Abstract
Background
Vagus Nerve Stimulation (VNS) delivers Autonomic Regulation Therapy (ART) for heart failure (HF), and has been associated with improvement in cardiac function and heart failure symptoms. VNS is delivered using an implantable pulse generator (IPG) and lead with electrodes placed around the cervical vagus nerve. Because HF patients may receive concomitant cardiac defibrillation therapy, testing was conducted to determine the effect of defibrillation (DF) on the VNS system.
Methods
DF testing was conducted on three ART IPGs (LivaNova USA, Inc.) according to international standard ISO14708-1, which evaluated whether DF had any permanent effects on the system. Each IPG was connected to a defibrillation pulse generator and subjected to a series of high-energy pulses.
Results
The specified series of pulses were successfully delivered to each of the three devices. All three IPGs passed factory electrical tests, and interrogation confirmed that software and data were unchanged from the pre-programmed values. No shifts in parameters or failures were observed.
Conclusions
Implantable VNS systems were tested for immunity to defibrillation, and were found to be unaffected by a series of high-energy defibrillation pulses. These results suggest that this VNS system can be used safely and continue to function after patients have been defibrillated.
Similar content being viewed by others
Introduction
Heart failure (HF) is characterized by hemodynamic abnormalities that result in, and are exacerbated by, a marked imbalance created by increased sympathetic activity and withdrawal of parasympathetic tone. This pronounced pathological adrenergic hyperactivation contributes to the progression of HF, and increases the risk of mortality and morbidity independent of left ventricular ejection fraction (EF) and ventricular arrhythmias [1].
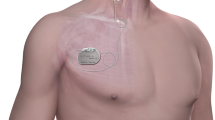
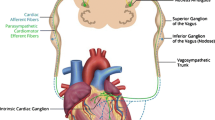
Autonomic Regulation Therapy (ART) is a novel investigational approach for the management of HF. ART utilizes cervical vagus nerve stimulation (VNS) to increase parasympathetic activity and to restore autonomic balance. ART is delivered using chronic stimulation through a self-sizing lead that is placed on the cervical vagus nerve without requiring any mapping for placement (Fig. 1). ART using open-loop VNS has been shown in a pilot study to be associated with long-term improvement in left ventricular function, 6-minute walk distance, NYHA class, heart rate, heart rate variability, and quality of life in patients with HF and reduced EF (HFrEF), [2,3,4] and is being evaluated further in an ongoing mortality and morbidity pivotal study in patients with HF and reduced left ventricular ejection fraction [5].
International guidelines recommend implantable cardioverter defibrillator (ICD) therapy for patients with HFrEF and at risk for ventricular arrhythmias [6,7,8,9]. Because HFrEF patients with implanted ART may be exposed to either external or internal high-energy defibrillation, it is important to evaluate whether defibrillation energy has an effect on the operation of the ART system. The purpose of this study was to determine the degree to which an ART system may be affected by an applied defibrillation shock.
Methods
International standards establish requirements for medical devices, and specify the parameters for defibrillation testing for active implantable devices. Testing was performed according to the protocol specified in ISO 14708-1, Implants for Surgery – Active Implantable Medical Devices – Part 1: General Requirements for Safety, Marking and for Information to be Provided by the Manufacturer [10].
Three Implantable Pulse Generators (IPG; Model 103, LivaNova, Houston, Texas) were tested using a defibrillation test voltage generator (MegaPulseDefib and MegaPulse Biphasic P, Compliance West, San Diego, CA). All equipment was calibrated and certified prior to performing the test. The outputs of the test voltage generator were sequentially connected to each of the two bipolar outputs of the IPG through a 300 Ω resistor and to the titanium case (Fig. 2).
A series of voltage spikes, monophasic pulses, and biphasic pulses were applied; three pulses with normal polarity 20 seconds apart, followed by a 60 second pause, followed by three pulses with reverse polarity 20 seconds apart (Fig. 3). The magnitude of the applied test pulses was 140 V, as specified by the international standard. Each IPG was exposed to three sets of pulses:
-
1.
A damped sinus defibrillation waveform with rise time of 1.5–2.5 ms, with a pulse shape specified by ISO 14708-1 clause 20.2.
-
2.
A monophasic, truncated exponential waveform with 10 ms duration.
-
3.
A biphasic, truncated exponential waveform with 10 ms duration.
According to ISO 14708-1 clause 20.2, there should be no irreversible damage to the IPG after completion of the test. Each IPG was interrogated before and after each test, and a comparison of programmed device parameters was performed. After all tests were completed, each IPG underwent a final electrical test, a duplicate of what is performed at the end of the manufacturing process to confirm that the device met all functional specifications. Programmed values (output current, output pulse width, output pulse rise time, output pulse fall time, output pulse overshoot, pulse rate accuracy, magnet response, device reset, battery voltage, and others) were programmed and measured to confirm that they produce an output that is within the allowable tolerance.
Results
All defibrillation tests were performed successfully in all devices, with no deviations from the ISO 14708-1 mandated protocol. The interrogated data from all three IPGs after each test was unchanged from the programmed values prior to the test. All three devices passed the post-test final electrical test, with no failures or parameter shifts.
Discussion
Patients with HF and reduced EF are at risk for life-threatening ventricular arrhythmias, and these patients commonly receive ICD therapy and external DF. These patients are also being evaluated for ART, a novel therapy that uses a VNS IPG to modulate autonomic balance. This raises the question of whether an implantable ART system is able to survive the application of DF energy and continue to deliver therapy as originally programmed. This study sought to test the ART system according to ISO 14708-1, the international standard for DF immunity for implantable medical devices.
All three devices met the requirements established by the international standard for DF immunity testing. Following the application of three different sets of DF test pulses (different waveforms, including opposite polarity pulses), the settings of all tested devices were unchanged, and the devices passed the final electrical test, indicating that all functions were within manufacturer specifications.
Although this DF immunity test was designed to simulate the conditions that an implantable device would experience in the presence of DF energy, it is still only a benchtop test. Future research is needed to complement this test by determining whether this ART system is able to withstand energy from internal and external DF in vivo.
Conclusions
Implantable VNS systems equivalent to the VITARIA ART system were tested for immunity to DF, and were found to be unaffected by a series of high-energy DF pulses. The results of this study suggest that the ART system can be used safely and continue to function after patients have been defibrillated.
Availability of data and materials
The datasets from the current study are available from the corresponding author on reasonable request.
References
Borovac J, D’Amario D, Bozic J, Glavas D. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J Cardiol. 2020;12:373–408.
Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH, Anand IS. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail. 2014;20:808–16.
Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B. KenKnight BH and Anand IS. Extended follow-up of patients with heart failure receiving autonomic regulation therapy in the ANTHEM-HF study. J Card Fail. 2016;22:639–42.
Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I. Long-term follow-up of reduced ejection fraction heart failure patients receiving autonomic regulation therapy in the ANTHEM-HF Pilot Study. J Am Coll Cardiol. 2019;73:770.
Konstam MA, Udelson JE, Butler J, Klein HU, Parker JD, Teerlink JR, Wedge PM, Saville BR, Ardell JL. Libbus I and DiCarlo LA. Impact of Autonomic Regulation Therapy in Patients with Heart Failure: ANTHEM-HFrEF Pivotal Study Design. Circ Heart Fail. 2019;12:e005879.
2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure. An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2016;22:659–69.
Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—executive summary: a report of the American College of Cardiology/American Heart Association Task Force and the European Society. Eur Heart J. 2006;27:2099–140.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327.
Implants for Surgery – Active Implantable Medical Devices – Part 1: General Requirements for Safety, Marking and for Information to be Provided by the Manufacturer. 2014;ISO 14708-1.
Acknowledgements
Testing was conducted by Mary Faltaous, a former employee of LivaNova.
Funding
No direct funding was provided for this study.
Author information
Authors and Affiliations
Contributions
IL and LAD contributed to writing the manuscript. SRS, STM, SM, and BHK contributed to the conceptualization of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
IL, SRS, SM, BHK, and LAD are employees and shareholders of LivaNova. STM is a consultant to LivaNova.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Libbus, I., Stubbs, S.R., Mazar, S.T. et al. Effect of defibrillation on the performance of an implantable vagus nerve stimulation system. Bioelectron Med 7, 3 (2021). https://doi.org/10.1186/s42234-021-00064-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42234-021-00064-w