Abstract
Background
Atelectasis can result during general anesthesia from mechanical ventilation and decrease in lung volume particularly in morbidly obese patients undergoing abdominal laparoscopic sleeve surgery, which may result in the development of postoperative pulmonary complications (PPCs), including hypoxemia and pneumonia, with an increased risk of postoperative morbidity and mortality.
Objective
To compare between volume-controlled ventilation (VCV) and pressure-controlled ventilation (PCV) in prevention of postoperative pulmonary atelectasis in morbidly obese patients undergoing laparoscopic gastric sleeve surgery.
Methods
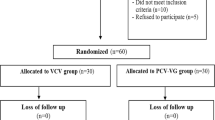
This is a randomized prospective comparative clinical study with a total of 52 morbidly obese patients who were randomly divided into 2 groups; 26 patients in group (V) for VCV and 26 in group (P) for PCV arterial blood samples were obtained, and PaCO2, PaO2, and SaO2 were obtained and recorded. ABG analyses were also obtained 30 min, 12 h, and 24 h post-extubation, and PaCO2, PaO2, and SaO2 were obtained and recorded.
Results
The results of this study revealed no significant differences between PCV and VCV as regards the incidence of postoperative lung atelectasis immediately postoperative (69.2% with VCV vs 61.5% with PCV, p = 0.368), 12 h postoperative (61.5% with VCV vs 53.8% with PCV, p = 0.282), and 24 h postoperative (53.8% with VCV vs 46.2% with PCV, p = 0.325). There were also no significant differences between VCV and PCV as regards baseline, intra-, and post-operative PaCO2 levels as well as baseline and postoperative SaO2 and PaO2 values. However, PCV showed better intraoperative oxygenation compared to VCV. SaO2 was 96.32% ± 1.85 and 97.25% ± 1.37 in VCV and PCV groups respectively (p = 0.027) while PaO2 was 212.75 mmHg ± 20.13 and 225.8 mmHg ± 18.69 in VCV and PCV groups respectively (p = 0.011).
Conclusion
Despite a slight improvement in intraoperative oxygenation parameters (PO2, SaO2) with PCV than VCV, there is no significant difference between VCV and PCV in the prevention of postoperative pulmonary atelectasis; moreover, there is no difference in postoperative oxygenation parameters in morbidly obese patients who undergo laparoscopic sleeve surgery.
Similar content being viewed by others
Introduction
Obesity is a worldwide serious problem. Obese patients are more likely than non-obese patients to develop atelectasis, which resolves more slowly. This may be attributed to marked impairment of the respiratory mechanics (decreased chest wall and lung compliance and decreased functional residual capacity) promoting airway closure with reduction of the oxygenation index (PaO2/PAO2) to a greater extent than in healthy weight subjects. For these reasons, avoiding atelectasis formation in obese patients remains challenging (Eldemrdash et al. 2017).
Volume-controlled ventilation (VCV) has been widely used for general anesthesia and has the merit of a guaranteed preset tidal volume. However, it presents the risk of increased airway pressure, when pulmonary compliances change. On the contrary, pressure-controlled ventilation (PCV) has less risk of barotrauma because peak airway pressure is limited, but it cannot ensure tidal volume. During pneumoperitoneum, PCV might be advocated because of a significant increase in airway pressure after CO2 insufflation (Choi et al. 2011).
The aim of this study was to compare volume-controlled ventilation and pressure-controlled ventilation in the prevention of post-operative atelectasis (detected by lung ultrasonography and ABG) in morbidly obese patients undergoing laparoscopic gastric sleeve operation.
Patients and methods
After institutional ethical approval, this randomized prospective controlled clinical study was carried out in Ain Shams University Hospitals during the period from March 2018 to March 2019 on 52 morbidly obese patients from both genders aged 21 years or older who were scheduled for laparoscopic gastric sleeve under general anesthesia with body mass index (BMI) of 45–60 kg/m2. A written informed consent was taken from each patient.
In addition to refusal to participate in the study, exclusion criteria included patients with severe cardiopulmonary co-morbidities or any clinical sign of cardiopulmonary disease during preoperative physical examination (such as jugular vein distension, gallop rhythm, hepatomegaly, tibial edema, or rales on auscultation of the chest, or any abnormalities in the preoperative 12-lead electrocardiogram or chest radiograph). Patients below 21 years of age were also excluded from the study.
Sample size justification
MedCalc® version 12.3.0.0 program “Ostend, Belgium” was used for calculations of sample size, statistical calculator based on 95% confidence interval, and power of the study 80% with α error 5%. According to a previous study, Movassagi et al. (2017) showed that the PaO2 at 55 min in group VCV (194.67 ± 9.42) significantly decreased, and the mean of group PCV (207.26 ± 9.97) with p value < 0.001 was highly significant with effect size of 1.298. So it can be relied upon in this study, based on this assumption, that sample size was calculated according to these values and a minimal sample size of 50 cases was enough to find such a difference. Assuming a drop-out ratio of 5%, the sample size will be 26 cases in each group (52 total).
Anesthetic management
All patients were kept fasting 8 h preoperative; in the pre-induction room, a wide bore IV cannula G18 was inserted and monitors were attached “pulse oximetry, electrocardiogram, and non-invasive arterial blood pressure.” All patients were premedicated by giving 1–2 mg IV midazolam. Preoperative lung ultrasonography and ABG were done as basal reference.
General anesthesia was induced as Ain Shams University Hospitals OR protocol with injection of fentanyl 1 μg kg − 1 i.v. followed by Na Thiopental 5 mg kg − 1 i.v. Atracurium 0.5 mg kg − 1 i.v. was used to facilitate tracheal intubation. Anesthesia was maintained with oxygen and isoflurane (minimal alveolar concentration 1–1.3); all doses were calculated according to lean body weight.
Recovery was carried out after closure wound by turning off isoflurane vaporizer and increasing FiO2 to 1.0. When respiratory attempts start, neostigmine (0.05 mg/kg) and atropine (0.01 mg/kg) were given to reverse residual neuromuscular block. This was followed by fully awake extubation (fully conscious, vitally stable, and good muscle power), and the patient was transferred to the ICU pain-free with oxygen supply, and monitored postoperative analgesia during the first 24 h postoperative was maintained by IV ketorolac amp 30 mg every 6 h.
Randomization
Patients were randomized based on closed envelope method into two groups based on the mode used for intra-operative ventilation.
Volume-controlled ventilation (group V)
The patient’s lungs were ventilated in constant-flow VCV mod; tidal volume (VT) was set at 8 mL/kg, inspiratory/expiratory (I/E) ratio 1:2, and inspired oxygen concentration (FIO2) 0.6. Positive end-expiratory pressure (PEEP) was 5 cmH2O. Respiratory rate (RR) was adjusted to maintain an end-tidal CO2 pressure (PETCO2) of 38 ± 2 mmHg.
Pressure-controlled ventilation (group P)
Pressure was adjusted to achieve VT of 8 mL/kg, I/E ratio1:2, and inspired oxygen concentration (FIO2) 0.6. PEEP was 5 cmH2O. RR was adjusted to maintain a PETCO2 of 38 ± 2 mmHg.
In both groups, arterial blood gases (ABG) were obtained every hour intraoperatively to measure PaCO2, PaO2, and SaO2 then 30 min,12 h, and 24 h after extubation.
Lung ultrasound using (MYsonou 6) curved probe was performed to each group of patients immediately postoperative then 12 and 24 h post-extubation in supine position placing the probe on lateral and inferior chest wall and longitudinally. According to the systematic protocol for LUS examination (Bouhemad et al. 2015), each hemithorax was divided into anterior, lateral, and posterior regions using anterior and posterior axillary lines as anatomic landmarks, and each region was further divided into two parts superior and inferior. Using a curved probe, detecting lung pulse, absence of A lines, and presence of B lines was used to detect atelectasis (Bouhemad et al. 2015).
The primary study outcome was postoperative pulmonary atelectasis detected by lung ultrasonography and ABG. Comparisons were also done between both groups as regards demographic data, duration of surgery, PaO2, SaO2, and PaCO2 (mean intraoperative values then 30 min, 12 h, and 24 h postoperative).
Statistical analysis
Recorded data were analyzed using the Statistical Package for Social Sciences, version 20.0 (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as mean ± standard deviation (SD). Qualitative data were expressed as frequency and percentage. Independent samples t test of significance was used when comparing between two means. Chi-square (χ2) test of significance was used in order to compare proportions between qualitative parameters. The confidence interval was set to 95%, and the margin of error accepted was set to 5%. p value < 0.05 was considered significant while p value < 0.001 was considered as highly significant.
Discussion
The results of this study revealed no difference between PCV and VCV as regards postoperative oxygenation or the incidence of post-operative lung atelectasis. However, PCV showed better intraoperative oxygenation (PaO2 and SaO2) compared to VCV.
Similar to the results of the current study, Movassagi and co-workers in 2017 carried a study on 70 obese patients undergoing laparoscopic cholecystectomy. Their results showed that PCV resulted in higher intraoperative PaO2 levels compared to VCV without significant differences in other post-operative complications including atelectasis (Movassagi et al. 2017).
Similarly, another study carried by Hans and colleagues in 2008 on forty morbidly obese patients undergoing laparoscopic gastric bypass surgery found no noteworthy differences between VCV and PCV regarding both postoperative complications (including atelectasis) and intraoperative oxygenation (Hans et al. 2008).
The results of the study carried by Gupta and colleagues in 2012 to assess the effects of PCV and VCV on intraoperative oxygenation in obese patients undergoing laparoscopic cholecystectomy matched the results of the current study. There were significantly higher intraoperative PO2 levels and better oxygenation in patients who received PCV compared to those who received VCV and no noteworthy differences regarding postoperative complications including atelectasis (Gupta et al. 2012).
Moreover, in the study carried by Kothari and colleagues in 2018 on 75 patients undergoing laparoscopic cholecystectomy, significantly higher intraoperative PO2 levels were found in patients who received PCV and PCV-VG compared to those who received VCV but no difference in post-operative oxygenation and complication including atelectasis. These results agree with the results of the current study (Kothari and Baskaran 2018).
Cadi and colleagues in 2008 carried a study on thirty-six morbidly obese patients who undergo laparoscopic gastric banding and found a significantly higher intraoperative PO2 levels in the PCV group compared to the VCV group. They stated that PCV generates higher instantaneous flow peaks and may allow better alveolar recruitment leading to improved oxygenation without any side-effects, but no difference in 2 h post-operative oxygenation and complications including atelectasis (Cadi et al. 2008).
Moreover, supporting the results of the current study, Aldenkortt and colleague in 2012 performed a meta-analysis of thirteen studies (505 obese surgical patients) reporting a variety of ventilation strategies: pressure- or volume-controlled ventilation (PCV, VCV), various tidal volumes, and different PEEP or recruitment manoeuvres (RM), and combinations and revealed that the ideal intraoperative ventilation strategy in obese patients remains obscure. There is no evidence of any difference between PCV and VCV as regards oxygenation and post-operative complications including lung atelectasis (Aldenkortt et al. 2012).
Conclusion
Despite slight improvement in intraoperative oxygenation parameters (PO2, SaO2) with PCV than VCV, there is no significant difference between VCV and PCV in prevention of postoperative pulmonary atelectasis; moreover, there is no difference in postoperative oxygenation parameters.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABG:
-
Arterial blood gas
- BMI:
-
Body mass index
- OR:
-
Operative room
- PCV:
-
Pressure-controlled ventilation
- PEEP:
-
Positive end-expiratory pressure
- PPCS:
-
Postoperative pulmonary complication
- VCV:
-
Volume-controlled ventilation
- VT:
-
Tidal volume
References
Aldenkortt M, Lysakowski C, Elia N, Brochard L, Tramèr MR (2012) Ventilation strategies in obese patients undergoing surgery: a quantitative systematic review and meta-analysis. Br J Anaesth 109(4):493
Bouhemad B, Mongodi S, Via G, Rouquette I (2015) Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology 122:437–447
Cadi P, Guenoun T, Journois D, Chevallier JM, DiehlJL SD (2008) Pressure controlled ventilation improves oxygenation during laparoscopic obesity surgery com-pared with volume-controlled ventilation. Br J Anaesth 100(5):709–716
Choi EM, Na S, Choi SH, An J, Rha KH, Oh YJ (2011) Comparison of volume-controlled and pressure-controlled ventilation in steep Trendelenburg position for robot-assisted laparoscopic radical prostatectomy. J Clin Anesth 23:183–188
Eldemrdash AM, Gamaleldeen NM, Ahmed MK, Ahmed SB (2017) A comparative study between protective lung ventilation versus conventional ventilation in obese patients undergoing abdominal laparoscopic surgery. J Anesth Clin Res 8:777
Gupta SD, Kundu SB, Ghose T, Maji S, Mitra K, Mukherjee M, Mandal S, Sarbapalli D, Bhattacharya S, Bhattacharya S (2012) A comparison between volume-controlled ventilation and pressure-controlled ventilation in providing better oxygenation in obese patients undergoing laparoscopic cholecystectomy. Indian J Anaesth 56:276–282
Hans GA, Pregaldien AA, Kaba A et al (2008) Pressure-controlled ventilation does not improve gas exchange in morbidly obese patients undergoing abdominal surgery. Obes Surg 18:71–76
Kothari A, Baskaran D (2018) Pressure-controlled volume guaranteed mode improves respiratory dynamics during laparoscopic cholecystectomy: a comparison with conventional modes. Anesth Essays Res 12:206–212
Movassagi R, Montazer M, Mahmoodpoor A, Fattahi V, Iranpour A, Sanaie S (2017) Comparison of pressure vs. volume controlled ventilation on oxygenation parameters of obese patients undergoing laparoscopic cholecystectomy. Pakistan J Med Sci 33(5):1117–1122
Acknowledgements
Not applicable
Funding
None
Author information
Authors and Affiliations
Contributions
Ramy Mohammed Hassan designed the study, revised literature, followed up the patients, and critically reviewed the manuscript. Hoda omar Mahmoud designed the study, analyze the data, and wrote and critically revised the manuscript. Wael ahmed abd el aal, Mona ahmed mohamed, and Tarek Samir shabana revised literature, followed up the patients, collected the data, performed the analysis, and wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval of the research ethical committee of Faculty of Medicine, Ain-Shams University, was obtained (code number: FMASU M D 98/2018), and written informed consent was obtained from patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, R.M., Mahmoud, H.O., Abd el Aal, W.A. et al. Comparative study between volume-controlled ventilation and pressure-controlled ventilation in prevention of postoperative pulmonary atelectasis in morbidly obese patients undergoing laparoscopic gastric sleeve surgery. Ain-Shams J Anesthesiol 12, 38 (2020). https://doi.org/10.1186/s42077-020-00080-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-020-00080-z