Abstract
Background
We successfully treated a case of acute type A aortic dissection in a patient with acute inferior wall infarction as well as severe circulatory and respiratory disorders.
Case presentation
A 69-year-old woman was diagnosed with acute type A aortic dissection. She received emergency partial aortic arch replacement and coronary artery bypass grafting. After the cardiopulmonary bypass, extracorporeal membrane oxygenation (ECMO) with central cannulation was performed due to severe right heart failure and extensive alveolar hemorrhage. Since the surgery, transesophageal echocardiography was used to monitor her hemodynamic status. The positive end-expiratory pressure (PEEP) was managed based on end-expiratory transpulmonary pressure. Replacement of the ECMO circuit was required every 2–3 days due to intra-circuit thrombus, and continuous renal replacement therapy was started on postoperative day (POD) 8. On POD 13, improvement of cardiac function was observed; we therefore attempted closure of the chest and conversion to veno-venous (V-V) ECMO. However, the patient’s hemodynamics were unstable due to diastolic impairment after the chest closure; thus, peripheral veno-arteriovenous (V-AV) ECMO was introduced. The patient was converted to V-V ECMO on POD 16 and weaned from ECMO on POD 17. The patient was extubated on POD 19. She left the intensive care unit with non-invasive ventilation on POD 20.
Conclusions
The favorable outcome in the current case can be attributed to the following three points: (1) appropriate ECMO strategies were employed according to the patient’s condition, (2) the patient’s lung condition improved due to transpulmonary pressure monitoring and fluid balance management from an early stage, and (3) we observed respiratory and hemodynamic status during the 50–90-s circulatory arrest periods that occurred during ECMO circuit changes, and this observation contributed to the evaluation of weaning from ECMO.
Similar content being viewed by others
Background
Circulatory collapse after open-heart surgery is a serious condition with a high mortality rate, even today. According to a recent study, the mortality rate when extracorporeal membrane oxygenation (ECMO) with central cannulation is used for shock after cardiac surgery is 67.3% (Kowalewski et al. 2021); thus, the management of post-cardiotomy shock has been extremely challenging. Herein, we describe a successfully treated case of acute type A aortic dissection in a patient with acute inferior wall infarction as well as severe circulatory and respiratory disorders in the intensive care unit (ICU) by employing situational strategies with ECMO. Written informed consent for the publication of this article was obtained from the family of the patient.
Case presentation
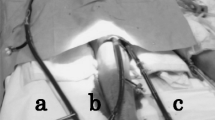
A 69-year-old Japanese woman (height 151 cm, weight 53 kg) without any past medical history was diagnosed with acute type A aortic dissection and acute coronary syndrome by contrast-enhanced computed tomography. She received emergency aortic root replacement (Bentall procedure), partial aortic arch replacement, and coronary artery bypass grafting to the anterior descending branch. After the cardiopulmonary bypass, ECMO with central cannulation (right atrial and left ventricular—ascending aorta) was used due to severe right heart failure and extensive alveolar hemorrhage (Fig. 1). A large amount of bloody fluid came up from the endotracheal tube due to severe pulmonary edema and alveolar hemorrhage. Intraoperatively, blood viscoelasticity test (TEG®6 s system, Haemonetics Corp., MA, USA) revealed that 3080 mL of red blood cells, 3360 mL of fresh frozen plasma, 800 mL of platelet concentrates, 6 g of fibrinogen concentrates, and 7 g of tranexamic acid were needed after the cardiopulmonary bypass for hemostasis. The cardiopulmonary bypass time was 530 min, the operative time was 843 min, the anesthesia time was 945 min, and the bleeding volume was approximately 11,500 mL. After the surgery, the patient was transferred to the ICU with an open chest and artificial respiration.
In the ICU, propofol 100–150 mg/h, ketamine 30 mg/h, and morphine 2.5 mg/h were used for sedation/analgesia during mechanical ventilation and ECMO use. Since the surgery, transesophageal echocardiography and pulmonary artery catheter were used to monitor her hemodynamic status. Based on these parameters, we titrated milrinone, nitroglycerin, and carperitide to maintain an ECMO flow of about 4.0 L/min and a mean blood pressure of about 65 mmHg and to prevent elevation of lactate levels. The positive end-expiratory pressure (PEEP) was maintained at 10–15 cmH2O to achieve the end-expiratory transpulmonary pressure stayed above 0 cmH2O. The transpulmonary pressure was measured using an esophageal balloon catheter kit with a dedicated ventilator (HAMILTON-C6 ventilator, Hamilton Medical AG, Switzerland).
The patient’s consciousness was confirmed on postoperative day (POD) 1, and sedative agents had managed to keep her score on the Richmond Agitation-Sedation Scale between − 2 and 0. Continuous administration of heparin was started to keep he activated clotting time around 200 s on POD 3, when the risk of bleeding decreased. However, replacement of the ECMO circuit was required every 2–3 days in the ICU due to intra-circuit thrombus. Additional blood tests ruled out heparin-induced thrombocytopenia.
Continuous renal replacement therapy for fluid management was started on POD 8. On POD 13, because hemodynamics could be maintained even when the ECMO flow was reduced to 2.0 L/min, we judged the patient’s cardiac function to have improved; we therefore attempted closure of the chest and conversion to veno-venous (V-V) ECMO. However, the patient’s hemodynamics were unstable due to diastolic impairment after the chest closure; thus, peripheral veno-arteriovenous (V-AV) ECMO was introduced. The patient was converted to V-V ECMO on POD 16 and weaned from ECMO on POD 17, after confirming that oxygenation was maintained when the ECMO flow was reduced to 1.5 L/min. The patient was evaluated every day with spontaneous-breathing trials with pressure-support ventilation (Thille et al. 2022), and she was extubated on POD 19. She left the ICU with non-invasive ventilation on POD 20.
Discussion
Based on the findings of a recent study investigating 7185 patients supported with ECMO for postcardiotomy cardiogenic shock (Kowalewski et al. 2021), the present case had multiple factors of poor prognosis, including ECMO with central cannulation, intervention of the thoracic aorta and coronary artery, and concomitant severe left and right heart failure, in addition to severe lung condition associated with pulmonary edema and acute respiratory distress syndrome. However, the favorable outcome in the current case can be attributed to the following three points: (1) appropriate ECMO strategies were employed according to the patient’s condition, which fluctuated daily; (2) the patient’s lung condition improved due to transpulmonary pressure monitoring and fluid balance management from an early stage; and (3) we observed respiratory and hemodynamic status during the 50–90-s circulatory arrest periods that occurred during ECMO circuit changes, and this observation contributed to the evaluation of weaning from ECMO.
ECMO with central canulation is side by side with the risk of mediastinitis. Thus, we tried closing the chest and transitioning from ECMO with central cannulation to peripheral access as soon as possible. In critically ill patients with combined respiratory failure and cardio-circulatory shock, V-AV ECMO is a valid rescue strategy (Mihu et al. 2021); therefore, we chose this approach. One of the other strategies is the use of an Impella® (Abiomed, Danvers, MA, USA), which is a catheter-based left ventricular assist device that is inserted into the left ventricular cavity by arterial access. Left ventricular unloading by using Impella® improves mortality in patients with cardiac shock (Schrage et al. 2020), and several studies have reported the usefulness of V-A ECMO with Impella® (called ECPELLA or ECMELLA) to treat such patients (Pappalardo et al. 2017). In the present case, because of the lack of experience with Impella® after aortic replacement, we did not choose to use ECPELLA for early weaning from central ECMO.
Fluid balance management to avoid volume overload is recommended for patients with acute respiratory distress syndrome from an early stage (Seitz et al. 2020), and fluid management was also performed in this patient from an early stage. In the present case, we performed fluid management based on her pre-operative weight.
Because there is no established protocol for weaning or changing ECMO (Al-Fares et al. 2021), we managed our patient with multifaceted assessments by using trans-esophageal/thoracic echocardiography, pulmonary artery catheter, X-ray, lung ultrasound, and transpulmonary pressure. In particular, ventilatory management using transpulmonary pressure may contribute to successful ECMO withdrawal (Wang et al. 2020). Hence, excessive high PEEP also affects hemodynamics, monitoring of transpulmonary pressure was useful in the management of PEEP to prevent both lung collapse and lung hypertension, with minimal impact on hemodynamics. Moreover, respiratory and hemodynamic status during ECMO circuit changes provided valuable information for weaning from ECMO.
Conclusions
We successfully treated a case of acute type A aortic dissection in a patient with acute inferior wall infarction as well as severe circulatory and respiratory disorders in the ICU by employing situational strategies with ECMO. In such cases, careful management with multifaceted assessments should be provided through close collaboration among anesthesiologists, intensivists, cardiovascular surgeons, ICU staff, and clinical engineers.
Availability of data and materials
Not applicable.
Abbreviations
- ICU:
-
Intensive care unit
- ECMO:
-
Extracorporeal membrane oxygenation
- PEEP:
-
Positive end-expiratory pressure
- V-V ECMO:
-
Veno-venous extracorporeal membrane oxygenation
- V-AV ECMO:
-
Veno-arteriovenous extracorporeal membrane oxygenation
- POD:
-
Postoperative day
References
Al-Fares AA, Ferguson ND, Ma J, Cypel M, Keshavjee S, Fan E, Del Sorbo L (2021) Achieving safe liberation during weaning from VV-ECMO in patients with severe ARDS: the role of tidal volume and inspiratory effort. Chest 160:1704–1713
Kowalewski M, Zieliński K, Brodie D, MacLaren G, Whitman G, Raffa GM, Boeken U, Shekar K, Chen YS, Bermudez C, D’Alessandro D, Hou X, Haft J, Belohlavek J, Dziembowska I, Suwalski P, Alexander P, Barbaro RP, Gaudino M, Di Mauro M, Maessen J, Lorusso R (2021) Venoarterial extracorporeal membrane oxygenation for postcardiotomy shock-analysis of the extracorporeal life support organization registry. Crit Care Med 49:1107–1117
Mihu MR, Mageka D, Swant LV, El Banayosy A, Maybauer MO, Harper MD, Koerner MM, El Banayosy A (2021) Veno-arteriovenous extracorporeal membrane oxygenation-a single center experience. Artif Organs 45:1554–1561
Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Müllerleile K, Colombo A, Sydow K, De Bonis M, Wagner F, Reichenspurner H, Blankenberg S, Zangrillo A, Westermann D (2017) Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 19:404–412
Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, Colson P, CudemusDeseda G, Dabboura S, Eckner D, Eden M, Eitel I, Frank D, Frey N, Funamoto M, Goßling A, Graf T, Hagl C, Kirchhof P, Kupka D, Landmesser U, Lipinski J, Lopes M, Majunke N, Maniuc O, McGrath D, Möbius-Winkler S, Morrow DA, Mourad M, Noel C, Nordbeck P, Orban M, Pappalardo F, Patel SM, Pauschinger M, Pazzanese V, Reichenspurner H, Sandri M, Schulze PC, Schwinger R HG, Sinning JM, Aksoy A, Skurk C, Szczanowicz L, Thiele H, Tietz F, Varshney A, Wechsler L, Westermann D (2020) Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation 142:2095–2106
Seitz KP, Caldwell ES, Hough CL (2020) Fluid management in ARDS: an evaluation of current practice and the association between early diuretic use and hospital mortality. J Intensive Care 12(8):78
Thille AW, Gacouin A, Coudroy R, Ehrmann S, Quenot JP, Nay MA, Guitton C, Contou D, Labro G, Reignier J, Pradel G, Beduneau G, Dangers L, Saccheri C, Prat G, Lacave G, Sedillot N, Terzi N, La Combe B, Mira JP, Romen A, Azais MA, Rouzé A, Devaquet J, Delbove A, Dres M, Bourenne J, Lautrette A, de Keizer J, Ragot S, Frat JP, REVA Research Network (2022) Spontaneous-breathing trials with pressure-support ventilation or a T-piece. N Engl J Med. 387:1843–1854
Wang R, Sun B, Li X, Tang X, He H, Li Y, Yuan X, Li H, Chu H, Tong Z (2020) Mechanical ventilation strategy guided by transpulmonary pressure in severe acute respiratory distress syndrome treated with venovenous extracorporeal membrane oxygenation. Crit Care Med 48:1280–1288
Acknowledgements
The authors would like to thank the Scientific English Editing Section of Fukushima Medical University for their work on this manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
KS, KY, and TH managed perioperative care and prepared the manuscript. ST performed anesthetic management during the surgery. SI helped to draft the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
At our institution, Institutional Review Board approval is not required for a case report. The IRB of Fukushima Medical University (Fukushima, Japan) determined that there were no problems with describing the course of treatment for this presentation if written informed consent was obtained.
Consent for publication
Written informed consent for publication of this case report and the accompanying images were obtained from the family of the patient. A copy of the written consent is available for review at the request the Editor-in-Chief of Ain- Shams Journal of Anesthesiology.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suzuki, K., Yoshida, K., Hakozaki, T. et al. Successful management by employing situational extracorporeal membrane oxygenation strategies in a patient with acute type A aortic dissection: a case report. Ain-Shams J Anesthesiol 15, 64 (2023). https://doi.org/10.1186/s42077-023-00360-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-023-00360-4