Abstract
Background
Ossified intramuscular hematomas (OIH) are an exceptionally rare condition that may be mistaken for alternative calcified intramuscular pathologies, such as myositis ossificans. Exceedingly few cases of OIHs have been reported to date, with no cases yet to be reported in the paraspinal muscles.
Case presentation
Here, we report on a patient who presented with a chronic back pain and swelling in the setting of trauma 15 years prior. Radiographic workup revealed a calcified mass in the erector spinae muscles. The lesion was surgically excised, and histologic examination confirmed the presence of an OIH. The patient did well postoperatively.
Conclusion
An OIH is a poorly understood pathology. Although benign, these lesions can cause significant morbidity, and surgical excision is a reasonable and safe treatment option. OIHs may be distinguished from related calcified intramuscular pathologies based on key clinical features and distinct histopathology. Clinically, they are characterized by a history of remote trauma and, on histopathology, by compact, mature bone in the setting of an old, organizing hematoma. Despite this, similarities with other calcified intramuscular pathologies persist, and further study is warranted to better understand and classify these lesions.
Similar content being viewed by others
Background
An ossified intramuscular hematoma (OIH) is a rare condition. It is exceedingly rare in the paraspinal muscles with no reported cases to date. Such lesions can likely be traced back to an intramuscular bleed emanating from local vasculature [1, 2]. In general, intramuscular hematomas can be either traumatic or spontaneous in nature [3]. Spontaneous intramuscular bleeds are usually symptomatic on presentation and can be life-threatening if not properly managed. Alternatively, traumatic intramuscular hematomas are associated with an inciting injury, may or may not become symptomatic, and are most often managed conservatively [2, 3]. Importantly, traumatic muscular hematomas are largely considered benign and are expected to self-resolve with time [1]. However, if the hematoma does not spontaneously resorb, downstream complications can arise [3]. One possible complication may be calcification or ossification of the remaining blood clot.
The pathophysiology of an ossified or calcified intramuscular hematoma remains poorly understood [4]. Ossification of hematomas outside of the musculature is relatively more well-studied. Specifically, studies of ossified chronic subdural hematomas may provide pathophysiologic insights [5]. Here, it is reported that when evacuation is not pursued and there is no spontaneous resorption, formation of microscopic calcium deposits within the membranes of the subdural hematoma alongside hyalinization of the connective tissues can occur [5]. This can progress to more significant calcification and ultimately ossification in some cases. Ossification would be the terminal stage of the calcification process [5, 6]. In general, bone formation occurs by both endochondral and intramembranous processes. Of interest, in endochondral bone formation there is formation of hyaline cartilage which is gradually calcified and replaced with ossified lamellar bone through extensive remodeling processes [7]. Given histologic evidence of hyalinization, calcification and ossification in calcified chronic hematomas to date, we propose that bone formation in an OIH could occur through this process [4, 6, 8].
Clinically, ossified or calcified intramuscular hematomas present as deep-seated masses that enlarge at variable rates and may be discovered as many as 30 years following the initial injury [4, 8]. If the lesion is small, it may be clinically insignificant. However, if large, these lesions can impose significant morbidity. For example, they can cause notable mass effect leading to exceptional pain, mechanical limitations, and impaired quality of life [8]. Further, depending on location, neurovascular impingement can occur, leading to neuromuscular deficits [4].
The work-up for these lesions spans 3 dimensions—clinical, radiologic and histologic. One should begin with a careful history with a particular focus on a history of prior trauma. Physical examination is essential and should be completed to evaluate for any subtle or salient signs of neurologic deficits or mechanical limitations. Palpation at the site of pain will often reveal focal swelling and a mass-like lesion [8]. Following a thorough history and physical, imaging is essential to better characterize the mass and is a critical step to narrow the differential. This should be completed with a plain radiograph followed by CT or MRI. CT may be considered the gold-standard as it is able to best characterize the calcifications [9]. However, it is important to note that MRI may be essential to best evaluate for the possibility of a soft tissue malignancy. Finally, if the lesion is resected, histopathologic examination should be pursued to obtain a definitive diagnosis.
When evaluating an intramuscular calcification, it is important to maintain a broad differential. In particular, OIH may be mistaken for alternative calcified intramuscular pathologies. For example, without histologic examination, it is reported that an ossified hematoma may be incorrectly diagnosed as myositis ossificans (MO) [4]. MO is a form of heterotopic ossification that occurs exclusively within the muscle. It is reported to be a reactive process of aberrant bone formation following trauma and most commonly occurs in large muscle groups of the extremities [10]. Furthermore, benign intramuscular calcifications can also closely resemble soft tissue sarcomas and care must be taken to rule such out [11]. For example, soft tissue neoplasms such as osteosarcoma and synovial sarcoma often contain calcifications and may mimic a calcified hematoma on imaging, as well as in clinical presentation. Ultimately, a thorough workup is essential to best differentiate these lesions.
Here, we report on a unique case of an ossified intramuscular hematoma in the erector spinae muscles that was discovered in a patient who presented with chronic back pain and a history of remote trauma. We present a review of the literature on this topic and highlight key findings that may help to differentiate these lesions from similar pathologies reported in the literature. Finally, we will also identify areas of future study to aid in advancing our understanding of ossified intramuscular hematomas.
Case presentation
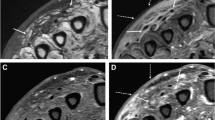
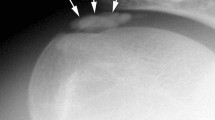
A 29-year-old female presented with chronic back pain and swelling and a history of back trauma 15 years prior. On examination, there was an increase in pain with flexion. Laboratory workup was unremarkable. MRI followed by CT imaging demonstrated an ossified lesion within the erector spinae muscle on the left side, spanning from the level of T12 to L2 (Figs. 1, 2). Due to functional limitation and pain, surgical excision was planned. Intraoperatively, the lesion was accessed through a paramedian skin incision centered over the mass, and we were able to excise the lesion en bloc, as a single mass (Fig. 3). Gross examination revealed a nodular yellowish and stony hard mass 9.5 × 4 × 3 cm, with a side groove that was 4 cm long and 1 cm in width. Histopathologic examination revealed compact bony tissue with irregular cement lines consistent with an ossified, old organizing hematoma (Fig. 4). There were no postoperative complications, and the patient recovered well. Follow-up CT revealed complete excision of the mass with clean surgical cavity (Fig. 5).
Discussion
Here, we present a case of an OIH within the erector spinae muscles of the thoracolumbar region, confirmed with pathologic examination. To our knowledge, this is the first report of an ossified intramuscular hematoma in the paraspinal muscles. Calcified intramuscular hematomas in muscles groups of the extremities have been reported in the literature, although rarely—limited to one case report and one case series [4, 8]. In 2015, Ladermann and colleagues reported on a case of a calcified intramuscular hematoma in the supraspinatus muscle [4]. This patient presented with symptomatic progression following a remote history of trauma to the shoulder 30 years prior. Similar to our case, CT demonstrated a well-delimited calcification. Grossly, a well-circumscribed ovoid mass was identified, and histologic examination revealed highly hyalinized collagenous stroma with peripheral dystrophic calcification, as well as evidence of mature lamellar bone formation.
Prior to this report, the only additional reports of calcified intramuscular hematomas are limited to a case series published in 1997 [8]. Here, four cases of what were termed “ancient hematomas” presented in the tensor fascia lata muscle of the thigh. All lesions presented clinically as deep-seated variably enlarging masses that developed over the course of 6 to 12 weeks and anywhere from 18 months to 20 years after initial traumatic insult. Gross examination revealed masses with ovoid or fusiform morphology. Each contained a dense fibrous pseudocapsule and, in one case, a calcified rim. Inner cavities consisted of a soft, cystic center with necrotic debris and blood clots. On histology, the peripheral rim contained hyalinized fibrous tissues with areas of dystrophic calcification and a center of reactive fibro- and myofibroblastic proliferation and hemorrhage. Of note, the lesions in this series did not appear to be as heavily calcified as our case or of that discussed prior. This may be secondary to earlier intervention due to the location of the lesions or, alternatively, these lesions may be of separate pathology. Nonetheless, altogether, a total of five prior cases suggestive of calcified or ossified intramuscular hematomas have been reported in the literature. Although these cases each presented with varying degrees of calcification and ossification, they maintain important similarities. Specifically, they are all similar in clinical presentation, morphology and with evidence of calcification and an old, organizing hematoma on histopathology.
It is important to emphasize that these lesions maintain some resemblance to additional calcified intramuscular pathologies reported in the literature. For example, Mentzel and colleagues reported that their histopathologic findings were similar, if not identical, to what had been reported in cases of calcific myonecrosis [8]. Calcific myonecrosis is a benign soft tissue lesions that most often occurs in muscular compartments of the lower limb. It is associated with a remote history of compartment syndrome and is characterized by an intramuscular necrotic, hemorrhagic calcified mass on histopathology [8, 11, 12]. However, calcific myonecrosis has a distinct clinical history of compartment syndrome, as well as unique radiographic features [11]. Furthermore, the presence of compact, mature bone would not be seen in the setting of calcific myonecrosis. Thus, we feel it to be a less likely diagnosis in our case.
Further, as previously mentioned, an OIH can be mistaken for MO [4]. MO is a more commonly reported condition that likewise presents similarly to a calcified or ossified intramuscular hematoma [10]. In fact, many have proposed that an organizing hematoma may be a step in the formation of MO [4, 10, 13, 14]. The increased incidence of MO in those with bleeding disorders further supports this hypothesis [14, 15]. Clinically, MO and OIHs are associated with a history of trauma and present with symptomatic progression following enlargement of a deep-seated soft tissue mass. On plain radiograph and CT, they present as a calcified lesion with varying degrees of calcification or mature lamellar bone formation, depending on stage of development [11]. However, key differences are apparent which support the idea of two separate pathologies. Foremost, the natural history of these pathologies is distinct. In our case and those of calcified intramuscular hematomas reported previously, lesions developed on a scale of years to decades following traumatic insult. On the other hand, MO develops within weeks to months following injury and, unlike calcified intramuscular hematomas, will exhibit spontaneous resolution if not resected. Furthermore, the histology of MO classically demonstrates a “zonal phenomena” [10]. This is characterized by peripheral bone maturation and inner zones of immature osteoid or cellular focus. These zones are notably absent in calcified intramuscular hematomas. Nonetheless, due to limited literature and unclear pathophysiology, we cannot exclude the possibility of a relationship between an OIH and formation of MO.
In regard to treatment, there are no formal recommendations. However, similar to MO, it can be assumed that either conservative or surgical management is appropriate depending on clinical context [10]. For lesions that are small, asymptomatic and without functional impairment, conservative therapy with surveillance may be sufficient. However, lesions that are sufficiently large and painful, or with evidence of functional limitation or neurovascular impingement, should be considered for surgical resection. Importantly, if surgical intervention is pursued, gross total resection is recommended as recurrence has been reported in one case with subtotal excision. [8]
Looking to the future, additional work is needed to characterize these lesions in order to better understand their natural history, histologic and radiologic features, and pathophysiology. Foremost, all calcified and ossified intramuscular hematomas reported thus far have been excised due to symptomatic progression, and we have been unable to determine natural history—for example, whether these lesions have the capacity to self-resolve without intervention. Surveillance of more benign lesions that would have been confirmed with biopsy will be key to elucidating natural progression. Further, among cases reported, there remains variation in radiographic and histologic findings alongside apparent similarities with alternative pathologies—for example, calcific myonecrosis and MO. As a result, a more broad, unifying condition is possible, and further reports of OIH will be needed to better classify these similar pathologies. Finally, the process of calcification and subsequent ossification in the setting of an intramuscular hematoma remains poorly understood. With reports of additional cases, continued histopathologic study may contribute to our understanding of the ossification process.
Conclusion
The occurrence of an OIH is rare in the literature, and here we report the first case of an OIH within the paraspinal muscles, specifically the left erector spinae muscles of the thoracolumbar region. The patient was symptomatic due to location and size of the lesion and successfully treated with resection of the mass. She did well postoperatively without complications.
There are few prior reports of calcified intramuscular hematomas, limited to one case report and one case series. The clinical, radiographic, and histopathologic findings among these lesions maintain important features in common. For example, a history of remote trauma, a calcified mass on radiographic workup, and evidence of an old, organizing hematoma with calcification or ossification on histopathology. However, commonalities between these cases and alternative calcified intramuscular pathologies reported in the literature persist, and further study may be needed to definitively classify calcified intramuscular pathologies.
Availability of data and material
All information and data that are available have been shared in the article and are genuine.
Abbreviations
- OIH:
-
Ossified intramuscular hematoma
- MO:
-
Myositis ossificans
- CT:
-
Computerized tomography
- MRI:
-
Magnetic resonance imaging
References
Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas: a clinicopathologic entity. JAMA. 1980;244(21):2441–2. https://doi.org/10.1001/jama.1980.03310210043026.
Sreenivas M, Nihal A, Ettles DF. Chronic haematoma or soft-tissue neoplasm? A diagnostic dilemma. Arch Orthop Trauma Surg. 2004;124(7):495–7. https://doi.org/10.1007/s00402-004-0698-x.
Davis, Donald D, Kane, Steven M. Muscular hematoma. StatePearls. 2022.
Lädermann A, Genevay M, Abrassart S, Schwitzguébel AJP. Supraspinatus intramuscular calcified hematoma or necrosis associated with tendon tear. Case Rep Orthop. 2015;2015:496313. https://doi.org/10.1155/2015/496313.
Pakrasi R, Pandey P, Das S, Datta S, Saha D. Calcified chronic subdural hematoma: illustrative case. J Neurosurg Case Lessons. 2021;2(15):CASE21468. https://doi.org/10.3171/CASE21468.
Áfra D. Ossification of subdural hematoma: report of two cases. J Neurosurg. 1961;18(3):393–7. https://doi.org/10.3171/jns.1961.18.3.0393.
Wlodarski K, Galus R. Histological aspects of bone fracture healing. Ortop Traumatol Rehabil. 2005;7:351–60.
Mentzel T, Goddlad J, Smith M, Fletcher C. Ancient hematoma: a unifying concept for a post-traumatic lesion mimicking an aggressive soft tissue neoplasm. Mod Pathol. 1997;10(4):334–40.
Freire V, Moser TP, Lepage-Saucier M. Radiological identification and analysis of soft tissue musculoskeletal calcifications. Insights Imaging. 2018;9(4):477–92. https://doi.org/10.1007/s13244-018-0619-0.
Meyers C, Lisiecki J, Miller S, et al. Heterotopic ossification: a comprehensive review. JBMR Plus. 2019;3(4):e10172. https://doi.org/10.1002/jbm4.10172.
Kwee RM, Kwee TC. Calcified or ossified benign soft tissue lesions that may simulate malignancy. Skelet Radiol. 2019;48(12):1875–90. https://doi.org/10.1007/s00256-019-03272-3.
Gangrade K, Yeotikar G, Wadhwani A, Naneria V. Calcific myonecrosis of the leg: a case report. Case Rep Orthop Res. 2019;2(1–3):47–53. https://doi.org/10.1159/000502031.
Jung D, Cho KT, Roh JH. Non-traumatic myositis ossificans in the lumbosacral paravertebral muscle. J Korean Neurosurg Soc. 2013;53(5):305–8. https://doi.org/10.3340/jkns.2013.53.5.305.
Järvinen TAH, Järvinen TLN, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745–64. https://doi.org/10.1177/0363546505274714.
Beiner JM, Jokl P. Muscle contusion injury and myositis ossificans traumatica. Clin Orthop Relat Res. 2002;403. https://journals.lww.com/clinorthop/Fulltext/2002/10001/Muscle_Contusion_Injury_and_Myositis_Ossificans.13.aspx
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical committee clearance was taken before including this case for study.
Consent for publication
Valid informed consent was obtained from the patient.
Competing interests
There are no financial and non-financial competing interests associated with this case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zelmanovich, R., Lucke-Wold, B. & Elghareeb, M. Ossified intramuscular hematoma of the paraspinal muscles: a case report. Egypt J Neurosurg 38, 69 (2023). https://doi.org/10.1186/s41984-023-00259-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-023-00259-0