Abstract
Background
Diffuse coronary artery spasm after coronary artery bypass grafting may cause disastrous circulatory collapse and death if not detected and managed promptly.
Case presentation
Four out of 387 patients who underwent uneventful off-pump coronary artery bypass grafting between January 2019 and December 2021 at our centre developed unexplained arrhythmias and hypotension within 2 to 6 h of the postoperative period. Coronary angiogram showed diffuse coronary vasospasm involving both grafted and ungrafted native coronary arteries. Vasospasm was refractory to intracoronary vasodilators in all these cases. All of the patients recovered completely with the use of intravenous verapamil infusion and intraaortic balloon pump support.
Conclusions
Coronary artery spasm should remain in the differential diagnosis in patients developing unexplained arrhythmias and haemodynamic instability following coronary artery bypass grafting. Prompt diagnosis with coronary angiogram and proper management strategy using calcium channel blockers and intraaortic balloon pump can revert this deadly complication. Surgical intervention may delay precious time needed for therapeutic intervention.
Similar content being viewed by others
Background
The term coronary artery spasm (CAS) refers to a sudden, intense vasoconstriction of an epicardial coronary artery that causes vessel occlusion or near occlusion (Hung et al. 2014). Coronary artery spasm may complicate the immediate postoperative period after coronary artery bypass grafting (CABG) leading to circulatory collapse and death (Pichard et al. 1980). Although cases have been reported worldwide with different outcomes and lines of management, there are not many reported in the Indian scenario (Lin et al. 2007; Unai et al. 2014; Lemmer and Kirsh 1988; Schena et al. 2007; Lorusso et al. 2012). To our knowledge, this is the first reported case series of refractory diffuse coronary artery spasm (CAS) after uneventful off-pump CABG (OPCABG) in India where a strategy of immediate coronary angiography, calcium channel blockers and mechanical circulatory support helped in complete recovery.
Case presentation
Three hundred eighty-seven patients underwent uneventful off-pump CABG between January 2019 and January 2021 at our institution. As per our existing protocol, all patients were preoperatively on long-acting oral nitrates and beta-blockers. All patients underwent surgery with general anaesthesia using 0.03 mg/kg midazolam, 2 mg/kg propofol 1% or 0.2 mg/kg etomidate (for patients with left ventricular dysfunction), 5mcg/kg fentanyl, and 1 mg/kg rocuronium for induction and maintenance with sevoflurane. Haemodynamic, respiratory, temperature and bispectral index monitoring was done. A well-experienced surgeon performed all surgeries through a median sternotomy with a take-down of the left internal mammary artery (LIMA). A heparin dose of 300 IU/kg was given with activated clotting time maintained at approximately 350 s. The LIMA was sprayed with 0.5% topical papaverine. Patients were kept on nitroglycerin infusion 2–3 mcg/kg/min and a low dose of inotropes, dopamine (2.5–3 mcg/kg/min) and norepinephrine (0.02–0.03 mcg/kg/min) for haemodynamic support. The grafting sequence was similar in all patients: left anterior descending (LAD) coronary artery, followed by the anterior, lateral and posterior vessels using intracoronary shunts and mechanical stabiliser. Distal coronary anastomoses were carried out before the proximal aortic analogues in the vein grafts. Patients were kept on intravenous fentanyl infusion along with paracetamol while on ventilatory support in the Intensive care unit (ICU), so as to keep a universal pain assessment score of less than four. Inotrope used for haemodynamic support in ICU was Dopamine in the range of 2.5–5 mcg/kg/min. All patients were on nitroglycerin infusion 0.5–1 mcg/kg/min.
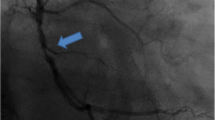
Among these patients, four had clinical deterioration between 2 and 5.5 h of arrival to the ICU after surgery which manifested as junctional bradycardia with a heart rate range between 50 and 58 beats per minute and hypotension with a mean arterial pressure (MAP) between 40 and 50 mm Hg. One patient demonstrated ST elevation in inferior leads while another developed an episode of ventricular fibrillation which was reverted with direct current electrocardioversion. Inotropes were stepped up to ensure MAP > 60 mm Hg and immediate coronary angiography (CAG) was performed to rule out graft failure. CAG showed diffuse CAS of all the grafted coronary arteries (Fig. 1) as well as arterial conduits. It is to be noted that two patients had vasospasm of the ungrafted right coronary artery (Fig. 2) which was not surgically handled at all. Repeated intracoronary injection of 0.2 mg nitroglycerin and nicorandil did not revert the vasospasm. Intraaortic balloon pump (IABP) was inserted for three of the patients for haemodynamic support. Intravenous verapamil infusion (0.05 mg/kg/h gradually increased to 0.1 mg/kg/h) was started for all of the patients and continued for 72 h. After starting verapamil infusion junctional bradycardia resolved after 1–2 h and normal sinus rhythm was restored for all patients. All patients tolerated verapamil therapy without further episodes of bradycardia or hypotension. Patients on IABP did not have complications like limb ischemia or local bleeding. IABP was weaned off after 48 h for two patients and after 72 h for one patient.
None of the four patients who developed CAS gave a history of rest angina. The mean age group of patients was 59.25 ± 6.55 years with the male-to-female ratio being 3:1 (Table 1). Half of the patients were smokers, three of them were diabetics and two of them had preexisting hypertension. There was no evidence of hypothermia, metabolic acidosis, respiratory alkalosis, hypoxia, hypercarbia, or dyselectrolytemia in the perioperative and immediate postoperative period.
All patients recovered completely and were discharged on oral verapamil. Renal dysfunction was not observed in any. All patients were asymptomatic after discharge, ECG and echocardiography done on the follow-up visit 1 month after discharge were normal. One patient underwent follow-up CT coronary angiography 1 week post discharge, which showed no evidence of CAS.
Discussion
Although Prinzmetal first described CAS associated with variant angina in 1959, it was first reported in the postoperative period after CABG in 1980 by (Pichard et al. 1980). CAS is more common in males than females, individuals aged between 40 and 70 years, (JCS Joint Working Group 2010; Matta et al. 2020) more in Japanese (24.3%) followed by Taiwanese (19.3%) and Caucasian (7.5%) populations (Matta et al. 2020).
Usually, vascular spasm involves a single implanted graft or coronary artery but reports have described a more diffuse form with dismal outcome (Lin et al. 2007). Diffuse refractory CAS represents a significant determinant of morbidity and mortality during the perioperative phase. It may occur intraoperatively, postoperatively upon emergence, or shortly after extubation with an incidence ranging from 0.8 to 1.3% (Lin et al. 2007). Perioperative CAS can occur after both on-pump and off-pump CABG (Lin et al. 2007; Unai et al. 2014). Patients with the normal dominant right coronary artery (RCA) without stenosis, history of smoking or variant angina before surgery are a high-risk group to develop coronary artery spasm (Unai et al. 2014; Hung et al. 2014).
The mechanisms underlying CAS are poorly defined and multifactorial. Important mechanisms involved are autonomic system dysfunction, inflammation and endothelial dysfunction, smooth muscle cell hypercontractility, degradation of nitric oxide (NO) by oxygen free radicals and genetic mutations or polymorphism of the endothelial NO synthase gene (Hung et al. 2014). Precipitating causes of CAS include manipulation of the heart, intimal injury by intracoronary shunts, external traction and compression by instruments or chest tubes, hypomagnesaemia, hyperkalemia, high endogenous catecholamine levels during emergence, high exogenous catecholamine administration, respiratory alkalosis, hypothermia, and underlying atherosclerotic disease (Lemmer and Kirsh 1988). Postoperative diffuse CAS is most commonly manifested by arrhythmias, ST-segment elevation and circulatory collapse without a specific cause (Lemmer and Kirsh 1988). Failure to promptly resolve the spasm may produce irreversible and life-threatening consequences.
Our case series of four patients developed severe refractory diffuse coronary vasospasm after uneventful OPCABG. This constituted 1.03% of the total surgical cases performed by us during that period. Majority were males in the age group of 50–65 years. There were no intraoperative events like hypotension or electrolyte abnormalities to precipitate CAS in any of the patients. CAG showed spasm involving the ungrafted RCA in two of the patients which makes surgical manipulation or intracoronary shunts an unlikely trigger of the event. Inotropes administered were low doses as used in all patients undergoing CABG in our institution. Adequate postoperative analgesia, ventilatory support and nitroglycerin infusion were maintained in all these patients. Vascular smooth muscle hyper-reactivity is thought to be central to the pathogenesis of CAS. We hypothesise that this particular set of patients demonstrated abnormal coronary vascular hyper-reactivity to the surge of endogenous catecholamines on emergence in ICU and developed diffuse CAS.
Immediate intervention is essential in this situation, after ruling out other reasons like graft failure, tamponade etc. Coronary angiogram is a powerful tool in the diagnosis and formulation of further management. Intracoronary injection of vasodilators has been shown to be effective but may not be sufficient in the presence of profound cardiogenic shock and marked myocardial stunning (Schena et al. 2007). In this situation, IABP support along with calcium channel blockers (CCBs) like verapamil may help to tide over this crisis. Rarely temporary ECMO support may be required to handle the severe cardiogenic shock related to CAS (Lorusso et al. 2012). CAS being a reversible condition, surgical intervention is unnecessary and might delay the critical period for therapy leading to a fatal outcome.
Conclusions
Diffuse coronary artery vasospasm is an existent rare underdiagnosed perioperative complication after CABG with dismal outcomes. Suspicion of CAS in the perioperative period should warrant immediate coronary angiogram for diagnosis. Prompt management with the help of CCBs and IABP can be life-saving while surgical intervention has no role. Larger clinical trials will be helpful to identify vulnerable patient groups and further study the exact cause of diffuse CAS.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- CAS:
-
Coronary artery spasm
- CCB:
-
Calcium channel blockers
- CABG:
-
Coronary artery bypass grafting
- IABP:
-
Intraaortic balloon pump
- LAD:
-
Left anterior descending artery
- LCX:
-
Left circumflex artery
- LIMA:
-
Left internal mammary artery
- LMCA:
-
Left main coronary artery
- MAP:
-
Mean arterial pressure
- OM:
-
Obtuse marginal artery
- PDA:
-
Posterior descending artery
- RA:
-
Radial artery
- RCA:
-
Right coronary artery
- RI:
-
Ramus intermedius
- VF:
-
Ventricular fibrillation
References
Hung MJ, Hu P, Hung MY (2014) Coronary artery spasm: review and update. Int J Med Sci 11(11):1161–71. https://doi.org/10.7150/ijms.9623
JCS Joint Working Group (2010) Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ J 74(8):1745–1762
Lemmer JH Jr, Kirsh MM (1988) Coronary artery spasm following coronary artery surgery. Ann Thorac Surg 46:108–115
Lin CY, Weng ZC, Loh SH, Hong GJ, Tsai CS (2007) Coronary artery spasm after off pump coronary artery bypass grafting. ANZ J Surg 77:126–129
Lorusso R, Crudeli E, Lucà F, De Cicco G, Vizzardi E, D’Aloia A, Gelsomino S (2012) Refractory spasm of coronary arteries and grafted conduits after isolated coronary artery bypass surgery. Ann Thorac Surg 93(2):545–551
Matta A, Bouisset F, Lhermusier T, Campelo-Parada F, Elbaz M, Carrié D, Roncalli J (2020) Coronary Artery Spasm: New Insights. J Interv Cardiol 14(2020):5894586. https://doi.org/10.1155/2020/5894586
Pichard AD, Ambrose J, Mindich B, Midwall J, Gorlin R, Litwak RS, Herman MV (1980) Coronary artery spasm and perioperative cardiac arrest. J Thorac Cardiovasc Surg 80(2):249–254 (PMID: 7401678)
Schena S, Wildes T, Beardslee MA, Lasala JM, Damiano RJ Jr, Lawton JS (2007) Successful management of unremitting spasm of the nongrafted right coronary artery after off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg 133:1649–1650
Unai S, Hirose H, Cook G, Lee Y, Miura S, Kigawa I, Fukuda S, Miyairi T (2014) Coronary artery spasm following off-pump coronary artery bypass surgery. Int Heart J 55(5):451–454
Acknowledgements
Dr George M Chandy, Director and Dr John Valliattu, Chief of Cardiac Surgery, Believers Church Medical College Hospital, Thiruvalla.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
BA did the concept, design, literature search, data collection and manuscript preparation. KRN interpreted, reviewed and added surgical perspectives to the manuscript. SS interpreted, reviewed and added anaesthetic perspectives to the manuscript. All the authors have reviewed and approved the manuscript prior to submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics Committee approval number IEC2023/02/14 dated 27.02.2023 granted by Institutional Ethics Committee Believers Church Medical College Hospital, Kerala. Informed written consent to participate was obtained from the subjects.
Consent for publication
Informed written consent for publication of personal and medical data acquired from medical records as well as coronary angiography images was taken from all participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abraham, B., Nair, K.R. & Sulaiman, S. Diffuse refractory coronary artery spasm after off-pump coronary artery bypass grafting: a case series. Ain-Shams J Anesthesiol 15, 16 (2023). https://doi.org/10.1186/s42077-023-00313-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-023-00313-x