. 2011). The rise of feed intake in broiler chicken fed diet 3 being observed only at the starter phase may imply that there exist some variations in the factors affecting feed intake in broiler chickens at the starter and grower phases. Feed consumption was reported to differ with the feed quality/composition, chicks’ growth rate and management conditions (Ferket and Gernat 2006). In addition, chicks regulate their feed intake to meet up with their energy requirement for growth (Ferket and Gernat 2006; Gajana et al. 2011). This may explain in part the reason for the observed increase in feed intake across the diet as there exists a marginal decrease in the energy level of the feed with the increase in inclusion levels of PCHM across the diets in this study. However, the increased feed intake at this starter phase does not translate to increased growth performance. This may be due to the adverse effects of the phytochemicals in PCHM. For instance, tannin was reported of being capable of altering the growth rate and feed efficiency in animals (Gemede and Ratta 2014).
Nutrition has marked effect on yield of quality meat of animals, and their relative organ weights are very useful in the prediction of toxic effect of the test materials or the diets (Ayodele et al. 2016; Oloruntola et al. 2018c). In addition, the toxins in diet could be absorbed and accumulated in the various target tissues or organs and cause injury to the cells and alter their normal structure or function. The similarity in the carcass traits and relative internal organ weights of the experimental birds fed the varying inclusion levels of PCHM is of health benefits and indicates that the phytochemicals in PCHM is within the tolerable level and did not produce injurious or fatal effects or that the dietary treatment in this study did not pose treats to the development of edible portion of the experimental birds and the normal gross anatomy of their internal organs.
Erythrogram is one of the indicators for assessing the nutritional and health status of animals, and there exists a marked influence of nutrition on haematology traits (Oloruntola et al. 2018d). The stability of packed cell volume, haemoglobin concentration and red blood cells of the birds fed diets containing varying levels of PCHM also shows that the dietary treatment used in this study did not have negative effects on the normal blood-forming processes in the experimental birds. This result agrees with Adeyeye et al. (2017), who reported similar haematological indices values among experimental rabbits fed processed cocoa pod husk meal-inclusive diets. The assessment of biochemical parameters is also another important method of assessment of health in animals (Milner et al. 2003). The non-difference in the serum biochemical indices values in broiler chicken fed the experiment diets also indicates that dietary PCHM inclusion up to 8% support normal health in the broiler chickens. This may be the product of activities of the phytochemicals in the PCHM. For instance, caffeine intake was associated with a lower risk of elevated alanine aminotranaferase (Ruhl and Everhart 2005). The use of phytochemical in ameliorating the negative effects of heat-induced oxidative stress in birds has been reported (Oloruntola et al. 2018d). These phytochemicals with antioxidant properties play roles in reducing the process of oxidation by reacting with free radicals during oxidative process (Goyal and Brahma, 2014). Serum GPx and CAT are among the antioxidant enzymes protecting cells from the harmful effects of reactive oxygen species (Oloruntola et al. 2018d). The GPx protects the cells against the damaging effects of oxidation by catalyzing the degradation of various peroxidase and oxidizing glutathione (Vara et al. 2009), while the CAT inhibits or prevents cell against hydrogen lipid peroxidation and peroxide toxicity (Oloruntola et al. 2018d). The higher serum GPx and CAT concentration recorded in broiler chickens fed PCHM-inclusive diet compared to those fed the control diet suggests PCHM contain antioxidant properties. This is supported by the earlier report by Dhama et al. (2015) that active ingredients of plants have antioxidant effect by increasing the concentration of antioxidant enzymes.
The heart functions mainly as a pump for the movement of blood through the body. Effects of the major secondary metabolites of cocoa plant, i.e. caffeine and theobromine, on the heart have been reported (Biehl and Ziegleder 2003; Lopez-Garcia et al. 2008). Caffeine intake has been associated with lower prevalence of cardiovascular death (Lopez-Garcia et al. 2008), while theobromine is being used as an aid in urination, as a vasodilator and as a heart stimulant (Biehl and Ziegleder 2003). The similar histological myocardiac cell appearances being observed among the birds fed control diet and PCHM-inclusive diets in this study are in another way unveiling the wholesomeness of this test ingredient and its suitability for broiler chicken production.
The liver has multiple functions among which is filtering the blood coming from the digestive tract prior to its passage to the rest of the body, detoxification of chemicals and metabolism of drugs. Histological study of the experimental broiler chicken liver reveals that the test ingredient (i.e. PCHM) may contain some components that cause some histological changes such as vascular congestion, peri-vascular inflammatory cell infiltration in the hepatic tissue, marked infiltration of polymorphonuclear cells around the vessels and activation of hepatic macrophage, the Kupffer cells. This result disagreed with earlier reports that caffeine (one of the components of PCHM) and in particular its main metabolite parazanthine can suppress the synthesis of connective tissue growth factor (CTGF) and subsequently slow down the progression of liver damages (Modi et al. 2010). Therefore, further studies are needed to really ascertain the particular compound responsible for these histological changes.
Conclusions
Dietary PCHM inclusion up to 8% supports the performance, stability of haemato-biochemical indices and improved antioxidant status of the broiler chickens. However, since histological changes were observed in the liver of broiler chickens fed the 8.0% of PCHM-inclusive diets, there is a need for further studies to ascertain the cause of these pathological changes, modify and improve the processing method adopted in this study to enhance the nutritive value of PCHM.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AOAC:
-
Association of Analytical Chemists
- AST:
-
Aspartate aminotransferase
- BW:
-
Body weight
- BWG:
-
Body weight gain
- CAT:
-
Catalase
- CTGF:
-
Connective tissue growth factor
- FCR:
-
Food conversion ratio
- FI:
-
Feed intake
- GPx:
-
Glutathione peroxidase
- MO:
-
Molasses
- NRC:
-
National Research Council
- PCHM:
-
Processed cocoa pod husk meal
- RL:
-
Rumen liquor
- SOD:
-
Superoxide dismutase
- SPSS:
-
Statistical Package for Social Science
- THI:
-
Temperature-humidity index
References
Adamafio NA, Cooper Aggrinage E, Onaye EO, Laary JK, Onaye J (2004) Effectiveness of corn stalk ash in reducing tannin level and improving in vitro enzymatic degradation of polysaccharides in crop residues. Ghana J Sci 44:87–92
Adebowale EA (1985) Organic waste ash as possible source of alkali for animal feed treatment. Anim Feed Sci Technol 13:237–248
Adeyeye SA, Agbede JO, Aletor VA, Oloruntola OD (2017) Processed cocoa (Theobroma cacao) pod husks in rabbits diet: effect on haematological and serum biochemical indices. Asian J Adv Agric Res 2(4):1–9
Adeyeye SA, Agbede JO, Aletor VA, Oloruntola OD (2018) Performance and carcass characteristics of growing rabbits fed diets containing graded levels of processed cocoa (Theobroma cacao) pod husk meal supplemented with multi-enzyme. J Appl Life Sci Int 17(2):1–11
Aebi H (1974) Catalase estimation. In: Bergmeyer HV (ed) Methods of enzymatic analysis. Verlag Chemic, New York, New York, NY
Afolayan SB, Dafwang II, Tegbe TSB, Sekoni A (2012) Response of broiler chickens fed maize-based diets substituted with graded levels of sweet potato meal. Asian J Poult Sci 6(1):15–22
Akbarian A, Michiels J, Degroote J, Majdeddin M, Golian A, Dmegt SD (2016) Association between heat stress and oxidative stress in poultry: mitochondrial dysfunction and dietary interventions with phytochemicals. J Anim Sci Biotech 7:37. https://doi.org/10.1186/s40104-016-0097-5
Akinfala EO, Aderibigbe AO, Matanmi O (2002) Evaluation of the nutritive value of whole cassava plant as replacement for maize in the starter diets for broiler chickens. Livest Res Rural Dev 14(6). http://www.lrrd.org/lrrd14/6/akin146.htm
Alemawor F, Dzogbefia VP, Oddoye EOK, Oldham JH (2009) Effect of Pleurotus ostreatus fermentation on cocoa pod husk composition: influence of fermentation period and Mn2+ supplementation on the fermentation process. African J Biotechn 8(9):1950–1958
AOAC (1995) Official methods of analysis, 16th edn. Association of Official Analytical Chemists, Inc, Arlington
Ayodele SO, Oloruntola OD, Agbede JO (2016) Effect of diet containing Alchornea cordifolia leaf meal on performance and digestibility of Weaner rabbits. World Rabbit Sci 24:201–2016
Bancroft TD, Stevens A, Turner DR (1996) Theory and practice of histological technique, 4th edn. Churchill, Livingstone, New York
Biehl B, Ziegleder G (2003) Cocoa/chemistry of processing. Encyclopedia of food sciences and nutrition (second edition). Academic Press, pp 1436–1448
Bisto MS, Veloso MCC, Pinheiro HLC, De Oliveira RFS, Reis JON, De Andrade JB (2002) Simultaneous determination of caffeine, theobromine and theophylline by high performance liquid chromatography. J Chromatog Sci 40:45
Dhama K, Latheef SK, Mani S, Samad HA, Karthik K, Tiwari R, Khan RU, Alagawany M, Farag MR, Alam GM, Laudadio V, Tufarelli V (2015) Multiple beneficial applications and modes of action of herbs in poultry health and production—a review. Int J Pharmacol 11:152–176 https://doi.org/10.3923/ijp.2015
Egbunike GN, Agiang EA, Owosibo A, Fatufe AA (2009) Effect of protein on performance and haematology of broiler fed cassava peel-based diets. Arch Zootecn 58(224):655–662
Eghosa OU, Rasheed AH, Martha O, Luqman AA (2010) Utilization of cocoa pod husk (CPH) as substitute for maize in layers mash and perception of poultry farmers in Nigeria. Int J Sci Nature 1(2):271–275
Ferket PR, Gernat AG (2006) Factors that affect feed intake of meat birds: a review. Int J Poult Sci 5(10):905–911
Gajana CS, Nkukwana TT, Chimonyo M, Muchenje V (2011) Effect of altering the starter and finisher dietary phases on growth performance of broilers. African J Biotechn 10(64):14203–14208 http://www.academicjournals.org/AJB
Gemede HF, Ratta N (2014) Antinutritional factors in plant foods: potential health benefits and adverse effects. Int J Nutr Food Sci 3(4):284–289 http://www.sciencepublishinggroup.com/j/ijnfs
Goyal AK, Brahma BK (2014) Antioxidant and nutraceutical potential of bamboo: an overview. Int J Fundamental Appl Sci 3:2–10
Jimoh AA, Ayedun ES, Oyelade WA, Oloruntola OD, Daramola OT, Ayodele SO, Omoniyi IS (2018) Protective effect of soursop (Annona muricata linn.) juice on oxidative stress in heat stressed rabbits. J Anim Sci Techn 60(28) https://doi.org/10.1186/s40781-018-0186-4
Lamb GN (1981) Manual of veterinary laboratory technique. CIBA-GEIGY, Kenya, pp 96–107
Lopez-Garcia E, van Dam RM, Li TY, Rodriguez-Artalejo F, Hu FB (2008) The relationship of coffee consumption with mortality. Ann Intern Med 148:904–914
Milner JM, Stien A, Justin Irvine R, Albon SD, Langvatin R, Ropstad E (2003) Body condition in Svalbard reindeer and the use of blood parameters as indicators of condition and fitness. Canad J Zool 81:1566–1578
Modi AA, Feld JJ, Park Y, Kleiner DE, Everhart JE, Liang TJ, Hoofnagle JH (2010) Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology 51(1):201–209
Nworgu FC, Adebowale EA, Oredein OA, Oni A (1999) Prospect and economics of broiler production using two plant protein sources. Trop J Anim Sci 2:159–166
Ogunsipe MH, Balogun KB, Oladepo AD, Ayoola MA, Arikewuyo MT (2017b) Nutritive value of cocoa bean shell meal and its effect on growth and haematology of weaning rabbits. Nigerian J Agric Food Environ 13(1):23–28
Ogunsipe MH, Ibidapo I, Oloruntola OD, Agbede JO (2017a) Growth performance of pigs on dietary cocoa bean shell meal. Livestock Res Rural Dev 29(1)
Okeeke GC, Obioha FC, Udeogu AE (1985) Comparison of detoxification methods of cassava borne cyanide. Nutrit Report Int 32:138–148
Oloruntola DA, Dada EO, Osho IB, Ogundolie OO (2017) Effects of hydro-ethanolic leaf extract of Tithonia diversifolia on parasitaemia level, serum metabolites and histopathology of organs in Swiss Albino mice infected with Plasmodium berghei NK65. Asian J Med Health 6(2):1–7
Oloruntola OD, Agbede JO, Ayodele SO, Oloruntola DA (2018d) Neem, pawpaw, and bamboo leaf meal dietary supplementation in broiler chickens: effect on performance and health status. J Food Biochem:e12723 https://doi.org/10.1111/jfbc.12723
Oloruntola OD, Agbede JO, Onibi GE, Igbasan FA (2015) Composition of cassava (Manihot spp.) peels fermented with bovine rumen liquor and different nitrogen sources. J Global Agric Ecol 2(1):26–35
Oloruntola OD, Agbede JO, Onibi GE, Igbasan FA (2016) Replacement value of rumen liquor fermented cassava peels for maize in growing rabbit diet. Arch Zootec 65(249):89–97
Oloruntola OD, Agbede JO, Onibi GE, Igbasan FA, Ogunsipe MH, Ayodele SO (2018b) Rabbits fed fermented cassava starch residue II: enzyme supplementation influence on performance and health status. Arch Zootecn 67(260):588–595
Oloruntola OD, Agbede JO, Onibi GE, Igbasen FA, Ayodele SO, Arogunjo SO, Ogunjo ST (2018a) Rabbits fed fermented cassava starch residue I: effect on performance and health status. Arch Zootecn 67(260):578–586
Oloruntola OD, Ayodele SO, Oloruntola DA (2018c) Effect of pawpaw (Carica papaya) leaf meal and dietary enzymes on broiler performance, digestibility, carcass and blood composition. Rev Elev Med Vet Pays Trop 71(3). https://doi.org/10.19182/remvt.31640
Parvin N, Mandal TK, Saxema V, Sarkar S, Saxena AK (2010) Effect of increasing protein percentage feed on the performance and carcass characteristics of broiler chicks. Asian J Poult Sci 4(2):53–59
Pauzenga U (1985) Feeding Parent Stock. Zoo Technical International. pp 22–24.
Rade I, Branislava S, Matevz P, Marija B, Katarina K, Borut S (2008) Determination of caffeine and associated compounds in food, beverages, natural products, pharmaceuticals, and cosmetics by micellar electrokinetic capillary chromatography. J Chromatog Sci 46:137–143
Rotruck JT, Pope AL, Ganther HE, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Sci 179:588–590 https://doi.org/10.1126/ science.179.4073.588
Ruhl CE, Everhart JE (2005) Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. Gastroenterol 129:1928–1936
Shad MA, Nawaz H, Rehma T, Ikram M (2013) Determination of biochemicals, phytochemicals and antioxidative properties of different part of Cichorium intybus L.: a comparative study. The J Anim Plant Sci 23(4):1060–1066
Vara LSS, Reddy P, Thangavel A, Leela V, Narayana Raju KVS (2009) Antioxidant enzyme status. Tamilnadu J Vet Anim Sci 5:251–256
Acknowledgements
Not applicable
Funding
This study was not funded by any institution.
Availability of data and materials
Please contact the author for data request.
Author information
Authors and Affiliations
Contributions
SAA and ODO designed the study. All authors managed all the activities of the experiment and interpreted the data collectively and gathered reference materials. SAA, ODO and SOA prepared the first draft of the manuscript. JOA reviewed the first draft. SAA, ODO and SOA prepared the second draft. All authors reviewed the second draft of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experimental protocols were approved by the Research and Ethics Committee of Animal Health and Production Technology Department, The Federal College of Agriculture, Akure, Nigeria.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Adeyeye, S.A., Ayodele, S.O., Oloruntola, O.D. et al. Processed cocoa pod husk dietary inclusion: effects on the performance, carcass, haematogram, biochemical indices, antioxidant enzyme and histology of the liver and kidney in broiler chicken. Bull Natl Res Cent 43, 54 (2019). https://doi.org/10.1186/s42269-019-0096-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-019-0096-8