Abstract
Background
Coronavirus disease 2019 (COVID-19), which started in Wuhan, China, in late December 2019, was declared a pandemic, infecting more than twelve million people worldwide. Few studies have reported the findings of lung biopsies in COVID-19. Here, granulomatous inflammation was reported for the first time in COVID-19 lung biopsy.
Case presentation
A 54-year-old woman presented to a primary care facility with fever, dry cough, and fatigue. Antibiotherapy was administered for 10 days with the diagnosis of upper respiratory tract infection. However, her condition did not improve and she was admitted to the hospital. In physical examination, crepitant rales were heard in both lungs. Anemia and thrombocytopenia were detected in laboratory tests and she was referred to the hematology clinic. Bone marrow aspiration and flow cytometry showed she had acute myeloid leukemia. Computed tomography-integrated positron emission tomography with a history of previous breast cancer revealed a heterogeneous mass-like lesion in the left lung. The primary malignancy could not be ruled out and tru-cut biopsy was performed. Tests for tuberculosis were negative. Throat swab sample was taken and a real-time polymerase chain reaction confirmed that she had COVID-19. Radiological findings were evaluated as the progression of COVID-19 pneumonia on computed tomography 6 days after biopsy. Alveolar damage, edema, vascular congestion, mild inflammatory infiltration, type-2 pneumocyte hyperplasia, interstitial fibrosis, early fibrotic changes, fibrinous, organized pneumonia pattern, noncaseating granulomatous inflammation, and desquamation in alveolar epithelial cells were noted in lung biopsy.
Conclusions
There were only a few case reports that described lung biopsy findings in COVID-19 at the time of manuscript preparation. This was the first case of noncaseating granulomatous inflammation described in a COVID-19 case.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19), which started in Wuhan, China, in late December 2019 and progressed with severe acute respiratory distress syndrome, has since spread to Asia, Europe, and North America, infecting more than twelve million people worldwide (Tian et al. 2020). COVID-19 patients most frequently present with clinical symptoms such as fever, fatigue, dry throat, cough, and more rarely with sputum, headache, diarrhea (Huang et al. 2020; Wang et al. 2020). The number of confirmed cases with severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) infection, the viral agent leading to COVID-19, has reached 12.964,809 worldwide, including 570,288 deaths as of July 14, 2020 (new reference website of world health organization). Deceased patients are often older patients with an underlying disease. Several studies communicated the characteristic clinical and radiological findings in COVID-19 patients (Huang et al. 2020; Wang et al. 2020; Bernheim et al. 2020). Although the number of deaths is quite high, invasive procedures could not be applied due to the highly contagious feature of COVID-19. At the time of manuscript preparation for this report, there were a few studies that included findings of lung biopsy in COVID-19 patients (Tian et al. 2020; Xu et al. 2020; Zhang et al. 2020). Interestingly, we encountered the pathological findings of this new infection in a biopsy taken from the lung mass, although it is not due to COVID-19.
Case report
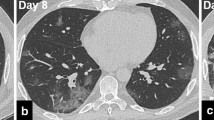
A 54-year-old woman presented to a primary care facility with fever, dry cough, and fatigue. Antibiotherapy was administered for 10 days with the diagnosis of upper respiratory tract infection. However, her condition did not improve and she was admitted to the hospital. In physical examination, crepitant rales were heard in both lungs. Her oropharynx had a natural appearance, and lymphadenopathy was not detected in the neck. Laboratory tests indicated anemia and thrombocytopenia; and she was directed to the hematology clinic. In her peripheral smear, 50–60% myeloid blast cells were detected. A bone marrow biopsy was performed. Bone marrow aspiration and flow cytometry showed approximately 25% blasts and was reported as acute myeloid leukemia transformed from myelodysplastic syndrome. Her medical history revealed that she was diagnosed with left breast cancer 6 years ago, underwent breast-conserving surgery, and had received chemotherapy and hormone therapy. She also had type-2 diabetes. The patient was on clinical follow-up and considered to be in remission. Computed tomography-integrated positron emission tomography (PET/CT) revealed a heterogeneous mass-like lesion with increased FDG (fluorodeoxyglucose) in the left upper lobe apicoposterior segment, with a size of 72 × 63 mm. Although the lung lesion was considered primarily to favor tuberculosis, the primary malignancy of the lung could not be ruled out, and a tru-cut biopsy was performed from the lesion (Fig. 1a). Cell culture and adenosine deaminase test for tuberculosis were negative. After taking the biopsy, throat swab sample was taken, and real-time polymerase chain reaction (RTPCR) analysis confirmed that the patient had COVID-19. Unlike the previous imaging study, computed tomography (CT) that was performed 6 days after biopsy, showed partial regression in the mass-like lesion in the upper lobe of the left lung (Fig. 1b). Besides that, multiple ground glass opacities and irregular nodular consolidations with air-bronchogram along the bronchovascular bundles and linear opacities was revealed especially in the bilateral lung lower lobes (Fig. 1c). Radiological findings were evaluated as progression of COVID-19 pneumonia.
CT imaging findings; a A mass like lesion was shown in the left upper lobe apicoposterior segment. b Dimensional regression of the mass like lesion and a consolidated area containing air bronchograms was observed in the left upper lobe apicoposterior segment 6 days later. c Follow-up CT scan 6 days later showed typical COVID-19 findings with multifocal segmental ground glass opacity infiltration and consolidation with air-bronchogram along the bronchovascular bundles and in subpleural areas with associated interlobular septal thickening and minimal pleural effusion in the left lower lobe
She was immediately admitted to the isolation ward in pandemic service. She was given hydroxychloroquine, antiviral (oseltamivir), azithromycin, and favipiravir. Her vital parameters worsened and she was admitted to the intensive care unit and put on a mechanical ventilator; she died 8 days later.
Malignancy was not considered in hematoxylin-eosin sections prepared from lung biopsy material. Alveolar damage, edema, vascular congestion, and mild inflammatory infiltration were noted (Fig. 2a). Type-2 pneumocyte hyperplasia, interstitial fibrosis, early fibrotic changes, and fibrinous and organizing pneumonia patterns were detected (Fig. 2b, c). Noncaseating granulomatous inflammation with neutrophils and desquamation in alveolar epithelial cells was noted (Fig. 2d). Inflammation consisted of mononuclear cells rich in CD3 positive lymphocytes and neutrophils (Fig. 2e). Pulmonary interstitial fibrosis was confirmed with Masson’s trichrome histochemical stain (Fig. 2f). The periodic acid-Schiff histochemical staining showed no evidence to suggest bacterial or fungal infections. Pathological findings were considered to be consistent with the histopathological findings of COVID-19 pneumonia based on limited studies available in the literature (Tian et al. 2020; Xu et al. 2020; Hwang et al. 2005).
Histopathological changes in the lung in COVID-19 pneumonia; a Alveolar damage, congestion, inflammation (HE × 100); b pneumocyte hyperplasia, interstitial fibrosis (HE × 400), c early fibrotic changes, fibrinous, organized pneumonia (HE × 100), d noncaseating suppurative granulomatous inflammation with neutrophils (HE × 100), e inflammation consisted rich in CD3 positive lymphocytes (HE × 40), f interstitial fibrosis was confirmed with Masson Trichrome histochemical stain (HE × 100)
Discussion and conclusions
Novel coronavirus pneumonia (COVID-19) is the disease caused by the viral agent SARS-CoV-2. SARS-CoV-2 has initially caused an epidemic in Wuhan, China, in late December 2019 and later spread to all continents, infecting more than twelve million people and causing more than 500 thousands deaths in six and half months (new reference website of world health organization). The knowledge about the epidemiological, clinical, and even radiological imaging findings for the disease is growing with new studies. However, due to the risk of high transmission, published autopsy findings are rare, and a few case reports describing the findings of lung biopsy in COVID-19 were available at the time of manuscript preparation for this report (Tian et al. 2020; Xu et al. 2020).
Our patient was immune-suppressed, had a history of breast cancer, and currently had acute myeloid leukemia. She did not have a history of a trip abroad or a contact with a sick person. However, we thought that she might have infected by another patient in the hospital while the underlying cause of bicytopenia detected in the laboratory test results was being investigated. In the patient, alveolar damage, alveolar edema, vascular congestion, and inflammatory cell infiltration were noted in the lung biopsy, similar to those in previous studies. Inflammation was made up of dominant mononuclear cells from lymphocytes and neutrophils. Noncaseating granulomatous inflammation was described for the first time in a COVID-19 case. Interstitial fibrosis and early fibrotic changes were also observed. The findings were consistent with the late-stage pathological findings of COVID-19 pneumonia and were evaluated as diffuse alveolar damage (Hwang et al. 2005). Separately, a case report by Tian et al. described intra-alveolar spherical globules and suspicious viral inclusions (Tian et al. 2020). Xu et al. observed desquamation and hyaline membrane formation in pneumocytes, and the prominent nucleoli cells in the intra-alveolar space were interpreted as other viral cytopathic effects (Xu et al. 2020). In the lung biopsy presented by Zhang et al., shedding of alveolar epithelial cells, type-2 pneumocytic hyperplasia, intra-alveolar fibrinous exudate, interstitial fibrosis, and chronic inflammatory infiltrate were observed similar to our case, and also there was an appearance of organized pneumonia with intra-alveolar fibrous plaques (Zhang et al. 2020). In studies of the SARS-coronavirus outbreak, the findings in SARS-CoV-positive patients were categorized as the early findings in the first 2 weeks of the disease and the late findings after the 14th day. Acute fibrinous exudate showing lung damage was observed more frequently in the early period, while organized exudate and pneumocyte hyperplasia were significantly more common in the late period (Hwang et al. 2005). Based on this categorization, our patient was in the late period.
In thorax CT performed 6 days after the first CT; bilateral irregular opacities, nodular consolidations, and ground-glass opacities were observed, which belonged to late-stage imaging findings of COVID-19 pneumonia progression. Bernheim et al. categorized 121 patients with COVID-19 pneumonia symptoms based on the CT findings as early (2 days after the onset of symptoms), intermediate (3-5 days after), and late group (6-12 days after). Accordingly, the coexistence of bilateral lung involvement, nodular consolidation, and ground-glass opacities mostly accompanies the late imaging findings (Bernheim et al. 2020).
Noncaseating granulomatous inflammation in COVID-19 was described for the first time in this case. Multisystemic granulomatous inflammation in ferrets carrying coronavirus antigen has been described in the literature (Martínez et al. 2008). Also, pyogranulomatous infiltration was observed in the domestic ferret carrying coronavirus (Lindemann et al. 2016). Therefore, granulomatous inflammation in coronavirus infection has been shown in animal studies in the literature and it is not surprising to see it in human cases (Martínez et al. 2008; Lindemann et al. 2016). We considered other infectious agents causing acute/chronic pneumonia and tuberculosis in the differential diagnosis of COVID-19 pneumonia. Separately, an association between COVID-19 and tuberculosis has been described by a cohort study (Tadolini et al. 2020). Before the tests were concluded, we thought that our patient most likely had tuberculosis accompanying COVID-19. However, cell culture and adenosine deaminase test results were negative. Tuberculosis bacilli were not observed in the Ehrlich Ziehl-Neelsen histochemical stain and PCR. Radiological findings were compatible with viral pneumonia rather than tuberculosis. Granulomas were suppurative, not caseified, like tuberculosis granulomas. Of course, we still cannot rule out the presence of tuberculosis bacilli that cannot be detected by these tests. Epidemiological and clinical findings, typical findings in computed tomography, and RTPCR testing supported the diagnosis of COVID-19 pneumonia. The biopsy findings of this newly encountered disease are still unclear. This issue will be better understood as studies report a larger number of cases with lung biopsy findings in COVID-19 patients.
Availability of data and materials
The data set supporting the conclusions of this article is included within the article. The detail of data analysed during the current case report are not publicly available due to patient privacy but are available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- PET/CT:
-
Computed tomography-integrated positron emission tomography
- FDG:
-
Fluorodeoxyglucose
- RTPCR:
-
Real time polymerase chain reaction
- CT:
-
Computed tomography
- SARS:
-
Serious acute respiratory distress syndrome
References
Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N et al (2020) Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 20:200463. https://doi.org/10.1148/radiol.2020200463
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Hwang DM, Chamberlain DW, Poutanen SM, Low DE, Asa SL, Butany J (2005) Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol 18(1):1–10
Lindemann DM, Eshar D, Schumacher LL, Almes KM, Rankin AJ (2016) Pyogranulomatous panophthalmitis with systemic coronavirus disease in a domestic ferret (Mustela putorius furo). Vet Ophthalmol 19(2):167–171. https://doi.org/10.1111/vop.12274 Epub 2015 Apr 28. PMID: 25918975; PMCID: PMC7169242
Martínez J, Reinacher M, Perpiñán D, Ramis A (2008) Identification of group 1 coronavirus antigen in multisystemic granulomatous lesions in ferrets (Mustela putorius furo). J Comp Pathol 138(1):54–58. https://doi.org/10.1016/j.jcpa.2007.10.002
Tadolini M, Codecasa LR, Garcia JM, Blanc FX, Borisov S, Alffenaar JW et al (2020) Active tuberculosis, sequelae and COVID-19 coinfection: first cohort of 49 cases. Eur Respir J 26:2001398. https://doi.org/10.1183/13993003.01398-2020
Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY (2020) Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. https://doi.org/10.1016/j.jtho.2020.02.010
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. https://doi.org/10.1001/jama.2020.1585
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C et al (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8(4):420–422. https://doi.org/10.1016/S2213-2600(20)30076-X
Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M et al (2020) Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. https://doi.org/10.7326/M20-0533
Acknowledgements
Not applicable.
Funding
No organizations funded my report.
Author information
Authors and Affiliations
Contributions
EUK have designed and drafted the manuscript, ET and NC revised, IU, NT, DK, and OK made interpretation of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Patient consent form was obtained.
Consent for publication
Consent for publication was obtained from the person in our case report (The consent form was uploaded as a file).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Usturalı Keskin, E., Tastekin, E., Can, N. et al. Granulomatous inflammation in pulmonary pathology of 2019 novel coronavirus pneumonia: case report with a literature review. Surg Exp Pathol 3, 21 (2020). https://doi.org/10.1186/s42047-020-00071-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42047-020-00071-2