Abstract
Background
Various studies have demonstrated the analgesic benefit of systemic lidocaine in the perioperative setting, especially during laparoscopic abdominal surgery. However, the best appropriate dose for an administered bolus and continuous infusion of lignocaine is unclear. Our aim is to compare the effect of two different doses of intravenous lidocaine for analgesia in patients undergoing elective laparoscopic cholecystectomy under general anesthesia. Fifty-four patients of ASA PS I or II, aged between 18 and 65 years undergoing elective laparoscopic cholecystectomy under general anesthesia were randomly divided into two groups of 26 patients each. Patients in group A received an intravenous bolus injection of lidocaine 1.5 mg/kg slowly over 10 min, prior to induction and then followed by a continuous infusion at the rate of 1.5 mg/kg/h via infusion pump whereas group B patients received intravenous lidocaine bolus of 1.5 mg/kg slowly over 10 min followed by infusion at the rate of 2 mg/kg/h. Postoperative analgesia was assessed by VAS score, time to first analgesic dose, and total consumption of rescue analgesic in 24 h.
Results
There was a statistically significant difference in mean VAS Scores between the two groups at different time intervals postoperatively. Time to first rescue analgesia was earlier in group A (30.65 min) compared to group B (49.42 min) and the difference was statistically significant. Total consumption of rescue analgesic was higher in group A with a mean of 178.85 mg compared to 126.92 mg in group B.
Conclusion
Both the infusion doses of Lidocaine provided clinically adequate analgesia postoperatively but the infusion dose of 2 mg/kg/h had a mean VAS score significantly lower than 1.5 mg/kg/h.
Similar content being viewed by others
Background
Effective postoperative analgesia in surgical patients is essential for encouraging an early recovery. Even though there is a decreased incidence of pain with laparoscopic surgery than with open surgery, it still needs to be adequately managed. Following laparoscopic cholecystectomy (LC), pain can arise from a number of different sources, including incisional, local visceral, peritoneal, and referred pain. Various multimodal approaches have been used to achieve effective postoperative analgesia because of multiple sources of pain in LC (Mitra et al. 2012).
Various studies have found that multimodal analgesia may decrease the need for opioids during surgery by lowering the pain scores in the postoperative period. Non-opioid analgesics such as lidocaine, ketamine, gabapentin, magnesium sulfate, etc. have been used to achieve this (Toleska et al. 2022). Lidocaine is unique among these analgesic adjuvants, as along with a favorable effect on pain scores it also improves enhanced recovery after surgery (ERAS) outcomes (Eipe et al. 2016, Ibrahim et al. 2018). The antinociceptive effects of intravenous (IV) lidocaine have been confirmed in various acute and chronic pain states and there is convincing evidence that IV lidocaine has antihyperalgesic effects (Hermanns et al. 2019). Lidocaine also offers the advantages of adaptability, affordability, accessibility, and familiarity of use (Weibel et al. 2016).
Many studies have demonstrated the analgesic benefit of systemic lidocaine in the perioperative setting, especially during laparoscopic abdominal surgery but the exact relationship between the regimen of lidocaine used and its postoperative analgesic effects remains to be further studied (Song et al. 2017; Zhao et al. 2018; Li et al. 2018). A Cochrane-based meta-analysis revealed inadequate evidence for IV lidocaine compared to placebo on early postoperative pain scores and overall opioid demand and urged further research to find out adequate dose to be used (Kranke et al. 2015, Weibel et al. 2018). The optimal dose for an administered bolus and continuous infusion of lidocaine giving good analgesia and fewer side effects is still undetermined. Since the published literature is not clear about the effective and safe dose of lidocaine to be used in laparoscopic surgeries, we planned a study comparing two different doses of intravenous lidocaine infusion with regard to postoperative analgesia, time to first rescue analgesia and total analgesics used in 24 h.
Methods
This was a prospective, randomized, single-center, double-blind study conducted adhering to the principles of the Declaration of Helsinki. Institute ethics committee approval was taken and the study was conducted from September 2020 to September 2021. Written informed consent was obtained from each patient to participate in this study.
Study participants
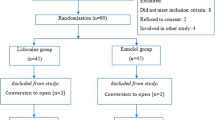
Fifty-four patients of ASA (American Society of Anaesthesiologists) physical status I or II, aged between 18 and 65 years undergoing elective laparoscopic cholecystectomy under general anesthesia were enrolled in the study. One patient from group A was converted to laparotomy because of bleeding and one patient from group B could not be followed postoperatively. So these two were excluded from the study and a total of 52 patients, 26 in each group were analyzed (Fig. 1).
Exclusion criteria
Patients with severe cardiac, respiratory, hepatic, renal, or endocrine disease, patients with a neurological disorder, electrolyte disorders, with a history of alcohol or any drug addiction, who were on analgesics pre-operatively, pregnant/breastfeeding females, and those who had known allergic to local anesthetic were excluded from the study.
Sample size and sampling design
To estimate the group size needed to show statistical significance, assuming a between-group difference in VAS pain score of 1.3 (mean difference) and standard deviation of 1.7 as reported by Gallagher et al. with a two-tailed α = 0.05 and power of 80%, it was calculated that a minimum of 24 patients per group were required (Gallagher et al. 2001). We increased the total number of patients to 27 per group to compensate for the dropouts.
Simple randomization was done using computer-generated random numbers. The Anaesthesia technician was assigned the role of group assignment using a computer-based randomization list, which was secured in sequentially numbered sealed envelopes concealed till enrollment of the patient. At the time of pre-anesthetic evaluation, all patients were explained about the study and briefed about the use of a visual analog scale (VAS) for pain assessment. VAS is a pain assessment tool in which pain is assessed by asking the patient to indicate on a 10-cm line the point that corresponds to the level of pain intensity they felt, where 0 is “no pain” and 10 is “worst imaginable pain”. Patients were advised to remain fasting for 8 h before surgery.
Method of data collection
On arrival in the operation theatre, peripheral venous access was secured with 20 G intravenous cannula. Patients were shifted to operation theatre and a multipara monitor was connected for monitoring ECG, pulse rate, noninvasive blood pressure (NIBP), and pulse oximetry. It was a double-blinded study and attempts were made to maintain allocation concealment from patients and anesthetists. One anesthetist was responsible for the preparation of the medication, and the other anesthetist who infused the medication was not aware of the strength of the infused medication. Patients in group A received an IV bolus of lidocaine 1.5 mg/kg slowly over 10 min, prior to induction followed by a continuous infusion at the rate of 1.5 mg/kg/h via infusion pump. Patients in group B patients received an IV lidocaine bolus of 1.5 mg/kg slowly over 10 min followed by infusion at the rate of 2 mg/kg/h.
Induction was done with Inj. propofol 2 mg/kg and Inj. fentanyl 2 µg/kg, followed by Inj. atracurium 0.5 mg/kg intravenously and the airway was secured by endotracheal tube. Maintenance of anesthesia in both groups was with 50% N2O in O2, isoflurane, and intermittent atracurium as required. Fentanyl 0.5 µg/kg was repeated every hour after the first hour. Fifteen minutes prior to the end of surgery, all patients received intravenous paracetamol 1 gm infusion and ondansetron 4 mg. The lidocaine infusion was continued throughout the surgery and was terminated just after the last suture. Neuromuscular blockade was reversed with IV neostigmine and glycopyrrolate and the patients were extubated after regaining consciousness and were transferred to the post-anesthesia care unit (PACU). Local anesthetic was not given in any other form. During the whole perioperative period heart rate, systolic blood pressure, diastolic blood pressure, mean arterial pressure, and ECG were monitored and documented. Bradycardia was defined as HR < 60 bpm or less than 20% of the baseline and was treated with atropine 0.6 mg (may repeat up to a total of 3 mg). Hypotension was defined as a fall in mean arterial pressure (MAP) of more than 20% of the baseline and was treated with an injection of mephenteramine 6 mg iv.
VAS score was evaluated in the PACU and the surgical ward by the investigator who was unaware of the study medication given, immediately after surgery, then at intervals of 30 min, 1 h, 2 h, 4 h, and thereafter every 4 hourly till 24 h. Postoperatively all the patients were given injection paracetamol 1gm IV 8 hourly. Patients having VAS scores of more than 3 were treated with diclofenac sodium 75 mg IV in 100 ml of saline to be repeated not less than 8-h intervals. If the patient’s VAS remained more than three even after 30 min of inj. diclofenac sodium then injection tramadol 2 mg/kbw was given as an infusion. The time to the first dose and total consumption of inj. diclofenac in 24 h was noted. Adverse effects of lidocaine, i.e., lightheadedness, perioral numbness, nausea and vomiting, sedation, arrhythmias, hypotension, and bradycardia were monitored and documented if present.
Statistical analysis
The data was tabulated in MS Excel and statistical analysis was done using SPSS software, version 19.0 (Statistical Package for the Social Sciences Inc., Chicago, IL, USA. Mean and standard deviation were used for descriptive analysis. An unpaired t test was used to compare the mean difference of VAS scores at different time points, mean time to demand for the first rescue analgesic (in minutes), and mean total analgesic requirement between the two groups. Demographic and other background characteristics were compared between the two groups using suitable statistical tests. For all statistical tests p < 0.05 was considered statistically significant.
Results
Background information like age, gender, BMI, duration of surgery, and ASA scores were not significantly different among groups A and B as shown in (Table 1). This study was adhered to CONSORT guidelines (Fig. 1).
The difference in mean VAS scores between the two groups was statistically significant at 0 min (p = 0.0047), 30 min (p = 0.002), 4 h (p = 0.0001), 8 h (0.0001), 16 h (0.0024), and 24 h (0.0005) (Fig. 2). In other time points it was not found to be statistically significant.
The mean time to the first dose of analgesic given was 30.65 ± 14.63 min in group A and it was 49.42 ± 17.47 min in group B. This was statistically significant (P < 0.001) (Table 2).
The first dose of diclofenac sodium was required in all the patients of group A and group B. The second dose was required in all patients of group A and 18 (69.23%) patients of group B. Ten patients in group A required a third dose of Diclofenac sodium whereas none of the patients in group B required the third dose (Fig. 3). Mean total consumption of Diclofenac sodium was significantly more in group A as compared to group B (p < 0.0001)(Table 3).
One of the patients had an episode of bradycardia in group A that was managed by injection of atropine 0.6 mg. Two patients in group A and three patients in group B had an episode of nausea and vomiting. No other side effects were reported in both groups.
Discussion
Multimodal analgesia involves the use of analgesics of different pharmacological groups acting on different receptors along the pain pathway. This results in improved analgesia with fewer side effects. Multimodal analgesia has been incorporated in almost all surgical procedures to promote enhanced recovery after surgery and intravenous lidocaine is emerging as an essential component of this.
The role of perioperative intravenous lidocaine in the management of post-operative pain following laparoscopic studies has been established in many studies (Song et al. 2017; Zhao et al. 2018; Li et al. 2018). Most of the previous studies have used an intravenous initial lidocaine bolus of 1.5–2 mg/kg followed by an infusion of 1.5–3 mg/kg/h (Li et al. 2018 Feb; Dunn and Durieux 2017). Cochrane review of continuous intravenous perioperative infusion of lidocaine observed the beneficial effect for abdominal laparoscopic surgeries, but the optimal dose and time to terminate lidocaine infusion remains unanswered (Kranke et al. 2015). The same review has categorized a perioperative dose of less than 2 mg/kg as low and more than 2 mg/kg as a high dose of lidocaine. For our study, we chose the doses of 1.5 mg/kg/h and 2 mg/kg/h and avoided larger doses because they might increase the likelihood of toxicity.
In our study, both the lidocaine bolus and infusion were started before induction of anaesthesia. Kawamata et al. in their study found that systemic lidocaine administered prior to a surgical incision lowers the excessive inputs from the injured peripheral nerves, limiting the development of flare formation and secondary hyperalgesia through peripheral and central processes, respectively (Kawamata et al. 2002). We avoided continuing the infusion postoperatively as that requires continuous monitoring of the vitals of the patient and it is uncomfortable in ambulatory or day care surgeries such as laparoscopic cholecystectomies where we aim at enhanced recovery after surgery.
The difference between the mean VAS Score among the two groups was statistically significant in group A as compared to group B at 0 min (p = 0.004), 30 min (p = 0.0002), 4 h (p = 0.0001), 8 h (p = 0.0001), 16 h (p = 0.0024), and 24 h (p = 0.0005). Although this difference in the two groups was statistically significant clinically in both groups the mean VAS Scores remained below 4 at any point in time and never went beyond that. Intravenous lidocaine is postulated to have a multifactorial mechanism of action. It prolongs the pain threshold by increasing acetylcholine concentration at the spinal level through the activation of both muscarinic and nicotinic receptors (Ghimire et al. 2020). The anti-hyperalgesic effect of IV lidocaine is due to the blockade of NMDA receptor-mediated post-synaptic depolarization (Lee et al. 2022). Analgesia produced by lidocaine is also because of its anti-inflammatory property as it decreases the plasma levels of proinflammatory cytokines and inhibits leucocyte activation and adhesion to the site of injury. Song et al. have used the perioperative dose of lidocaine similar to our study in laparoscopic cholecystectomy and have demonstrated that it reduces postoperative pain, improves recovery profile, and attenuates the initiation of the excessive inflammatory response following laparoscopic surgery (Song et al. 2017). In our study, the mean VAS score remained below four in both the groups at different time intervals but group B patients had better analgesia than group A patients.
The mean time for the first rescue analgesic was significantly higher in patients in group A as compared to group B. The mean total rescue analgesia used was also significantly higher in group A as compared to group B. Yang et al. in their study concluded that the use of IV lidocaine infusion significantly reduced postoperative pain and opioid consumption in LC patients, compared with control infusions (Yang et al. 2014). Meta-analysis of randomized controlled trial on intravenous lidocaine infusion for pain control after laparoscopic cholecystectomy illustrated the efficacy and safety of intravenous lidocaine for pain management in these patients as evidenced by reduced postoperative pain scores, opioid consumption, and fewer adverse effects in the lidocaine group (Zhao et al. 2018).
We started the lidocaine infusion prior to anesthesia and it was continued throughout the surgery and terminated just after the last suture. The persistence of the analgesic action beyond the infusion time, and plasma half-life, indicates that it may affect other targets in addition to voltage-gated sodium channels. It has been suggested that it may act via prevention of the hypersensitivity of the central, or peripheral nervous system, or both (Ibrahim et al. 2018). Another mechanism for the persistence of analgesic action even after the infusion is discontinued is that local anesthetics block polymorphonuclear cells priming at a very low concentration by inhibition of specific intracellular G protein signaling molecules (Dunn et al. 2107, Song et al. 2017).
Additional benefits of perioperative use of lidocaine are reduced anesthetic requirements by about one-third blunting the intubation response and stabilizing the heart rate and blood pressure intraoperatively (Ibrahim et al. 2018). Perioperative lidocaine use in laparoscopic surgeries has the added advantage of decreasing shoulder tip pain that is annoying and can last for 3 days (Yang et al. 2014). Lidocaine is also a suitable non-opioid alternative for multimodal pain relief, especially in case of patient refusal or contraindication for regional blocks for analgesia (Weibel et al. 2018).
We did not encounter any major side effects in both the groups. One patient in group B had an episode of bradycardia intraoperatively which was managed by inj atropine. Dose, speed, and duration of infusion are important determinants of lidocaine toxicity (Dunn et al. 2017). CNS toxicity occurs when plasma concentration exceeds 5 mcg/ml and is definite at 10 mcg/ml and CVS toxicity occurs when serum levels exceed 10 mcg/ml. The doses used in our studies have been proven safe by other researchers also (Awal et al. 2022, Sarakatsianou et al. 2021). Meta-analysis by Sarakatsianou et al. 2021 reported that lidocaine infusions at low thresholds (max 2 mg/kg/h) were not associated with any toxicity. We continued the infusion intraoperatively and stopped after the last suture and have not continued the infusion in the post-operative period, that might be the reason we have not encountered any major side effects perioperatively. Lee et al. have reported that the longer the duration of infusion, the higher the rate of toxicity as enzymes become saturated and clearance rates are decreased (Lee and Schraag 2022).
Limitations of our study
(1) We started the lidocaine infusion prior to anaesthesia which was continued throughout the surgery and terminated just after the last suture. We cannot ascertain whether prolonging the lidocaine infusion could have improved analgesia further. We did not measure the serum level of lidocaine but we conducted our study based on the evidence from previous studies which have shown that the plasma level of lidocaine remains in the range of 0.5–5 µg/ml when doses ranging from 1.5 to 3 mg/kg/h are used (Dunn et al. 2017). Recent consensus statements on the efficacy and safety of the use of intravenous lidocaine for postoperative pain and recovery have recommended that a loading dose of not more than 1.5 mg/kg and infusion of no more than 1.5 mg/kg/h, for no longer than 24 h should be used (Foo et al. 2021). Further studies with a larger sample size may be required for the same. Also, we did not include patients with higher body mass index and with contraindications to regional anesthesia in our study. The infusion can be useful in patients with high BMI as an alternative to opioids which have a higher risk of respiratory depression. Similarly, it can provide analgesia in patients in whom regional blocks for analgesia cannot be given.
Conclusions
In this research, it was found that postoperatively most of the time patients of both groups had a mean VAS score of less than three. This indicates that both doses of lignocaine were found to provide adequate analgesia. But the dose of 2 mg/kg/h was found to be providing better analgesia compared to 1.5 mg/kg/h as is evident by significantly lower VAS scores (0 min, 30 min, 4 h, 8 h, 16 h, and 24 h), increased mean time for first analgesic demand and decreased total consumption of analgesics in 24 h among those patients treated with the maintenance dose of 2 mg/kg/h. Although we did not encounter any major adverse effects in our study and found it to be safe in the doses we used, further studies with a large sample size are required for the same.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ASA PS:
-
American Society of Anesthesiologists Physical status
- LC:
-
Laparoscopic cholecystectomy
- IV:
-
Intravenous
- ECG:
-
Electrocardiogram
- NIBP:
-
Non-invasive blood pressure
- ERAS:
-
Enhanced recovery after surgery
- VAS:
-
Visual analog scale
- BMI:
-
Body mass index
References
Awal S, Bhalotra AR, Sharma S (2022) Effects of intra-operative infusion of lidocaine on postoperative pain and quality of recovery in patients undergoing gynecological laparoscopic surgery: a randomized controlled trial. J Anaesthesiol Clin Pharmacol 38(2):300–308
Dunn LK, Durieux ME (2017) Perioperative use of intravenous lidocaine. Anesthesiology 126:729–737
Eipe N, Gupta S, Penning J (2016) Intravenous lidocaine for acute pain: an evidence-based clinical update. BJA Education 16(9):292–298
Foo I, Macfarlane AJ, Srivastava D, Bhaskar A, Barker H, Knaggs R, Eipe N, Smith AF (2021) The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety. Anaesthesia 76(2):238–250
Gallagher EJ, Liebman M, Bijur PE (2001) Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med 38(6):633–638. https://doi.org/10.1067/mem.2001.118863
Ghimire A, Subedi A, Bhattarai B, Sah BP (2020) The effect of intraoperative lidocaine infusion on opioid consumption and pain after totally extraperitoneal laparoscopic inguinal hernioplasty: a randomized controlled trial. BMC Anesthesiol 20(1):137
Hermanns H, Hollmann MW, Stevens MF, Lirk P, Brandenburger T, Piegeler T, Werdehausen R (2019) Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth 123(3):335–349
Ibrahim A, Aly M, Farrag W (2018) Effect of intravenous lidocaine infusion on long-term postoperative pain after spinal fusion surgery. Medicine (baltimore) 97(13):e0229
Karan P, D’souza N, Patil R (2021) A prospective randomized study to evaluate the analgesic efficacy and quality of recovery of perioperative intravenous lidocaine infusion in laparoscopic surgeries. Res Inno in Anesth 6(2):36–43
Kawamata M, Takahashi T, Kozuka Y, Nawa Y, Nishikawa K, Narimatsu E, Watanabe H, Namiki A (2002) Experimental incision-induced pain in human skin: Effects of systemic lidocaine on flare formation and hyperalgesia. Pain 100:77–89
Kranke P, Jokinen J, Pace NL, Schnabel A, Hollmann MW, Hahnenkamp K, et al (2015) Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database Syst Rev (7):CD009642.
Lee IWS, Schraag S (2022) The use of intravenous lidocaine in perioperative medicine: anaesthetic, analgesic and immune-modulatory aspects. J Clin Med 11(12):3543
Li J, Wang G, Xu W, Ding M, Yu W (2018) Efficacy of intravenous lidocaine on pain relief in patients undergoing laparoscopic cholecystectomy: a meta-analysis from randomized controlled trials. Int J Surg 50:137–145
Mitra S, Khandelwal P, Roberts K, Kumar S, Vadivelu N (2012) Pain relief in laparoscopic cholecystectomy–a review of the current options. Pain Pract 12(6):485–496
Sarakatsianou C, Perivoliotis K, Tzovaras G, Samara AA, Baloyiannis I (2021) Efficacy of intravenous use of lidocaine in postoperative pain management after laparoscopic colorectal surgery: a meta-analysis and meta-regression of RCTs. in vivo 35(6):3413–21.
Song X, Sun Y, Zhang X, Li T, Yang B (2017) Effect of perioperative intravenous lidocaine infusion on postoperative recovery following laparoscopic Cholecystectomy-A randomized controlled trial. Int J Surg 45:8–13
Toleska M, Dimitrovski A, Shosholcheva M, Kartalov A, Kuzmanovska B, Dimitrovska NT (2022) Pain and multimodal analgesia in laparoscopic cholecystectomy. Prilozi 43(2):41–49
Weibel S, Jokinen J, Pace NL, Schnabel A, Hollmann MW, Hahnenkamp K et al (2016) Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth 116(6):770–783
Weibel S, Jelting Y, Pace NL, Helf A, Eberhart LH, Hahnenkamp K, et al (2018) Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev 6(6):CD009642.
Yang SY, Kang H, Choi GJ, Shin HY, Baek CW, Jung YH et al (2014) Efficacy of intraperitoneal and intravenous lidocaine on pain relief after laparoscopic cholecystectomy. J Int Med Res 42(2):307–319
Zhao JB, Li YL, Wang YM, Teng JL, Xia DY, Zhao JS et al (2018) Intravenous lidocaine infusion for pain control after laparoscopic cholecystectomy: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 97(5)
Acknowledgements
Nil
Funding
Nil.
Author information
Authors and Affiliations
Contributions
HS: Clinical studies, Data acquisition, Data analysis, Statistical analysis, Study Supervision, Manuscript editing and Manuscript review. SG: Design, Clinical studies, Statistical analysis, Study Supervision, Manuscript editing and Manuscript review. PA: Design, Definition of intellectual content, Literature search, Data analysis, Statistical analysis, Study Supervision, Manuscript preparation and Manuscript editing. NM: Design, Definition of intellectual content, Literature search, Data acquisition, Data analysis, Statistical analysis and Manuscript preparation. SB: Design, Definition of intellectual content, Literature search, Data acquisition, Data analysis, Statistical Analysis, Study Supervision, Manuscript preparation and Manuscript editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institute Ethics Committee number (ASCOMS/IEC/RP&T/2020//382 dated 25/07/2020). Chairperson Dr Anil Kumar Gupta. Name of the institute Acharya Shri Chander College of Medical Sciences and Hospital Sidhra, Jammu, Jammu and Kashmir. Date 25/07/2020. Written informed consent was obtained from each patient to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gupta, S., Attal, P., Mehta, N. et al. Comparison of dose–response to two different doses of intravenous lidocaine for analgesia in patients undergoing elective laparoscopic cholecystectomy under general anesthesia. Ain-Shams J Anesthesiol 15, 89 (2023). https://doi.org/10.1186/s42077-023-00390-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-023-00390-y