Abstract
Background
Interaction with medical providers is a stressful experience for a child. The current study aimed to assess the efficacy and safety of intranasal midazolam alone versus midazolam/ketamine combination for preoperative sedation prior to ophthalmic procedures in preschool children. This randomized, controlled trial included male and female children (3 to 7 years old) who were American Society of Anesthesiologists (ASA) physical status I or II, with either disability or special needs (such as autism or Down syndrome) or were undergoing multiple operative procedures. Participants were given either intranasal midazolam (0.5 mg/kg) or a combination of intranasal midazolam (0.25 mg/kg) and ketamine (1 mg/kg). Primary outcome measures were the preoperative level of sedation, agitation, and easiness of separation. Secondary outcomes included oxygen saturation and pulse rate. Any adverse effects, such as nausea and vomiting were reported.
Results
The mean rank of the Six-point Pediatric Sedation Scale was significantly (p = 0.001) higher in the midazolam/ketamine group compared to the midazolam group (28.15 vs 18.85, respectively). The median pulse rate was significantly (p < 0.001) lower in the midazolam group than the combination group at 5, 10, 15, and 20 min after induction of anesthesia.
Conclusions
These findings indicate that intranasal ketamine and midazolam combination produced better sedation than intranasal midazolam alone in preschool children prior to ophthalmic procedures. Moreover, ketamine and midazolam combination was safer with less incidence of bradycardia.
Similar content being viewed by others
Background
Interactions with medical providers are a stressful experience for children (Lerwick, 2013). Preoperative anxiety and uncooperativeness experienced by pediatric patients are commonly associated with postoperative behavioral problems (Chokshi et al., 2013). Because of this stress and anxiety, even the minor procedures require preoperative sedation, especially when non-pharmacological behavioral guidance is unsuccessful (Gomes et al., 2017).
The appropriate method and medication chosen for sedation depends on the clinical situation. Oral or rectal medications may be adequate for sedation; however, these delivery routes require a considerable amount of time to produce effect, leading to delays in care and interrupted patient flow. Intramuscular injections have similar problems in terms of delay in effect besides the major problem being painful and frightening to the patient. Intravenous therapy is the gold standard for sedation allowing rapid onset and titrable effect, but establishing an intravenous line is painful and frightening for many patients, time-consuming, and might increase the risk of respiratory depression (Pansini et al., 2021).
Intranasal and oral transmucosal routes offer an alternative. There are several benefits to using the intranasal route including high vascularization of the nasal mucosa, wide absorption area, avoidance of first pass metabolism, avoidance of intravenous placement, high patient tolerance of drug administration, and quick onset of action (Fantacci et al., 2018).
Ketamine and midazolam are two of the most commonly used medications for intranasal sedation. Midazolam is a useful drug in pediatrics for situations where anxiolysis and amnesia are needed. It is used intranasally in doses ranging from 0.2 mg/kg to 0.5 mg/kg. It has been shown to have rapid onset of action and adequate sedation. One drawback is that it is irritating by the intranasal route more than the other routes. It is a great choice when anxiolysis is needed but analgesia is not the main focus (Feng et al., 2017; Lane & Schunk, 2008).
Intranasal ketamine is used as a sedative analgesic and a premedication agent. It can achieve an adequate level of sedation when administered intranasally in doses ranging from 0.5 mg/kg to 5 mg/kg for anesthetic pre-induction. Its most common side effect is vomiting with no serious adverse events (Peltoniemi et al., 2016).
The combination of both drugs may add more benefits and counteract some side effects. It is supposed to potentiate the sedative effects of both drugs and add more synergetic value with less side effects (Sado-Filho et al., 2019). For example, while midazolam alone can cause some respiratory depression, ketamine is capable of maintaining the child’s airway. Midazolam can cause some bradycardia, which can be overcome by the tachycardia that ketamine causes (Liu et al., 2019). Ketamine can cause some agitation or hallucinations postoperatively while midazolam causes more sedation. In addition, ketamine has an analgesic effect while midazolam can cause amnesia (Peltoniemi et al., 2016).
The current study aimed to assess the efficacy and safety of intranasal midazolam alone versus midazolam/ketamine combination for preoperative sedation prior to ophthalmic procedures in preschool children.
Methods
Ethical considerations
This study was approved by our Institutional Review Board and was conducted in accordance with the principles of the Declaration of Helsinki. We obtained informed written consents from the parents or guardians of the patients, and we were responsible for maintaining the confidentiality of the data.
We intend to share the individual de-identified participants’ data. Data will be accessible through direct contact with the corresponding author, beginning 12 months and ending 24 months following article publication.
Study design, settings, and date
This parallel-group (1:1 allocation ratio), randomized, controlled clinical trial was conducted at the Research Institute of Ophthalmology, Egypt between January 15, 2021 and February 8, 2021.
Sample size calculation
Sample size was calculated using G*power 3.1.9.2 software. The alpha error level was set at 0.05, the power at 0.80, and the allocation ratio at 1:1. The effect size was calculated based on the difference of the means of sedation and separation scores between the two study groups. Intranasal midazolam was reported by Chokshi et al. (2013) to produce a mean (standard deviation) separation score of 1.8 (0.5) at 10 min after sedation. As for the sedation score at 10 min, Wasfy et al. (2020) reported a median score of 3 (interquartile range 3–4) in 20 patients. We postulated that combined midazolam and ketamine may result in lowering sedation and separation scores by 25% than those reported by midazolam alone. This hypothesis resulted in effect sizes of 1.038 and 0.90 for the sedation and separation scores, respectively. The sample size based on the sedation score is 16 subjects per group, while based on the separation score, it is 21 subjects per group. As both scores are primary outcomes for the present study, we adopted the higher sample size. We then added 10% to account for loss to follow-up, thus the final sample size is 23 subjects per group (a total of 46 subjects).
Eligibility criteria
We recruited male and female preschool children, aged from 3 to 7 years, who were American Society of Anesthesiologists (ASA) physical status I or II, with either disability or special needs (such as autism or Down syndrome) or were undergoing multiple operative procedures.
Exclusion criteria included anticipation of difficult airways (e.g., facial deformity or cervical spine injury), increased risk of aspiration due to anatomical abnormalities (e.g., cleft palate), neuromuscular diseases, or cerebral palsy, central or obstructive sleep apnea, and previous allergy to one or more of the used drugs.
Interventions
Patients were randomized using the sequentially numbered envelope method to either midazolam or midazolam/ketamine combination in a ratio of 1:1.
The first group was given midazolam intranasally in a dose of 0.5 mg/kg, and the second group was given a combination of intranasal midazolam (0.25 mg/kg) and ketamine (1 mg/kg). The drugs were administered 15 min prior to the induction of anesthesia.
All patients were subjected to history taking including sociodemographics, medical illnesses, and prior surgery or anesthetic experiences; physical examination including assessment of vital date (heart rate, blood pressure, respiratory rate, temperature, and oxygen saturation), level of consciousness, and respiratory examination; and routine laboratory investigations.
Outcome measures
The primary outcomes were the preoperative level of sedation (using the 6-point Pediatric Sedation State Scale 10 min after sedation was given), postoperative agitation (using the Emergence Agitation Scale at the time of patient recovery), and easiness of separation (using the Separation and Induction Score at the time of separation). The secondary outcomes included intraoperative oxygen saturation and pulse rate (assessed every 5 min intraoperatively) as well as postoperative nausea and vomiting (assessed 30 min after recovery).
Statistical analysis
Data analysis was carried out using the SPSS version 22. All numerical variables were checked for normality by Shapiro-Wilk test. Numerical variables were not normally distributed and were presented as the median and interquartile range (25th–75th percentile), and differences between the two groups were tested using the Mann-Whitney U test. Paired data of oxygen saturation and the pulse rate were compared in each group by Freidman test, followed by pairwise comparison using Wilcoxon-signed rank test. Categorical variables were summarized as frequencies and percentages, and association between variables was tested using X2 tests (Pearson’s chi-square for independence or Fisher’s exact tests as appropriate). A p value of < 0.05 was considered statistically significant.
Results
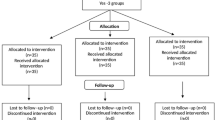
Fifty-six children undergoing multiple operative procedures were assessed and 46 met eligibility criteria and were randomly allocated to receive either midazolam (N = 23) or midazolam/ketamine combination (N = 23) between January and February 2021 (Fig. 1).
The two groups were comparable regarding their age, gender, and ASA with no significant (p > 0.05) differences (Table 1).
We found a significant (p = 0.001) difference between both groups regarding the six-point Pediatric Sedation Scale. The mean rank of the sedation scale was higher in the midazolam/ketamine group than the midazolam group (28.15 vs. 18.85, respectively). The Emergence Agitation Scale and the Separation and Induction Score showed no significant (p > 0.05) differences between the two groups as shown in Table 2.
The median pulse rate was significantly lower in the midazolam group than the midazolam/ketamine group at 5, 10, 15, and 20 min after induction of anesthesia (p < 0.001). Pairwise comparison with each group revealed a significant time-dependent decrease in the pulse rate in comparison with the baseline pulse rate (indicated by b) in the midazolam group. The midazolam/ketamine group showed a significant decrease in the median pulse rate at 20 min only (98.0) compared to the baseline reading (100.0).
We found no significant differences between the studied groups regarding oxygen saturation at all the studied time points (p > 0.05). The median oxygen saturation showed a significant decrease at 15 min after induction of anesthesia compare to the baseline reading in the midazolam group (indicated by a) (Table 3). Furthermore, we recorded no postoperative nausea and vomiting 30 min after recovery in both groups.
Discussion
Children who fail to cooperate before induction of general anesthesia are vulnerable to develop postoperative psychological trauma. Behavioral and medications interventions are commonly used before induction of anesthesia to decrease preoperative nervousness and anxiety in children (Fronk & Billick, 2020).
The ideal premedication should have a rapid onset of action, short duration, readily accepted route of administration by children, minimal side effects, besides analgesic properties, and regulation of autonomic responses. To date, there remains no widely-accepted premedication drug (Mohite et al., 2019).
The present study aimed to assess the efficacy and safety of intranasal midazolam alone versus midazolam/ketamine combination for preoperative sedation prior to ophthalmic procedures in preschool children. Participants received either intranasal midazolam alone or intranasal midazolam/ketamine combination. Both groups were comparable regarding their demographic data as well as the ASA status.
Midazolam and ketamine possess good criteria for premedication such as rapid onset, good anxiolysis, sedation, and rapid recovery (García-Velasco et al., 1998). Theoretically, a combination of midazolam and ketamine have complementary pharmacological characteristics and can produce acceptable sedative and analgesic effects, and less adverse effects (Chudnofsky et al., 2000).
The current study revealed that intranasal midazolam/ketamine combination produced better preoperative sedation than intranasal midazolam alone in preschool children who underwent ophthalmic procedures. Moreover, midazolam/ketamine combination was safe with less incidence of bradycardia compared to midazolam. These findings coincide with Khatavkar & Bakhshi (2014) who compared the effects of intranasal midazolam (0.2 mg/kg) versus intranasal midazolam with ketamine (0.15 mg/kg and 1 mg/kg, respectively) for premedication of children aged 1–12 years who underwent intermediate and major surgeries. They reported significantly better sedation score, anxiolysis, attitude, reaction to intravenous cannulation, and face mask acceptance in the midazolam/ketamine group. However, they did not report on postoperative agitation, which is an important adverse effect arising from the use of inhalational anesthesia such as sevoflurane. The current study found lower postoperative agitation in the midazolam/ketamine group compared to the midazolam group, but the difference between the two groups was nonsignificant. Another study investigated a combination of intranasally administered racemic ketamine (5 mg/kg) and midazolam (0.2 mg/kg) and did not report significant cardiovascular or respiratory side effects (Audenaert et al., 1995).
Furthermore, Weber et al. (2003)) randomly allocated 90 children to receive intranasally administered s-ketamine (1 mg/kg) and midazolam (0.2 mg/kg), s-ketamine (2 mg/kg) and midazolam (0.2 mg/kg), or midazolam (0.2 mg/kg) as premedications. They reported that sedation and anxiolysis occurred from 2.5 min after premedication until induction of anesthesia in intranasal s-ketamine and midazolam combination groups compared to 5 min in the midazolam group. Also, they reported a similar acceptable range of adverse effects for the three groups.
An earlier study reported a significantly higher success rate for intranasal ketamine than intranasal midazolam to produce moderate sedation for pediatric dental care (Bahetwar et al., 2011). However, other studies distinguished comparable levels of sedation in both intranasal midazolam and ketamine groups after premedication in children scheduled for elective surgery (García-Velasco et al., 1998; Narendra et al., 2015).
The findings of the present study are also in line with Aly (2020) who concluded that a combination of intranasal ketamine, in a dose of 5 mg/kg, added to dexmedetomidine was associated with significantly more satisfactory venous cannulation conditions and faster onset of sedation than using intranasal dexmedetomidine alone in pediatric patients undergoing cardiac catheterization. As well, intranasal premedication with a combination of dexmedetomidine and 2 mg/kg dose of ketamine produced better sedation than dexmedetomidine alone for children 3 to 7 years who underwent tonsillectomy (Qian et al., 2020).
A systematic review of intranasal ketamine for procedural sedation and analgesia in children aged up to 14 years reported that intranasal ketamine administration is well tolerated and without serious adverse effects. Additionally, intranasal ketamine produced superior sedation to different comparators in four of the seven studies reporting its use (Poonai et al., 2017).
A similar effectiveness/safety profile of intranasal in comparison with the intramuscular midazolam/ketamine combination has been elucidated in children aged 4 to 12 years and having mental disability (Wasfy et al., 2020).
Conclusions
These findings indicate that intranasal ketamine and midazolam combination produced better sedation than intranasal midazolam alone in preschool children prior to ophthalmic procedures. Moreover, ketamine and midazolam combination were associated with lower incidence of bradycardia.
Availability of data and materials
We intend to share the study protocol as well as the individual de-identified participants’ data. Data will be accessible through direct contact with the corresponding author, beginning 6 months and ending 24 months following article publication.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- IQR:
-
Interquartile range
References
Aly AA (2020) A comparison of intranasal ketamine, intranasal dexmedetomidine, and their combination as premedication in pediatric patients undergoing cardiac catheterization. Res Opin Anesth Intensive Care 7:232
Audenaert SM, Wagner Y, Montgomery CL, Lock RL, Colclough G, Kuhn RJ et al (1995) Cardiorespiratory effects of premedication for children. Anesth Analg 80:506–510
Bahetwar SK, Pandey RK, Saksena AK, Chandra G (2011) A comparative evaluation of intranasal midazolam, ketamine and their combination for sedation of young uncooperative pediatric dental patients: a triple blind randomized crossover trial. J Clin Pediatr Dent 35:415–420
Chokshi AA, Patel VR, Chauhan PR, Patel DJ, Chadha IA, Ramani MN (2013) Evaluation of intranasal midazolam spray as a sedative in pediatric patients for radiological imaging procedures. Anesth Essays Res 7:189–193
Chudnofsky CR, Weber JE, Stoyanoff PJ, Colone PD, Wilkerson MD, Hallinen DL et al (2000) A combination of midazolam and ketamine for procedural sedation and analgesia in adult emergency department patients. Acad Emerg Med 7:228–235
Fantacci C, Fabrizio GC, Ferrara P, Franceschi F, Chiaretti A (2018) Intranasal drug administration for procedural sedation in children admitted to pediatric Emergency Room. Eur Rev Med Pharmacol Sci 22:217–222
Feng JF, Wang XX, Lu YY, Pang DG, Peng W, Mo JL (2017) Effects of dexmedetomidine versus midazolam for premedication in paediatric anaesthesia with sevoflurane: A meta-analysis. J Int Med Res 45:912–923
Fronk E, Billick SB (2020) Pre-operative anxiety in pediatric surgery patients: multiple case study analysis with literature review. Psychiatr Q 91:1439–1451
García-Velasco P, Román J, Beltrán de Heredia B, Metje T, Villalonga A, Vilaplana J (1998) Nasal ketamine compared with nasal midazolam in premedication in pediatrics. Rev Esp Anestesiol Reanim 45:122–125
Gomes HS, Miranda AR, Viana KA, Batista AC, Costa PS, Daher A et al (2017) Intranasal sedation using ketamine and midazolam for pediatric dental treatment (NASO): study protocol for a randomized controlled trial. Trials 18:172
Khatavkar SS, Bakhshi RG (2014) Comparison of nasal Midazolam with Ketamine versus nasal Midazolam as a premedication in children. Saudi J Anaesth 8:17–21
Lane RD, Schunk JE (2008) Atomized intranasal midazolam use for minor procedures in the pediatric emergency department. Pediatr Emerg Care 24:300–303
Lerwick JL (2013) Psychosocial implications of pediatric surgical hospitalization. Semin Pediatr Surg 22:129–133
Liu J, Du M, Liu L, Cao F, Xu Y (2019) Sedation effects of intranasal dexmedetomidine combined with ketamine and risk factors for sedation failure in young children during transthoracic echocardiography. Paediatr Anaesth 29:77–84
Mohite V, Baliga S, Thosar N, Rathi N (2019) Role of dexmedetomidine in pediatric dental sedation. J Dent Anesth Pain Med 19:83–90
Narendra PL, Naphade RW, Nallamilli S, Mohd S (2015) A comparison of intranasal ketamine and intranasal midazolam for pediatric premedication. Anesth Essays Res 9:213–218
Pansini V, Curatola A, Gatto A, Lazzareschi I, Ruggiero A, Chiaretti A (2021) Intranasal drugs for analgesia and sedation in children admitted to pediatric emergency department: a narrative review. Ann Transl Med 9:189
Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI (2016) Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy. Clin Pharmacokinet 55:1059–1077
Poonai N, Canton K, Ali S, Hendrikx S, Shah A, Miller M et al (2017) Intranasal ketamine for procedural sedation and analgesia in children: A systematic review. PloS One 12:e0173253
Qian B, Zheng W, Shi J, Chen Z, Guo Y, Yao Y (2020) Ketamine enhances intranasal dexmedetomidine-induced sedation in children: a randomized, double-blind trial. Drug Des Dev Ther 14:3559–3565
Sado-Filho J, Viana KA, Corrêa-Faria P, Costa LR, Costa PS (2019) Randomized clinical trial on the efficacy of intranasal or oral ketamine-midazolam combinations compared to oral midazolam for outpatient pediatric sedation. PLoS One 14:e0213074
Wasfy SF, Hassan RM, Hashim RM (2020) Effectiveness and safety of Ketamine and Midazolam mixture for procedural sedation in children with mental disabilities: A randomized study of intranasal versus intramuscular route. Egypt J Anaesth 36:16–23
Weber F, Wulf H, el Saeidi G (2003) Premedication with nasal s-ketamine and midazolam provides good conditions for induction of anesthesia in preschool children. Can J Anaesth 50:470–475
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
NO, SM, AS, and DT have full access to all the data in the study and take responsibility for the integrity of the data. Study concept and design: NO and SM; acquisition of data: NO, AS, and DT; analysis of data and critical revision of the manuscript: AS, SM, and DT. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study obtained approval from the Research Institute of Ophthalmology (date of approval: 8-11-2020). The study was registered at the Iranian Registry of Clinical Trials (IRCT20201220049777N1; December 31, 2020; https://www.irct.ir/trial/53129). Informed written consents were obtained from the parents or guardians of the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osama, N.A., Mahmoud, S.R., Salem, A.S. et al. Intranasal midazolam alone versus midazolam/ketamine combination for preoperative sedation in pediatric patients undergoing ophthalmic procedures: a randomized controlled trial. Ain-Shams J Anesthesiol 14, 12 (2022). https://doi.org/10.1186/s42077-022-00212-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-022-00212-7