Abstract
Low grade endometrial stromal sarcoma (LGESS) typically presents as a solid intracavitary or intramural uterine mass. On imaging, LGESS is usually seen as an endometrial or myometrial solid mass. Cystic change is unusal and may lead to a differential diagnosis of an ovarian mass as was seen in the present case. Here we present a case of a 38 year old woman who presented with a cystic ovarian mass clinically and radiologically. Per-operatively, it was found to be a multiloculated cystic mass, filled with serosanguinous fluid, in the uterine fundus. Histopathological examination showed a tumor mass composed of mainly spindle cells arranged in fascicular pattern showing marked myxoid degeneration and cystic areas. On immunohistochemistry(IHC), tumor cells showed positivity for CD 10, ER, PR, SMA and desmin while HMB45 was negative. Based on histopathological and IHC, a diagnosis of LGESS was made. Most cases of cystic uterine masses have a benign course but, LGESS exhibits a relatively poorer outcome and a risk of metastasis. Hence, we present this case for its unusual presentation which mimics an ovarian mass but has worse prognosis.
Similar content being viewed by others
Background
Low grade endometrial stromal sarcoma (LGESS) constitutes less than 1% of all uterine malignancies (Lee et al. 2019). This rare form of cancer typically manifests as abnormal uterine bleeding or abdominal discomfort. Upon gross examination, LGESS may appear as an intracavitary polypoid or an intramural solid mass. These masses often exhibit indistinct borders and infiltrate the myometrium (Lee et al. 2019).
A seldom reported morphological characteristic of primary LGESS is cystic change (Dionigi et al. 2002; Perez-Montiel et al. 2004). In the case we encountered, the patient presented with a cystic adnexal mass clinically and radiologically, thus, a provisional diagnosis of ovarian tumor was made. However, histopathological examination showed it to be a LGESS of uterus with cystic change – an unusal presentation and an important entity as it closely mimics adnexal cystic mass.
Case presentation
A 38-year-old female presented with complaints of lower abdominal pain of 12 weeks duration. She had six live births and one abortion. Physical examination suggested a firm immobile pelvic mass. On laboratory workup, Carcinoembryonic Antigen was 2.55 ng/mL (normal < 5 ng/mL), Carbohydrate Antigen-19.9 was 4.11 U/mL (normal < 37.0 U/mL), Alpha Fetoprotein was 3.73 ng/mL (normal 0.89–8.78 ng/mL), Cancer Antigen-125 was 28.5 U/mL (normal < 35.0 U/mL).
On ultrasonography and computed tomography(CT) scan (Fig. 1a), uterus and right sided adnexa could not be visualized separately. A multiseptated well-defined cystic mass measuring 142.0 mm X 136.5 mm with anechoic/ hypo-attenuated content was seen in the right adnexa, with normal uterus and cervix. A clinicoradiological opinion of right ovarian/pelvic cyst was given.
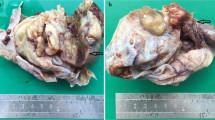
a. CT findings suggested an adnexal mass with multisepate cystic mass with hypoattenuat b. Gross Specimen – cut section of uterus shows a multiloculated solid-cystic mass in the fundus.Histological and IHC findings of multicystic LGESS c. The cyst wall composed of monotonous spindle cells (10X; H&E) d. infiltrative mass composed of spindle cells invading into the myometrium in tongue like fashion (arrow)(4X; H&E) e. spindle cells showing moderate atypia; eosinophilic cytoplasm with low mitotic activity (arrow) (40X; H&E) f. tumor cells showing perivascular whorling (40X; H&E). Tumor cells showing positivity for (40X)g. CD10 h. ER i. SMA
A total abdominal hysterectomy with bilateral salpingo-oopherectomy was done. Differential diagnoses of fibroid with degenerative changes and adenomyosis with cystic degeneration were considered. Grossly, uterus with cervix with bilateral fallopian tubes and ovaries was received measuring 14.5 cm X 10.5 cm X 6.5 cm. The uterine serosal surface was smooth with a large mass in the fundal region measuring 8.2 cm X 8.0 cm X 7.0 cm consisting of multiple fluid filled cystic spaces. The cut surface showed an intramural poorly demarcated multiloculated cystic mass with few solid areas. On cutting the cysts, serosanguinous fluid came out. The cyst wall thickness varied from papery thin to 0.4 cm (Fig. 1b). Areas of hemorrhage were seen in the cystic mass. Rest of the myometrium was unremarkable. Endometrial cavity and cervical canal were normal (Fig. 1b). Grossly, both sided adnexa were unremarkable.
Microscopic examination showed a tumor composed of densely cellular areas admixed with paucicellular areas showing marked myxoid degeneration. Cystic areas were also seen. The cellular areas showed mainly spindle cells arranged in fascicular pattern (Fig. 1c). At places, tongue-like projections of these spindled to ovoid cells were seen invaginating into the surrounding myometrium. These projections extended > 3 mm into the myometrium (Fig. 1d). These tumor cells showed moderate atypia with hyperchromatic to vesicular nuclei, inconspicuous nucleoli and moderate amount of eosinophilic cytoplasm. Mitotic count was 1–2 per mm2 (Fig. 1e). Numerous capillaries with peri-arteriolar whorling of spindle cells were also seen (Fig. 1f). Necrosis was absent. Differential diagnoses of low-grade-endometrial-stromal-sarcoma, multicystic leiomyosarcoma, leiomyoma with cystic degeneration and perivascular-epithelioid-cell-tumor (PEComa) were kept.
Immunohistochemical (IHC) panel to confirm the final diagnosis was done (Table 1) (Folkins et al. 2019, Oliva et al. 2019).
Tumor cells showed positivity for Cluster of Differentiation 10 (CD 10) (Fig. 1g), ER (Fig. 1h), PR, SMA (Fig. 1i) and Desmin while Human Melanoma Black 45 (HMB45) was negative.
Based on histopathological and IHC findings, a final diagnosis of low-grade-endometrial-stromal-sarcoma was made.
On follow-up patient is alive and well with no residual disease.
Discussion and conclusion
LGESS is the second most common malignant mesenchymal tumor of uterus. It occurs over a wide age range with a mean of 52 years, but patients tend to be younger than those with other uterine sarcomas (Lee et al. 2019). Our patient was also of a younger age group. The findings on imaging match with those of epithelial or mesenchymal type of uterine tumors. On imaging, LGESS usually presents as a solid lesion but occasionally shows cystic degeneration (Rha et al. 2003). Radiologically, the present case was interpreted as a cystic ovarian mass which posed a diagnostic dilemma.
Grossly, LGESS usually presents with a yellow to tan fleshy cut surface, occasionally showing hemorrhage and/or necrosis. Size usually ranges from 5 to 10 cm. These may be intracavitary, polypoid or intramural masses, often with ill-defined but sometimes with well-defined margins and overt myometrial infiltration and/or intravascular plugs of tumor inside intramyometrial and parametrial veins (Lee et al. 2019). Our case presented as a predominantly cystic mass with few solid areas. No areas of necrosis were present.
The uterine lesions that may present as cystic masses are endometrial hyperplasia, endometrial polyps, leiomyoma with cystic change, endometrial-stromal-nodule, adenomyoma, adenofibroma, adenosarcoma, carcinosarcoma, adenomatoid tumor, adenomyomatous polyp, leiomyosarcoma, low-grade-endometrial-stromal-sarcoma, high-grade-endometrial-stromal-sarcoma and PEComa. Among these, most entities have a benign course. However, adenocarcinoma, leiomyosarcoma, adenosarcoma, carcinosarcoma and endometrial-stromal-sarcoma present with a poorer outcome with high incidence of metastasis. Hence, accurate histopathological diagnosis is of utmost importance in lesions of uterus presenting as a cystic mass.
The microscopy in our case showed tongue-like infiltrative growth of spindle cells (> 3 mm), along with no areas of necrosis and no foci of adenomyosis. IHC showed positivity for CD10. It was also ER and PR positive. Thus, various hormonal therapies for LGESS have been proposed over time but no consensus has been reached till date and needs further studies (Wang et al. 2018; Borella et al. 2022). Since, endometrial-stromal-sarcoma does not have a very specific IHC panel, a judicious combination of histopathological findings and IHC markers are helpful in making the diagnosis.
First-line treatment of LGESS is total hysterectomy with bilateral salpingo-oopherectomy even in case of recurrence while lymphadenectomy, endocrine therapy and regional radiotherapy are debatable and needs a risk to benefit ratio analysis along with further studies (Thiel et al. 2018).
As already mentioned, cystic change in LGESS is an unusal morphologic finding (Dionigi et al. 2002). Extensive literature search showed only a few cases of this sarcoma presenting as cystic mass, of which most were metastatic lesions at extra-uterine sites (Kim et al. 2009, 2022; Efared et al. 2019; McCarthy et al. 2019; Moral et al. 2022; Yu et al. 2022). Only one case of primary uterine low-grade-endometrial-stromal-sarcoma with cystic change has been reported till date by Perez-Montiel et al. 2004. All other cases of this sarcoma presenting as a cystic mass are metastatic in nature, ovary being the most common site (Kim et al. 2009; Efared et al. 2019; Moral et al. 2022; Yu et al. 2022), other sites being mesentery (Yu et al. 2022), lung (Kim et al. 2022), tail of pancreas (McCarthy et al. 2019) and right atrium (Wood et al. 2011). Our case is a case of primary uterine LGESS presenting as a cystic adnexal mass posing diagnostic dilemma.
Clinicoradiologically, cystic pelvic masses are almost always ovarian in origin but the possibility of a uterine mass presenting as a pelvic cyst should also be kept in mind. A detailed histopathological and immunohistochemical examination is essential for the correct diagnosis of such unusual presentations of low grade endometrial stromal sarcoma. Most cases of cystic uterine masses have a benign course but, LGESS exhibits a relatively poorer outcome and a risk of metastasis. Therefore, a possibility of LGESS should also be kept in the differential diagnoses of cystic uterine tumors to enable proper diagnosis and treatment.
Data availability
The clinical case data used to support the findings of this study are included within the article.
Abbreviations
- LGESS:
-
Low grade endometrial stromal sarcoma
- HGESS:
-
High grade endometrial stromal sarcoma
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- SMA:
-
Smooth muscle actin
- IHC:
-
Immunohistochemistry
References
Borella F, Bertero L, Cassoni P, et al. Low-Grade Uterine endometrial stromal sarcoma: prognostic analysis of Clinico-pathological characteristics, Surgical Management, and adjuvant treatments. Experience from two Referral centers. Front Oncol. 2022;12:883344. https://doi.org/10.3389/fonc.2022.883344. Published 2022 Jun 30.
Dionigi A, Oliva E, Clement PB, Young RH. Endometrial stromal nodules and endometrial stromal tumors with limited infiltration: a clinicopathologic study of 50 cases. Am J Surg Pathol. 2002;26(5):567 – 81. https://doi.org/10.1097/00000478-200205000-00003. PMID: 11979087.
Efared B, Sidibe IS, Hammas FEN, Chbani L, Fatemi HE. Extra-uterine low grade endometrial stromal sarcoma arising from ovarian endometriosis: a case report and review of literature. Gynaecoloncol res Pract. 2019;6:2. https://doi.org/10.1186/s40661-019-0067-7.
Folkins AK, Longacre TA. Immunohistology of the female genital tract. In: Dabbs DJ, editor. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications: Elsevier, Inc, Philadelphia; 2019. 5th Edition: p 662–717. https://doi.org/10.1007/978-3-319-46334-6
Kim JY, Hong SY, Sung HJ, Oh HK, Koh SB. A case of multiple metastatic low grade endometrial stromal sarcoma arising from an ovarian endometriotic lesion. J Gynecol Oncol. 2009;20:122–5. https://doi.org/10.3802/jgo.2009.20.2.122.
Kim GW, Baek SK, Han JJ, Kim HJ, Sung JY, Maeng CH. Pulmonary metastasising low grade endometrial stromal sarcoma: case report and review of diagnostic pitfalls. Diagnostics. 2022;12:271. https://doi.org/10.3390/diagnostics12020271.
Lee CH, Chiang S. Low-grade endometrial stromal sarcoma. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, eds. WHO Classification of Tumors of Female Reproductive Organs. International Agency for Research on Cancer: 5th edition; Lyon; 2019. p 287 – 88.
McCarthy AJ, Clarke BA, McGilvray I, Dickson BC, Khalili K, Chetty R. Metastatic low-grade endometrial stromal sarcoma of uterus presenting as a primary pancreatic tumor: a case presentation and literature review. DiagnPathol. 2019;14:30. 10. 1186/s13000-019-0807-3.
Moral AIB, Jimenez JCV, Banon CM, Huesca MJD, Gonzalez MV, Lopez JSJ. Primary ovarian endometrioid stromal sarcoma presenting with reno-ureteral colic. BMC Women’s Health. 2022;22:491. https://doi.org/10.1186/s12905-022-02046-9.
Oliva E, Zaloudek CJ, Soslow RA. Mesenchymal tumors of the uterus. In: Kurman RJ, Ellenson LH, Ronnett BM, editor. Blaustein’s Pathology of the Female Genital Tract: Springer Nature, Switzerland; 2019. 7th Edition: p 535–647. https://doi.org/10.1007/978-3-319-46334-6_10
Perez-Montiel D, Salmeron AA, Malagon HD. Multicystic endometrial stromal sarcoma. Ann DiagnPathol. 2004;8:213–8. https://doi.org/10.1053/j.anndiagpath.2004.04.004.
Rha SE, Byun JY, Jung SE, Lee SL, Cho SM, Hwang SS, et al. CT and MRI of uterine sarcomas and their mimickers. AJR Am J Roentgenol. 2003;181(5):1369–74.
Thiel FC, Halmen S. Low-Grade Endometrial Stromal Sarcoma - a Review. Oncol Res Treat. 2018;41(11):687–692. https://doi.org/10.1159/000494225. Epub 2018 Oct 13. PMID: 30317238.
Wang F, Lei R, Yang H, Guo M, Tan G. Endometrial stromal sarcoma: a clinicopathological analysis of 14 cases. Int J Clin Exp Pathol. 2018;11:2799–804.
Wood CL, Sederberg IIJ, Russ P, Seres T. Cystic appearance of low- grade endometrial stromal sarcoma in the right atrium: case report. Cardiovasc Ultrasound. 2011;9:23. https://doi.org/10.1186/1476-7120-9-23.
Yu HY, Jin YL. Metastatic low- grade endometrial stromal sarcoma with variable morphologies in the ovaries and mesentery: a case report. World J Clin Cases. 2022;10:8384–91. https://doi.org/10.12998/wjcc.v10.i23.8384.
Acknowledgements
None.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Author SK was responsible for the, acquisition of patient data, and drafting the manuscript. Author AS contributed to the writing of the Introduction and Case.
presentation sections. Author DS contributed to the conception of the case report, writing of the Discussion and critically revised the manuscript for important intellectual content. Author KV and author VM provided clinical expertise, contributed to the interpretation of the IHC results, and critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Consent for publication
Informed consent was obtained from the patient. The participant has consented to the submission of the case report to the journal.
Conflict of interest
There was no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, D., Khan, S., Varma, K. et al. Cystic endometrial stromal sarcoma mimicking a cystic ovarian tumor : an unusual presentation. Surg Exp Pathol 7, 17 (2024). https://doi.org/10.1186/s42047-024-00159-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42047-024-00159-z