Abstract
Background
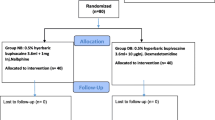
Spinal anesthesia with bupivacaine is very common for infraumbilical surgeries. Various adjuvants are added to it to improve the quality of the block and post-operative analgesia. The study period for this study was from October 2017 to March 2018, and it was a randomized double-blinded prospective observational study. In this study, we aim to compare nalbuphine and MgSO4 (magnesium sulfate) as adjuvant to hyperbaric bupivacaine in terms of sensorimotor blockage characteristics, hemodynamic stability, and postoperative analgesia. Ninety patients of ASA grades I and II, between 18 and 60 years of age of either sex posted for elective infraumbilical surgeries, after approval from the institutional review board and written informed consent, were allocated into 3 groups of 30 patients each. With the help of the randomization table, random numbers were generated, and the randomization was done at the time of giving intrathecal anesthesia.
Group A: 3 mL of 0.5% heavy bupivacaine 15 mg + 0.2 mL of 0.9% normal saline to a total volume of 3.2 mL
Group B: 3 mL of 0.5% heavy bupivacaine 15 mg + 0.1 mL of 1 mg preservative-free nalbuphine with 0.1 ml of 0.9% normal saline to a total volume of 3.2 mL
Group C: 3 mL of 0.5% heavy bupivacaine 15 mg + 0.2 ml of 50% preservative-free (100 mg) magnesium sulfate to a total volume of 3.2 mL
The primary outcome was to assess the postoperative analgesia, and the secondary outcome was to assess the perioperative hemodynamic stability and adverse effects during the study period.
Results
The onset of sensory and motor blockade was earlier in the nalbuphine group as compared with the other two groups. It was also observed that the duration of postoperative analgesia was longer in the patients who received magnesium sulfate as compared with the patients in the other two groups. Adverse effects (pruritus, nausea, vomiting) were more in the nalbuphine group as compared with the other two groups.
Conclusions
In a nutshell, preservative-free intrathecal 1 mg nalbuphine and 100 mg magnesium sulfate both are good adjuvants to hyperbaric bupivacaine. Nalbuphine provides faster sensory and motor onset than magnesium sulfate, whereas magnesium sulfate provides prolonged postoperative analgesia than nalbuphine.
Similar content being viewed by others
Background
Multimodal anesthetic techniques are available for infraumbilical surgeries. Intrathecal anesthesia is still the first choice because of its advantage like rapid onset, superior blockade, less failure rates, and cost-effectiveness but has the drawbacks of shorter duration of blockade and lack of postoperative analgesia. Bupivacaine is the most commonly used local anesthetic agent having satisfactory sensory and motor blockade with limited duration of action. Various preservative-free adjuvants are now being used with hyperbaric bupivacaine for intrathecal anesthesia.
Nalbuphine, an opioid with mixed μ antagonist and κ agonist properties, is related chemically to oxymorphone and highly lipid soluble (Verma et al. 2013). It has been used since 10 years, but no evidence of neurotoxicity has been found (Rawal et al. 1991). MgSO4 blocks NMDA channels in a voltage-dependent way, and addition of magnesium produces a reduction of NMDA-induced currents and potent analgesia. Intrathecal magnesium has been found to prolong duration of analgesia (Biswadeep et al. 2016; Pascual-Ramirez et al. 2013).
Methods
Ninety patients of ASA grade I and II, between 18 and 60 years of age of either sex and average height and weight undergoing elective infraumbilical surgeries, were taken for our study after the approval from the institutional review board.
-
A.
Patient exclusion criteria:
-
Patients with ASA grades III, IV, and V
-
Patient with gross spinal deformity, peripheral neuropathy, and pregnancy/lactation
-
Patients with coagulation disorders, systemic hypertension, hepatic dysfunction, renal dysfunction, endocrine dysfunction, and cardiac dysfunction
-
Patients with known allergy to local anesthetics, hypersensitivity to study drugs, and local site infection
-
Patient on chronic analgesic therapy
-
Patients having h/o drug or alcohol abuse
-
B.
Pre-anesthetic evaluation:
Pre-anesthetic checkup was carried out in all patients the day before the surgery.
Preoperative preparation
All patients fasted overnight. Vitals noted in the pre-operative room were considered as baseline values. Preoperatively, a peripheral venous access was secured with 18-gauge cannula, and preloading with lactated Ringer’s solution was initiated at the rate of 10 ml/kg over 30 min.
Premedication
Inj. glycopyrrolate 0.02 mg and inj ondansetron 4 mg IV were given.
No analgesics or sedatives were given preoperatively. In the operation theatre, anesthesia machine was checked, and emergency drugs were kept ready. VAS was explained to the patients.
Upon entering the OT, noninvasive monitoring was in the form of SpO2, ECG, and NIBP.
Study group allocation
Patients were divided into three groups with 30 patients each according to the drugs they received randomly.
Randomization was done by using the randomization table (Randomisation n.d.).
Allocation of randomized group was done by sealed opaque envelope at the time of spinal anesthesia.
Group A (bupivacaine group)—15 mg bupivacaine heavy (0.5%) (3 ml) with 0.2 ml NaCl (0.9%)
Group B (bupivacaine nalbuphine group)—15 mg bupivacaine heavy (0.5%) (3 ml) with 0.1 ml of 1 mg of preservative-free nalbuphine with 0.1 ml of 0.9% of normal saline to a total volume of 3.2 ml (we have used 10 mg of 1 ml ampoule of nalbuphine, and 0.1 ml was taken from it with help of insulin syringe)
Group C (bupivacaine magnesium sulfate group)—15 mg bupivacaine heavy (0.5%) (3 ml) with 0.2 ml of 50% preservative-free magnesium sulfate (100 mg) to a total volume of 3.2 ml
All patients received total volume 3.2 ml intrathecally.
Anesthesia technique
All the patients were explained with the procedure, and it was the same in all patients. Under strict aseptic and antiseptic precautions, in lateral position, between L3 and L4 intervertebral space, subarachnoid block was performed with 25-gauge Quincke’s needle via midline approach. After free flow of CSF, the test drug was injected for over 10–15 s. Patients were given in supine position after the completion of the block. Thereafter, the position was unchanged.
The surgical anesthesia was considered effective when T6-T8 dermatome was anesthetized, and grade 3 motor block was achieved.
Intraoperatively HR, SBP, DBP, RR, and SpO2 were recorded at 2, 4, 6, 8, 10, 12, and 15 min, then every 5 min up to 30 min, every 15-min intervals up to 1 h, and thereafter at frequent intervals till 24 h in the postoperative ward.
At the end of the procedure, patients were shifted to the postoperative ward where further monitoring was continued.
Observations were made considering following points:
-
Hemodynamic stability (HR, SBP, DBP, RR, SpO2)
-
Onset of sensory and motor block
-
Time of onset of sensory block (up to T10 dermatome) (T1)
-
Time to achieve highest sensory level(T2)
-
Time for 2 dermatome segment regression of sensory block (T3)
-
Time for sensory regression to S2 dermatome (T4)
-
Time for 1st rescue analgesia given when VAS score was > 3 (T5)
-
Duration of effective analgesia (T6 = T5–T1)
-
Time for onset of grade 3 motor block (grade 0 to grade 3) (Ta)
-
Regression of motor block from grade 3 to grade 0 (Tb)
-
Duration of motor blockade TC = Tb–Ta
Definitions of variables
-
Onset of sensory block was defined as time to loss of sensation of cold to spirit swab at the level of T10 dermatome
Highest level of sensory block T6–T8 and time to attain it were recorded. It was assessed by the loss of sensation of cold to spirit swab at 2-min intervals till surgical anesthesia was achieved. Further sensory testing was performed at 30-min intervals till the recovery of S2 dermatome.
Regression of sensory block by 2 segments was also recorded suggestive of offset of sensory blockade.
-
Onset of motor block: Motor block was assessed by using modified Bromage scale.
Grade 0—No motor block
Grade 1—Inability to raise extended leg; able to move knees and feet
Grade 2—Inability to raise extended leg and move knee; able to move feet
Grade 3—Complete motor block of lower limb
Motor block was assessed from its onset till achievement of the grade 3 motor blockade and at the end of surgery and at 30-min intervals till the patient had no motor block. This time to achieve grade 0 motor blockade from grade 3 motor blockade was noted and considered as the duration of motor blockade.
-
Duration of surgery was defined as time of last skin stitch − time of skin incision (in minutes)
-
Duration of sensory blockade was defined as time to sensory onset up to time to S2 segment regression
-
Duration of effective analgesia was considered as the interval from time of intrathecal injection to the time of first analgesic demand post operatively or when VAS score > 3 and at that time inj. tramadol 50 mg i.v. slowly with inj. ondansetron 0.08 mg/kg i.v. was given as rescue analgesia. The total number of analgesic requests in postoperative 24 h were noted. Intraoperative fluid loss and blood loss were assessed. Intraoperative urine output was also assessed.
Complications:
-
Hypotension if observed was treated with bolus of IV fluids and if required inj. ephedrine 6 mg i.v. was given.
-
Bradycardia (HR < 60/min or fall in HR > 20% from baseline value) was treated with 0.6 mg i.v. atropine
-
Respiratory depression if observed was treated with supplemental O2 via either Bain’s circuit or face mask as and when required
-
Sedation score: postoperative sedation assessed by OAA score (Chernik et al) (Chernik and Gilling 1990)
Sedation score was noted at 30 min after SAB up to 6 h.
All the patients were observed for any adverse effects in the postoperative period for 24 h.
-
Nausea and vomiting were assessed and rescue antiemetic in the form of inj. ondansetron hydrochloride 4 mg stat i.v. given
-
Shivering: grading of shivering was as per WRENCH (Wrench and Singh 1997) and was treated via warming the patient by covering the patient, decreasing the cooling of the OT and covering the patient. No antihistaminics or opioids causing sedation were administered
-
Pruritus was observed in patients and was assessed as mild, moderate, and severe as follows:
Mild—itching was only minor concern
Moderate—itching was a primary concern, although bearable, and the patient said that he/she would itch rather hurt
Severe—unbearable, patient requested treatment
In severe form of pruritus, antihistaminic was kept ready.
-
Post-dural puncture headache (PDPH) was mainly occipital or frontal headache aggravated by erect or sitting position, relieved on lying flat and increased on coughing, sneezing, or straining
-
Transient neurological symptoms (TNS) was defined as pain and/or dysesthesia in the back, buttocks, and legs or pain radiating to lower extremities after initial recovery from spinal anesthesia and resolved within 72 h. Patients were followed up to 7 days to check for any other neurological symptoms.
Patient were assessed for delay in voiding.
Sample size calculation
According to the previous studies of Gupta KL et al. (Lal et al. 2017) and Charu et al. (Pandya 2014), in order to increase the postoperative analgesia more than 35 min than the control group having power of 80% and confidence interval of 95, a total of 90 patients have been enrolled in the study and divided 30 in each group randomly. The results of our pilot study (done with 5 patients in each group) and discussion with the institutional review board were also considered during sample size calculation.
Statistical analysis
The data obtained was statistically analyzed using the SPSS software version 10 (IBM, NY,USA).
Quantitative data was expressed as mean and standard deviation.
Qualitative data was expressed in number, N, %.
Data was compared using analysis of variance (ANNOVA), for age, height, weight, and duration of surgery in demographic data.
Categorical data was analyzed by chi-square test, Fischer extract tests like adverse effects, ASA grade, and sex distribution in demographic data.
P value > 0.05 was considered non-significant (NS).
P value < 0.05 was considered statistically significant (S).
P < 0.001 was considered highly significant (HS).
VAS score (Revill et al. 1976)

Results
The present study was undertaken to evaluate efficacy and potency of preservative-free nalbuphine and magnesium sulfate as an adjuvant with intrathecal bupivacaine for effect on sensory and motor blockade, sedation hemodynamic stability, duration of effective analgesia, post-operative pain relief, post-operative analgesic requirement, and adverse effect of drugs used.
According to Wrench score, grade 1 shivering was noted in 2 patients of group A and 1 patient in group B.
Mild pruritus was observed in 1 patient of group A and 2 patients of group B.
Nausea and vomiting were noticed in 1 patient of group A and 2 patients of group B and 1 patient of group C.
Postoperative monitoring
We have monitored vitals of all patients in post-operative period till 24 h. OAA score was monitored up to 360 min, and it was 5 in each group (P > 0.05)
-
➢ Hemodynamic parameters of all patients were in normal limits.
-
➢ Rescue analgesics were repeated after VAS was more than 3.
-
➢ All patients were conscious and co-operative.
-
➢ No adverse effect was noted during post-operative monitoring.
Discussion
With more than 100 years of use, neuraxial anesthesia has gained much success. It is a safe and effective alternative to general anesthesia when surgical site is located in the infraumbilical region. To improve spinal anesthetic efficacy, adjuvants are used to enhance and prolong analgesia, to lower dose requirements, and to reduce dose dependent side effects of local anesthetics (R.K n.d.).
Nalbuphine is a mixed agonist-antagonist drug. When it binds to kappa receptor, it competitively displaces other mu agonists from mu receptor, thereby exhibiting less respiratory depression (R.K n.d.).
Magnesium is an NMDA receptor antagonist. Intrathecal magnesium blocks NMDA receptors at the dorsal horn, thus preventing central sensitization to pain and improving anesthetic and analgesic quality (Biswadeep et al. 2016; Pascual-Ramirez et al. 2013).
Drug and dosage
Gupta KL et al. (Lal et al. 2017) had taken in their study 3 ml hyperbaric bupivacaine 0.5% 15 mg and 1 mg of nalbuphine (preservative-free) injection made in 0.5 ml normal saline intrathecally.
Choudhury et al. (Biswadeep et al. 2016) had also taken in their study 100 mg (0.2 ml) magnesium sulfate and 0.8 mg of 0.2 ml of nalbuphine along with 3 ml (15 mg) 0.5% heavy bupivacaine. Total volume was 3.2 ml which is similar to our study.
Charu et al. (Pandya 2014) had also taken in their study 50% of 100 mg (0.2 ml) magnesium sulfate along with 3 ml (15 mg) 0.5% hyperbaric bupivacaine.
Patient characteristics
Table 1 shows that in our study demographic variables are comparable in each group in form of age, weight, height, sex, ASA grade, and duration of surgery (P > 0.05).
Gupta KL et al. (Lal et al. 2017) and Choudhury et al. (Biswadeep et al. 2016) also have comparable demographics.
Hemodynamic characteristics
In all groups, hemodynamic variables were stable in the intraoperative period (P > 0.05).
Gupta KL et al. (Lal et al. 2017) and Parveen et al. (Parveen et al. 2015) have observed statistically non-significant changes.
Characteristics of spinal block
Table 2 shows various characteristics of spinal blockade.
Time of T10 sensory onset
Parveen et al. (Parveen et al. 2015) concluded it was 3.23 ± 1.03 min in the plain bupivacaine group and 1.63 ± 0.57 min in the nalbuphine group (P < 0.001).
Choudhury et al. (Biswadeep et al. 2016) observed that it was 4.20 ± 0.67 min. in the nalbuphine group and 5.75 ± 0.74 min in the magnesium group (P < 0.001).
Khalili et al. (Khalili et al. 2011) have observed delayed time for onset of sensory block in the magnesium group (13.3 ± 4.0 min) as compared with the bupivacaine group (11.6 ± 3.5 min) (P< 0.05)
In our study, we have observed a delayed time of sensory onset in magnesium group as compared with the other two groups which are consistent with the above study findings.
Time to reach highest sensory level (T6-8)
It is 7.76 ± 0.52 min in group A, 7.52 ± 0.70 min in group B, and 11.56 ± 0.63 min. in group C. P (A and B) was P > 0.05 but P (B and C) and P (C and A) were P < 0.001.
Arora et al. (Arora et al. 2015) have observed that it is 5.3 ± 0.5 min in the fentanyl-bupivacaine group and 8.7 ± 0.5 min in the magnesium group (P< 0.05).
Gupta K et al. (Gupta et al. 2016) found that the time to reach highest sensory level in the fentanyl group is 7.4 ± 2.72 min, and in the nalbuphine group, it is 7.13 ± 3.81 min (P > 0.05).
Time to reach grade 3 motor blockade
It was similar in groups A and B and longer in group C which is highly significant statistically.
Choudhury et al. (Biswadeep et al. 2016) have concluded that the time to reach Bromage grade 3 was 5.33 ± 0.41 min in the nalbuphine group and 7.54 ± 1.18 min in the magnesium group (P< 0.001)
Ramírez et al. (Apeksha et al. 2014) showed that the time to maximal motor block was 2.4 min slower with intrathecal magnesium similar to our study.
Time to regression by two dermatome (min)
In our study, it was prolonged in group C > B > A.
Gupta KL et al. (Lal et al. 2017) have concluded that it was prolonged in the nalbuphine group compared with the bupivacaine group. These findings correlate with our study.
Parveen et al. (Parveen et al. 2015) found that in the nalbuphine group, it is 99.6 ± 9.86 min, and in the bupivacaine group, it is 72.33 ± 9.35 min (P < 0.001).
Khalili et al. (Khalili et al. 2011) have observed that the time for two segment regression was 85.5 ± 15.3 min in the bupivacaine group and 106.5 ± 22 min in the magnesium sulfate group (P = 0.001) similar to our study.
Time to regression by S2 dermatome (min)
In group A, it is 183.33 ± 8.56 min; in group B, it is 197.76 ± 7.95 min; and in group C, it is 236.66 ± 12.09 min (P< 0.001).
Choudhury et al. (Biswadeep et al. 2016) concluded that it is 154.84 ± 8.11 min in the nalbuphine group and 199.44 ± 10.41 min in the magnesium group (P < 0.001).
Patwa et al. (Apeksha et al. 2014) concluded that the duration of the sensory blockade is 153.33 ± 25.33 min in the bupivacaine group and 242.5 ± 22.46 min in the nalbuphine group (P < 0001).
Time to motor regression from Bromage grades 3–0
Khalili et al. (Khalili et al. 2011) and Manjula et al. (Manjula et al. 2017) in their study showed no significant difference in the time to complete motor recovery (P> 0.05).
Patwa et al. (Apeksha et al. 2014) concluded that the duration of motor block was 192.33 ± 23.80 min in the bupivacaine group and 205.33 ± 16.70 min in the nalbuphine group (P > 0.05) like our study.
Time of first rescue analgesia (min)
Gupta KL et al. (Lal et al. 2017) concluded that the duration of postoperative analgesia was 6–8 h in the nalbuphine group and 3–4 h in the bupivacaine group,(P = 0.0001), which correlates with our study.
Parveen et al. (Parveen et al. 2015) observed that the duration of effective analgesia was 420.4 ± 25.30 min in the nalbuphine group and 170.83 ± 27.59 min in the bupivacaine group (P < 0.001), which correlate with our study.
Choudhury et al. (Biswadeep et al. 2016) concluded that the time for 1st rescue analgesia in the nalbuphine group was 257.30 ± 28.50 min, and in the magnesium group, it was 241.06 ± 19.6 min (P < 0.001). In our study, we have longer duration of effective analgesia with the magnesium sulfate group.
Pascual et al. (Pascual-Ramirez et al. 2013) concluded that the “time to first analgesic request” was at least 35 min longer when intrathecal magnesium was included in the intervention (SDM 0.94, 95% CI 0.51 to 1.37, P < 0.001)
-
Total analgesic request in 24 h
Khalili et al. (Khalili et al. 2011) have found decrease in number of total analgesic request in the magnesium group compared with bupivacaine group which correlates with our study.
Adverse effects
Table 3 shows the adverse effects observed in each group.
Shivering was observed in 6.66% in group A and 3.33% in group B.
Pruritus was observed in 3.33% of group A and 6.66% of group B.
Nausea and vomiting were observed in 3.33% of group A, 6.66% of group B, and 3.33% of group C.
No other adverse effects were observed in any group.
Adverse effect profile in each group was related to pharmacological property of study drug.
Culebras et al. (Culebras et al. 2000) had observed dose-dependent incidence of pruritus and nausea vomiting (P< 0.05).
Parveen et al. (Parveen et al. 2015) observed no hemodynamic and respiratory adverse effects in the nalbuphine group.
Our results regarding adverse effects differ with study of Choudhary et al. (Biswadeep et al. 2016) who observed nausea in 4% patients of the\ MgSO4 group and 6% in the nalbuphine group. They also observed vomiting in 2% in the nalbuphine group. Bradycardia was seen in 16% in the MgSO4 group and 22% of the nalbuphine group. Pruritus was not observed in any group (they have defined hypotension as decrease in MAP > 20%).
Regarding PDPH and TNS, our observations correlate with the study of Biswadeep et al.
Conclusions
Preservative-free 1 mg nalbuphine and 100 mg MgSO4 both are good adjuvants to intrathecal hyperbaric bupivacaine. Nalbuphine provides faster sensorimotor onset than MgSO4. MgSO4 provides prolonged Postoperative analgesia.
Availability of data and materials
The data sets used/analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- ASA:
-
American Society of Anaesthesiology
- BP:
-
Blood pressure
- CI:
-
Confidence interval
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CVS:
-
Cardiovascular system
- DBP:
-
Diastolic blood pressure
- Dr.:
-
Doctor
- ECG:
-
Electrocardiogram
- et al.:
-
(et alibi)
- etc:
-
Etcetera
- Hg:
-
Mercury
- HR:
-
Heart rate
- i.m.:
-
Intramuscular
- i.v.:
-
Intravenous
- Inj.:
-
Injection
- IT:
-
Intrathecal
- n/No:
-
Number
- NIBP:
-
Non-invasive blood pressure
- NMDA:
-
N-methyl-d-aspartate
- NS:
-
Normal saline
- OAA:
-
Observer’s assessment of awareness
- RR:
-
Respiratory rate
- RS:
-
Respiratory system
- SAB:
-
Subarachnoid block
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- SDM:
-
Standard deviation of mean
- SPO2 :
-
Partial pressure of oxygen saturation
- STG:
-
Split thickness graft
- TKR:
-
Total knee replacement
- VAS:
-
Visual analog scale
- %:
-
Percentage
References
Apeksha P et al (2014) A comparison of intrathecal nalbuphine hydrochloride with hyperbaric bupivacaine 0.5% and hyperbaric bupivacaine 0.5% alone in patients undergoing abdominal hysterectomy. Int J Res Med 3(2):7–11
Arora B, Pathak DG et al (2015) Comparison of intrathecal magnesium and fentanyl as adjuvants to hyperbaric bupivacaine in preeclamptic parturients undergoing elective cesarean sections. J Obstet Anaesth Crit Care 5:9–15
Biswadeep C et al (2016) Effect of intrathecal nalbuphine and magnesium sulphate used as adjuvants with bupivacaine in spinal anesthesia for lower abdominal surgery: a comparison. J Evol Med Dent Sci 68(5):4922–4926
Chernik DA, Gilling D (1990) Validity and reliability of the observers assessment of alertness/sedation score. J Clin Pharmacol 10:244–257
Culebras X et al (2000) Advantages of intrathecal nalbuphine, compared with intrathecal morphine, after cesarean delivery: an evaluation of postoperative analgesia and adverse effects. Anesth Analg 91(3):601–605
Gupta K et al (2016) Intrathecal nalbuphine versus intrathecal fentanyl as adjuvant to 0.5% hyperbaric bupivacaine for orthopedic surgery of lower limbs under subarachnoid block: a comparative evaluation. Indian J Pain 30:90–95
Khalili G et al (2011) Effects of adjunct intrathecal magnesium sulphate to bupivacaine for spinal anesthesia: a randomized, double-blind trial in patients undergoing lower extremity surgery. J Anesth 25(6):892–897
Lal GK et al (2017) Efficiency of nalbuphine as an adjuvant to bupivacaine in lower limb orthopaedic surgery- a prospective study. Int J Res Med Sci 5:623–626
Manjula R et al (2017) Comparative study of bupivacaine with nalbuphine and bupivacaine alone for post-operative analgesia in subarachniod block for lower limb surgeries. J Anest Inten Care Med 2(2):555581
Parveen S et al (2015) Evaluation of the effect of intrathecal nalbuphine as an adjuvant to spinal bupivacaine for post-operative analgesia in patients undergoing abdominal hysterectomy: a randomized, double-blinded control trial. Int J Sci Stud 3(8):141–146
Pascual-Ramirez J et al (2013) Intrathecal magnesium as analgesic adjuvant for spinal anesthesia: a meta-analysis of randomized trials. Minerva Anestesiol 79(6):667–678
Rawal N, Nuutinen L et al (1991) Behavioral and histopathological effects following intrathecal administration of butorphanol, sufentanil, and nalbuphine in sheep. Anesthesiology 75(6):1025–1034
Revill SI, Robinson JO, Rosen M, Hogg MIJ (1976) The reliability of a linear analogue for evaluating pain. Anaesthesia 31:1191–1198
Verma D et al (2013) Postoperative analgesic efficacy of intrathecal tramadol versus nalbuphine added to bupivacaine in spinal anesthesia for lower limb orthopaedic surgery. J Evol Med Dent Sci 2:6196–6206
Wrench IJ, Singh P (1997) The minimum effective doses of pethidine and doxapram in the treatment of post-anaesthetic shivering. Anaesthesia. 52(1):32–36
Pandya CJ (2014) Effect of adding magnesium sulphate as an adjuvant to bupivacaine in spinal anesthesia for lower abdominal surgery. Indian J Appl Basic Med Sci (2014):698–701
R.K. (n.d.) Stoelting, 5th ed. Chapter- 7 page no. 243–245.
Randomisation: (n.d.) Available from//www.Randomisation.com.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SD analyzed, observed, and interpreted the patient data, and MK and DS performed the spinal anesthesia in the patients and were major contributors in writing the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The above study was presented in front of the NHL institutional review board (NHLIRB) on the 14 September 2017 and was approved (approval letter received on 23 October 2017). The approval letter can be provided if needed. There is no reference number mentioned in the approval letter.
Written and informed consent was taken from all the patients involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kapdi, M.S., Shah, D. & Desai, S. Comparison of nalbuphine versus magnesium sulfate as an adjuvant to intrathecal hyperbaric bupivacaine (0.5%) in infraumbilical surgeries. Ain-Shams J Anesthesiol 14, 52 (2022). https://doi.org/10.1186/s42077-022-00250-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-022-00250-1