Abstract
Background
Post-coarctoplasty aortic pseudoaneurysms constitute a lethal problem occurring in up to 38% of patients with a history of aortic coarctation surgical repair. Such pseudoaneurysms are prone to rupture if managed conservatively and high mortality and morbidity if treated with open surgery. Therefore, the endovascular approach has been proposed for their management.
Case report
We describe a patient with a post-coarctoplasty aortic pseudoaneurysm complicated by an aortobronchial fistula. The case was treated via the endovascular approach (thoracic endovascular aortic repair and endovascular coarctoplasty) with an atrial septal defect occluder device.
Conclusions
Endovascular repair is a feasible, safe, and promising treatment for thoracic aortic pseudoaneurysms secondary to coarctation repair.
Similar content being viewed by others
Background
Coarctation is one of the causes of aortic aneurysm formation as a result of a more flexible vessel wall, even after repair (Preventza et al. 2013). Aortic coarctation repair has been based on surgical methods from the outset, although catheter intervention techniques are progressively improving (Preventza et al. 2013). Different surgical approaches are available for coarctation repair, including resection with end-to-end anastomosis, transverse suture repair, patch-graft aortoplasty, subclavian flap aortoplasty, and resection with end-to-end conduit interposition. Despite a successful repair, however, there is a likelihood of long-term complications such as aortic coarctation recurrence, aortic aneurysms, and pseudoaneurysms, which are at risk for dissection, rupture, or fistulization to adjacent structures (Oliver et al. 2004; Moosavi et al. 2022). We herein describe a patient with an aortic pseudoaneurysm at the site of a Dacron tube graft used for coarctoplasty, resulting in an aortobronchial fistula.
Main text
Case report
A 43-year-old man presented to our hospital due to massive hemoptysis. He had a history of surgical repair of interrupted aorta via bypass grafting 19 years previously. The patient stated that on the day before his referral, he had suffered dyspnea and pleuritic chest pain, which were subsequently relieved.
At presentation, the patient had a blood pressure of 138/74 mm Hg, a heart rate of 98 beats per minute, a respiratory rate of 16 breaths per minute, and an oxygen saturation level of 97% in room air. He had no fever. Cardiac auscultation revealed normal regular heart sounds. Additionally, the lungs in auscultation were clear, and no crackles or any other abnormal sounds were heard. Other organs were also normal on physical examination.
The patient’s laboratory results are summarized in Table 1.
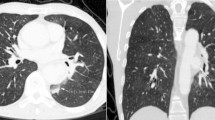
Spiral chest computed tomography (CT) depicted a consolidation in the posterior aspect of the left lung, just adjacent to the tube graft, in favor of hemorrhage (Fig. 1). Spiral CT angiography of the pulmonary arteries showed no evidence of pulmonary thromboembolism.
Spiral CT angiography of the aorta revealed aortic interruption after left subclavian artery debranching and a patent tube graft with its proximal anastomosis onto the left subclavian artery origin and the aorta immediately distal to the left subclavian artery with 2 pseudoaneurysms of about 20 mm: one emanating from the mid-posterior part of the tube graft and the other from the distal anastomotic point with wall thrombosis fistulating into the adjacent pulmonary parenchyma (Figs. 2 & 3). As well, it showed ectatic left Subclavian artery, reflecting a more diffuse arteriopathy.
CT angiography of thorasic aorta (3D reconstruction). Red Arrow:the left Subclavian artery is ectatic, reflecting a more diffuse arteriopathy, Arrow head: Aortic interruption, Red Dashed arrow: pseudoaneurysm of distal anastomotic site, White arrow: pseudoaneurysm of mid posterior part of the tube graft. White Dashed Arrow: Ascending Aorta
Accordingly, the patient underwent catheterization.
Technique
In the catheterization laboratory, arterial access was obtained via the right femoral and radial arteries. Contrast injection in the proximal end of the graft showed that the proximal anastomosis was attached to the ostium of the left subclavian artery. Hence, a sole thoracic endovascular aortic repair (TEVAR) procedure would entail a high risk for endoleaks from the left subclavian artery (type II endoleak).
A Judkins right (JR) catheter was advanced on a 0.035-inch J-tipped guidewire via right radial access to the ascending aorta. Next, the wire was exchanged with a 0.014 BMW guidewire, which was passed through the interruption site. Via right femoral artery access, the BMW was snared with the aid of a 6 F JR catheter and a snare device, and the JR catheter was pulled out. After that, a pigtail catheter was advanced through this wire to the ascending aorta. The tip of the catheter was kept there in order for the contrast material to be injected through it with a view to defining the interruption site and other structures such as anastomotic sites (Fig. 4). The catheter was, then, connected to the pressure system.
In the next step, via left femoral artery access the JR catheter was advanced with a 0.035-inch J-tipped guidewire to the ascending aorta via the tube graft. Then, removed, and was exchanged with a 14 F long sheath (45 cm) using a super-stiff wire. The distal tip of the sheath was left in the proximal portion of the left subclavian artery, and its dilator and the super-stiff guidewire were removed.
In the next stage, for the prevention of retrograde perfusion into the fistula or the pseudoaneurysm site and also for the prevention of endoleaks from the proximal anastomotic site, a Figulla Flex II atrial septal defect occluder device (30 mm, Occlutech) was inserted into the tube graft. Thus, the device, as well as its delivery cable and loader, was prepared, and the loader was attached to the delivery sheath. Next, the device was advanced carefully until it reached the tip of the delivery sheath in the ostium of the left subclavian artery. Under fluoroscopic guidance, the sheath was retracted over the delivery cable, and the left atrial disk was deployed in the proximal portions of the left subclavian artery and the tube graft to prevent endoleaks and exclusion of the fistula and the pseudoaneurysms. Subsequently, with tension on the delivery cable, the sheath was retracted further to deploy the right atrial disk. Before the release of the device, appropriate position and flow limitation were confirmed through contrast injection via the pigtail catheter.
In the following step, a super-stiff wire was advanced into the pigtail catheter, and its tip was fixed in the ascending aorta. The pigtail catheter was, then, extracted. Afterward, the coarctation site was predilated with a Powerflex Pro Balloon (10 mm × 2 cm) and an Oceanus 35 Balloon (6 mm × 40 mm). TEVAR was performed with an endovascular graft (28–80) (ESBE, Cook Medical) and an endovascular graft (26–10) (Zenith Alpha Thoracic Endovascular Stent Graft, Cook Medical) to exclude the proximal and distal anastomotic sites of the graft. After that, a 4.5 cm CP stent (NuMED Inc, Hopkinton, New York), which was premounted on a BIB balloon (5 cm × 20 cm), was implanted in the aortic interruption site (Fig. 5). The final angiogram showed complete exclusion of the tube graft without any endoleaks.
CP stent insertion into the graft stent eliminated the residual 15 mmHg pressure gradient across the lesion after TEVAR.
Follow up
After the procedure, the patient’s hemoptysis was eradicated. At 1 year’s follow-up, he was in good condition and had experienced no recurrence of symptoms. Aortic CT angiography, conducted at 1 month and 1 year’s follow-ups, revealed complete exclusion of the tube graft without endoleaks and a patent CP stent (Figs. 6 & 7).
Follow up CTangiography the day after (a), one month later (b) and after one year (c) showed complete exclusion of tube graft without endoleak and successful endovascular coarctoplasty. Red arrow: TEVAR device, dashed arrow: CP stent, Red arrow head: Left Subclavian artery, White arrow: Occlutechdevice, White arrow head: thrombosed excluded tube graft
In spite of fistula formation, the patient follow up and more evaluation did not unmask any sign related to graft infection.
Discussion
Surgical treatment of aortic coarctation has a high success rate. However, irrespective of the surgical technique used, a significant portion of patients develop late complications, including re-coarctation, aneurysms, pseudoaneurysms, systemic hypertension, infections including mycotic aneurysm, premature coronary artery disease, aortic valve abnormalities, dissection, and aortic rupture (Oliver et al. 2004). Aneurysms, true or false, are the most common complications, with an incidence rate of 7% to 38%. Asymptomatic enlargement of these kind of aneurysms or pseudoaneurysms has an unacceptable high rate of sudden rupture according to literature even in smaller sizes (Oliver et al. 2004; García-Pavíaa 2010; Kodolitsch 2002). Aneurysms occur following all types of surgical and even transcatheter repair procedures, especially after Dacron patch graft aortoplasty.
Pseudoaneurysms, with an incidence rate of 3% to 38%, develop from suture lines or at the site of isthmic restenosis. Conservative management is associated with an unbelievably high rupture rate and a rupture-related mortality rate of 7% (Oliver et al. 2004; García-Pavíaa 2010; Kodolitsch 2002; Marcheix et al. 2007).
Aneurysm pathogenesis can be explained by congenital weakness (thinning or cystic medial necrosis) of the aortic wall, foreign-body reaction, acquired atherosclerotic changes in the aortic wall, hemodynamic changes (hypertension or turbulence), excessive wall stress originating from the rigid patch onto the more elastic wall opposite to the patch, intimal damage, excessive excision of the coarctation, infection, aortic wall necrosis, and suture fracture (Oliver et al. 2004).
Other risk factors for aneurysm formation include the type of graft (knitted Dacron interposition grafts compared with woven Dacron interposition grafts), bicuspid aortic valves, advanced age at primary coarctation repair, hypoplastic transverse aortic arches, and high preoperative systolic peak pressure gradients (Oliver et al. 2004).
Conventional management of large thoracic aneurysms after aortic coarctation repair is similar to surgical treatment of nonspecific aneurysms. It is a complex procedure in that it necessitates hypothermic circulatory arrest more frequently and is associated with high mortality (14%– 23%) and morbidity, including paralysis of the recurrent laryngeal and phrenic nerves, bleeding, and paraplegia (García-Pavíaa 2010; Yazar et al. 2011). The endovascular approach has been proposed as a promising alternative for managing these patients (García-Pavíaa 2010).
Bertrand Marcheix (2007) described 4 patients with a history of surgical repair of congenital aortic coarctation who suffered from pseudoaneurysms. There were 2 graft interpositions:1 subclavian flap aortoplasty and 1 aorto-aortic bypass. All the patients were treated via the endovascular approach with a mean interval of 24 years from the surgery. One of the patients had massive hemoptysis resulting from an aortobronchial fistula and, thus, received emergent treatment. In all the cases, the Zenith TX2 thoracic stent-graft was used, and 1 patient underwent predilation at the coarctation site. Moreover, no major complications occurred during the procedure, and there was no mortality during the follow-up. One patient presented with a type II endoleak, which spontaneously healed during the first month. Another patient presented with claudication of the left arm resulting from coverage of the left subclavian artery and underwent carotid-subclavian bypass. After a median follow-up of 7.5 months, the patients were asymptomatic, and CT scans revealed complete exclusion of all the aneurysms without any stent-graft-related complications.
Omid Shafe (2018) described a 60-year-old man with a history of surgical repair of aortic coarctation who presented with inferior ST-segment-elevation myocardial infarction and simultaneous massive hemoptysis. CT angiography of the thoracic aorta revealed a large and ruptured pseudoaneurysm adjacent to the graft insertion site between the left subclavian artery and the descending thoracic aorta. After primary angioplasty on the occluded right coronary artery via right radial access, the patient underwent exclusion of the ruptured pseudoaneurysm and the graft itself with the aid of a covered CP stent. Thereafter, stent coarctoplasty was performed on the native site of the aortic coarctation using a long self-expandable bare-metal stent. Finally, an occluder was inserted into the previous graft. The final angiogram, as well as follow-up CT angiography, showed no endoleaks.
TEVAR is not free of morbidity and is associated with such complications as endoleaks, neurological and vascular dissection, pneumonia, upper limb claudication, and neurological complications of extracranial vessel rerouting. Still, the design of the newer generations of stents-grafts has conferred more ease of use and durability. Further, redo surgery is associated with complications, and patients tend to opt for minimally invasive procedures. Endovascular stent grafting is considered as the preffered option for the treatment of patients with post-coarctoplasty aortic pseudoaneurysm (García-Pavíaa 2010; Yazar et al. 2011).
Conclusions
Endovascular repair, given its more feasibility and less invasiveness, is a promising alternative to redo surgery for thoracic aortic pseudoaneurysms secondary to coarctation repair. Nevertheless, clinical and imaging long-term follow-ups are essential to assess the durability of stent-graft repair and to detect possible long-term complications, particularly endoleaks.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
Change history
29 December 2022
A Correction to this paper has been published: https://doi.org/10.1186/s42155-022-00346-7
Abbreviations
- CT:
-
Computed tomography
- TEVAR:
-
Thoracic endovascular aortic repair
- JR:
-
Judkins right
References
García-Pavíaa P, Ruigómeza JG, López-Mínguezb JR, Roldánc PF, Asensiob JMN, Domíngueza JR, Segoviaa J, Alonso-Pulpóna L (2010) Endovascular treatment of long-term complications following surgical repair of aortic coarctation. Rev Esp Cardiol 63(4):473–477
Marcheix B, Lamarche Y, Perrault P, Cartier R, Bouchard D, Carrier M, Perrault LP, Demers P (2007) Endovascular management of pseudo-aneurysms after previous surgical repair of congenital aortic coarctation. Eur J Cardiothorac Surg 31(6):1004–1007
Moosavi J, Ahmadi S, Firouzi A, Sadeghipour P, Mohebbi B, Shafe O, Alizadeasl A, Asadian S, Hoseini M (2022) Cardiac solution for a vascular scenario. CVIR Endovasc 5:9
Oliver JM, Gallego P, Gonzalez A, Aroca A, Bret M, Mesa JM (2004) Risk factors for aortic complications in adults with coarctation of the aorta. J Am Coll Cardiol 44(8):1641–1647
Preventza O, Livesay JJ, Cooley DA, Krajcer Z, Cheong BY, Coselli JS (2013) Coarctation-associated aneurysms: a localized disease or diffuse aortopathy. Ann Thorac Surg 95(6):1961–1967
Shafe O, Sadeghipour P, Moosavi J (2018) Simultaneous thrombosis and hemorrhage: endovascular approach to both conditions. J Am Coll Cardiol Intv 11(9):906–908
von Kodolitsch Y, Aydin MA, Koschyk DH, Loose R, Schalwat I, Karck M, Cremer J, Haverich A, Berger J, Meinertz T, Nienaber CA (2002) Predictors of aneurysmal formation after surgical correction of aortic coarctation. J Am Coll Cardiol 39(4):617–624. https://doi.org/10.1016/s0735-1097(01)01784-3
Yazar O, Budts W, Maleux G, Houthoofd S, Daenens K, Fourneau I (2011) Thoracic endovascular aortic repair for treatment of late complications after aortic coarctation repair. Ann Vasc Surg 25(8):1005–1011. https://doi.org/10.1016/j.avsg.2011.05.031
Acknowledgements
Not applicable.
Funding
The authors received no financial support for authorship and publication of this article.
Author information
Authors and Affiliations
Contributions
Somaye Ahmadi: supplied the acquisition of data and wrote the manuscript. Jamal Moosavi: provided the conception and design of the procedure and performed the procedure. Parham Sadeghipour: was involved in planning and supervised the procedure. Bahram Mohebbi: was involved in performing the procedure. Parham Rabiei: interpretation of pre-procedural and follow-up CT angiography study. Maryam Parham: supplied the acquisition of data. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In the originally published version of this article, the name of the patient was mistakenly included in the consent form.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmadi, S., Sadeghipour, P., Mohebbi, B. et al. Cardiac device to plug an aorto-bronchial fistula. CVIR Endovasc 5, 49 (2022). https://doi.org/10.1186/s42155-022-00327-w
Published:
DOI: https://doi.org/10.1186/s42155-022-00327-w