Abstract
Objectives
Post-infection hydrocephalus with multiple intraventricular septations is a complex issue in neurosurgery, with multiple treatment options available. The authors reviewed the results of neuroendoscopic cyst wall fenestration for managing this disease.
Materials and Methods
Medical records of 76 patients with post-infection hydrocephalus and multiple intraventricular septations who underwent endoscopic treatment were collected and analyzed.
Results
The patient group consisted of 40 males (52.6%) and 36 females (47.4%), with a mean age of 22.36 months (range: 4–132 months). Bacterial meningitis was the most common cause of hydrocephalus with multiple intraventricular septations in 37 patients (48.6%), while 24 patients had post-shunt infection (31.6%) that was complicated with multiloculated hydrocephalus. After confirming clearance of CSF infection, all patients underwent ventriculoscopic cyst fenestration and insertion of a ventriculoperitoneal shunt to create a single communicating system drained by one ventricular catheter. Fifty-five patients underwent De novo shunt implantation, while 20 patients required shunt revision. Endoscopy reduced the shunt revision rate from 3.4 per year before fenestration to 0.4 per year after fenestration. During the mean follow-up period of 7.7 months (range: 1–20 months), complications were reported in 13 patients (17.1%), including CSF leakage in eight (10.5%), VPS malfunction in five (6.5%), and two deaths (2.6%).
Conclusion
The authors concluded that neuroendoscopic fenestration with the aid of CSF drainage by intraventricular catheter is an effective treatment for managing multiloculated post-infection hydrocephalus with much lower rates of morbidity and mortality than traditional procedures.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Hydrocephalus with multiple intraventricular septations poses a challenging neurosurgical condition, often attributed to various etiological factors. In the neonatal age, the most common causes include intraventricular hemorrhage and infection, while shunt-related infection, direct ependymal trauma during catheter insertion, head injury, and intracranial surgery can also contribute to its development [1,2,3].
The main pathological process in different etiologies is ventriculitis, which can progress to ventricular septations within an average of 2–4 months. Intraventricular compartmentalization is the appearance of new septae within the ventricle that gradually progress to the indiscriminate ventricular pattern, leading to a single, large, multiloculated cavity surrounded by a layer of the cerebral mantle. The primary targets for management include controlling hydrocephalus, managing increased intracranial pressure symptoms, and reducing the risk of compartmentalization through proper management and anticipation of ventriculitis [2, 4, 5].
Given its progressive nature, hydrocephalus with multiple intraventricular septations necessitates difficult surgical procedures that are often repeated over time due to the formation of new membranes within the decompressed loculi. Endoscopic fenestration followed by cerebrospinal fluid diversion is the primary management approach, and various surgical treatment modalities have been reported, including multiple shunt implantation, multiperforated ventricular catheter, craniotomy (transcallosal fenestration of intraventricular septations), stereotactic aspiration, and endoscopic fenestration [6,7,8,9].
Neuroendoscopy has revolutionized the treatment of complex multiloculated hydrocephalus by consolidating multiple cavities into fewer cavities in one or more stages. This approach allows for fenestration to be performed before or after the initial shunt, avoiding multiple shunting procedures and minimizing the number of ventricular catheters required inside the cranium [10, 11].
However, patients with distorted ventricular anatomy may present challenges during neuroendoscopy, and unifying multiple cavities may not be feasible. Additionally, the introduction of the neuroendoscope into the cavity can cause a loss of cerebrospinal fluid (CSF), resulting in the shifting of anatomical points and the failure of predicted locations for fenestration. Therefore, relying solely on preoperative neuroimaging and a neuroendoscope may not ensure a successful operation [12].
The high incidence of infection as the primary cause of ventricular loculation in Egypt underscores the need for further research and intervention to address the issue. These study findings may potentially aid in the diagnosis, treatment, and management of post-infectious hydrocephalus with multiple intraventricular septations.
Material & methods
Study population
In a retrospective study that was carried out after approval from the local ethical and scientific committee (R.22.11.1933), medical records of 76 patients with a diagnosis of hydrocephalus with multiple intraventricular septations were managed at Mansoura university hospitals during the period of January 2017 and March 2022. We reviewed the outpatient clinic visits medical records to collect the follow-up data through the follow-up period that ended in March 2022.
Management plan for each patient starts with meticulous history taking with emphasis on previous infections such as meningitis and history of shunt placement. The clinical examination includes general (signs of infection: irritability, fever, increasing seizures) and neurological examination (anterior fontanelle, head circumference, suture diastasis, seizures, consciousness level, and delayed milestones of growth). Laboratory investigation including laboratory check for infection indexes: blood infection screen (ESR, CRP). Neuroradiological evaluation through computed tomography (CT) scanning and magnetic resonance (MR) imaging; special consideration was given to MRI/T2 with flow study and 3D MR sequences (FIESTA, CISS) (presence of evolving loculations) and finally CSF sampling through trans-fontanellar tapping or lumbar tapping and all the existing reservoirs for analysis as CSF cytology, biochemistry, and culture.
The selection criteria we used for post-infectious hydrocephalus through CSF analysis included a white blood cell count of greater than 100/mm3, as this would indicate the aftermath of a bacterial infection, whereas lower counts may be due to viral meningitis or seizures. This information is supported by Morgenlander JC [13]. Protein concentration in CSF is one of the most sensitive indicators of pathology in the central nervous system. Newborns typically have up to 150 mg/dl of protein, whereas the adult range is 18–58 mg/dl and is reached between 6 and 12 months of age. Therefore, a value above 150 mg/dl in an infant is certainly elevated. As for CSF glucose levels, there is no definitive normal range. In adults, CSF glucose is approximately two-thirds of the serum glucose measured in the preceding 2–4 h. However, the CSF-to-serum glucose ratio is generally higher in neonates. It is important to note that up to 50% of patients with bacterial meningitis may have normal CSF glucose levels, according to Seehusen et al. [14]. Therefore, a level below 20 mg/dl would be a clear indication of preceding meningitis. In our study, we found coagulase-negative staphylococci to be the most prevalent organism followed by other gram-positive, group B streptococcus, and gram-negative organisms, such as Haemophilus influenzae and Neisseria meningitides. If the shunt system was infected (CSF samples are positive for infection), we removed the infected shunt and replaced it with an external ventricular drainage (EVD), and a selective antibiotic was given on the basis of CSF cultures and sensitivity. Endoscopic lavage of the infected ventricles was done to wash out CSF infection in selected cases (heavy purulent infection).
Following the resolution of CSF infection (clinically and laboratory guided), endoscopic procedures were tailored and selected to plan the site of entry and trajectory aiming to connect as much as possible the isolated loculi within the ventricles.
Equipment and operative procedures
The basic approach has been endoscopic fenestration of most of the possible cysts with conversion of the multiple ventricular cavities into a single ventricular cavity that can be diverted through a single ventriculoperitoneal shunt (VPS) if possible or in selected cases through a third ventriculostomy.
Ultrasonography had been used to identify the location, size, and shape of the fluid-filled spaces (locules) within the brain, and to identify the best entry for the endoscope. Preoperative radiology was also evaluated.
The site of the burr hole for the introduction of the endoscope was planned based on the largest cyst site, the ideal trajectory for easier manipulation of the endoscope, and the best angle of vision. Fenestrations were performed at the level of the most avascular areas of the cyst walls with a Bugbee wire, monopolar cautery, micro-scissors, and balloon catheter. A rigid endoscope with 0- and 30-degree optics (0.7 mm, Karl Storz) was used in all cases. The subsequent enlargement of the fenestrations was performed with a no three and sometimes four French Fogarty balloon, and widening of the stoma was done using micro-scissors and micro-forceps. Continuous irrigation was obtained in all cases using a warm ringer solution.
Whenever possible we tried to connect both sides of the ventricular system with each other. We maintain that by fenestration of the septum pellucidum when we were able to identify it. Usually, we puncture the septum at the thinnest avascular area and dilate the opening as much as we can by the previously mentioned methods. Sometimes when the third ventricle is identified there were trials to do endoscopic third ventriculostomy. For sure it is very difficult and may be an impossible mission to accomplish in the labyrinth of multiple isolated chambers with no recognizable anatomy.
At the end of the endoscopic procedure, we insert the ventricular catheter of the shunt under endoscopic vision. We try to drive the tube through as many cysts as we can across the prepared fenestrations. We add manually some extra holes to the shaft of the tube to enhance the drainage from a lot of chambers. This way the tube could function as a draining stent which guards against possible future re-encystation.
Postoperative follow-up
Control CT scan was done on the first day after surgery. Following the patient’s discharge from the hospital, outpatient clinic follow-up visits were scheduled every week during the first month, then postoperative clinical and radiological surveillance including a CT scan on the first day and MRI were obtained at one-, three-, or six-month intervals later. Two-month follow-up period is the minimum follow-up time, and 12 months is the mean duration.
Statistical analysis
Statistical analysis was performed using SPSS software (standard version 20; SPSS, Inc.). Continuous variables were reported as the mean ± SD or median. Categorical variables were recorded using numbers and percentages.
Results
The study enrolled 76 pediatric patients diagnosed with hydrocephalus with multiple intraventricular septations. Of the enrolled patients, 40 were males (52.6%) and 36 were females (47.4%), with ages ranging from 4 months to 132 months (mean 22.36 months). All patients had hydrocephalus with intraventricular multiple septations. The different etiologies of the hydrocephalus were reported, with 52 patients (68.4%) having post-infection hydrocephalus. Among these patients, 37 (48.6%) had meningitis, eight (10.5%) had ventriculitis, three (3.9%) had meningeoventriculitis, and four (5.2%) had post brain abscess. Additionally, 24 patients (31.6%) had previous shunt implantation with shunt infection as shown in (Table 1).
The most common clinical presentation is a tense anterior fontanelle in 73 patients (96%), followed by delayed milestones of growth in 70 patients (92.10%), increased head circumference in 66 patients (86.84%), suture diastasis in 40 patients (52.63%), seizures in 22 patients (28.94%), and disturbed consciousness level in nine patients (11.84%).
The radiological findings in the enrolled patients in the study were obliteration of the subarachnoid space in 54 patients (71%), midline shift in 47 patients (61.84%), and transependymal CSF permeation in 39 patients (51.3%), with multiple intraventricular septations in all patients (Table 2).
We performed endoscopic cystic fenestration (ECF) in all patients, with revision of the already implanted shunt in 11 patients (14.4%), and insertion of a De novo ventriculoperitoneal shunt in 65 patients (85.6%).
During the follow-up period (1–20 months) with a mean time of 7.76 months after the endoscopic procedures in all cases, signs and symptoms of intracranial hypertension resolved in all cases. Increased head circumference was stabilized in 63 out of 66 patients (95.45%), tense fontanelle became lax in 70 out of 73 patients (95.9%), suture diastasis improved in 39 out of 40 patients (97.5%), seizures were controlled in 19 out of 22 patients (86.36%), disturbed consciousness improved in eight out of nine patients (88.8%), and eight out of 70 patients (11.4%) showed improved growth milestones.
The complications of our surgical procedures affected 13 patients (17.1%): five patients (6.5%) had shunt malfunction which manifested as increased ICP. They were managed with revision in three cases and insertion of new ones for the remaining two cases. Eight patients (10.5%) had CSF leakage, out of which six patients had an infection, and four were treated conservatively with antibiotics and unfortunately, two patients (2.6%) died due to the consequences of sepsis (shown in Table 3).
In total, we performed 136 endoscopic procedures. Five patients (6.5%) necessitated endoscopic shunt revision, while seven patients (9.2%) required adding of a new device; four out of them had a history of previous shunt infection with only one patient (1.3%) required three shunt devices after repeating endoscopic procedures. The shunt revision rate per year (0.4) and mean surgical time was 2.1 h (Table 4).
Case illustration
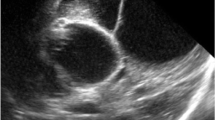
An 11-month-old female patient was diagnosed with hydrocephalus due to intraventricular hemorrhage when she was 6 months old. A VPS was initially implanted, but at 8 months old, she developed bacterial meningitis and the shunt was removed. An EVD was then repeatedly implanted to control the hydrocephalus, but over the next five weeks, there was a clinical and radiological progression of the condition. An MRI at 9 months revealed an active multiloculated hydrocephalus with multiple septa (Fig. 1). On admission, the infant showed symptoms of an active hydrocephalus such as fontanelle bulging and an increasing occipital-frontal circumference. The child was treated with endoscopic fenestration of multiple septa into a larger cavity and a VPS was inserted under endoscopic guidance under general anesthesia. After long-term follow-up, there was significant improvement in the child's neurological state. An MRI performed 2 months after surgery showed a significant collapse of the ventricles and no evidence of active hydrocephalus, confirming the patency of most of the septostomies that were performed (Fig. 2).
Discussion
A large number of infants who suffer from a serious CNS infection, such as meningitis or ventriculitis, may later develop hydrocephalus. Most of these patients have a risk of developing multiple intraventricular septations [2]. Neonatal meningitis is often associated with ventriculitis in 75–92% of cases. Inflammation of the ependymal wall of the ventricle induces the proliferation of sub-ependymal glia, and exudates of inflammation collect, resulting in the formation of webs of fibro gliosis. These webs project inside the ventricles, and intraventricular septations begin to be evident [10, 11].
Most studies of hydrocephalus with multiple intraventricular septations and its management options describe the pathology following all causes, including the post-hemorrhagic scenario. In our study, we described only cases following the occurrence of CNS infection because this group of patients represents the vast majority of cases in our locality in Egypt. Also, they are more challenging to treat, and until now, their incidence and prognosis in the literature were not discussed separately except for a few reports [7]. Different approaches for management have been reported in the literature. Multiple shunting has been associated with a high risk of shunt infection and high morbidity rates [12].
Some surgeons have reported good results with microscopic fenestration of the intraventricular septa. However, craniotomy with trans-cortical or transcallosal approaches has a lot of surgical risks, especially in children with such bloody approaches. Stereotactic aspiration of the cysts is associated with a high rate of recurrence and is not recommended by many surgeons [15, 16].
Nowadays, after many trials of previous approaches, there is a consensus that endoscopic approaches should be the first line of management for patients with multiple ventricular septations. In most of these endoscopic studies, it has been concluded that endoscopic fenestrations significantly reduce the rate of shunt revision per year and could even eliminate the need for shunts in a few cases. In our study, we found that our shunt revision rate was reduced from 3.1 per year to 0.4 after the introduction of endoscopy in treating cases with multiloculations [5, 17,18,19].
Avoiding the insertion of shunts in patients with multiloculations is a very difficult objective and is not usually feasible. The great achievement of endoscopy is in simplifying encystations as much as possible, decreasing the number of needed draining catheters. Patients will still need shunts to treat hydrocephalus due to the immaturity of the subarachnoid CSF dynamics. Another cause is that the pathophysiological mechanisms that lead to the formation of septa are accused of decreasing the absorptive power of the subarachnoid space, especially with the presence of arachnoid granulation scars. In our case series, we succeeded in avoiding shunting in only one patient (1.7%) with severe neurological deficit, who did not require a shunt after repeating endoscopic procedures, regression of ventriculomegaly, presence of encephalomalacia changes, and disappearance of increased intracranial pressure symptoms and signs. The majority of patients ultimately required a shunt system with only one ventricular catheter implanted in 64 patients (84.2%), while two ventricular catheters were implanted in 12 patients (15.8%). Teo et al. reported that in their series of 114 patients, 82 patients had only one shunt (72%), and 32 had none (22%). Zuccaro et al. showed that 46 patients had one shunt (98%), and one had none. Spennato et al. presented a case series of 30 patients, where at the end of the follow-up time, there were 17 patients with one shunt (73%), four had two shunts, one patient had three, and eight patients had none. We should clarify that most of these studies included different cases of complex multiloculated hydrocephalus resulting from many variant pathologies and not only post-infection cases like our study [18, 20, 21].
Revision of endoscopic procedures in these children is so common and expected, as it is well known that septations are an unstable pathology that usually progresses and changes over time. In our series, repeated endoscopic procedures were needed in 37 patients (48.6%). The maximum number of endoscopic procedures in one patient was five times, and this number was reported only in a single case. El Ghandour, in his case series, found that a repeated endoscopic procedure was necessary for eight (33%) of 24 patients during the overall mean follow-up period (30 months). We agree with him that patients in whom shunts were placed before the endoscopic procedures had a higher risk of having a repeated endoscopic procedure than patients in whom shunt placement was after the endoscopic fenestration. Almost all the studies dealing with multiple encystations in hydrocephalus reported their need for at least a single revision of the procedure [18, 20,21,22].
In our trial, we aimed to decrease the need for repeating the intervention by trying to open as much as possible of the septa with widened stomas, and even we sometimes made multiple openings in the same wall to work as a reserve for probable closure in the future. Another trick that we use in our procedure is to put the shunt at the end of the procedure under endoscopic vision. We make the shunt cross through as many chambers as we can through the already performed fenestrations by the endoscopic tools. We think that way will make the shunt works as a stent against future encystation. Also, we follow the same technique as Elkheshin et al. by modifying included fenestration of the proximal shunt hardware by adding extra side holes tailored according to the required length of ventricular system drainage and made to simulate the 360° all-around ports of the factory default catheter, to improve its ability to drain [23].
Although few cases were reported previously, El Ghandour et al. [12] performed septum pellucidotomy in 11 patients (14.4%). Also, a third ventriculostomy was done in one case. Of course, the anatomical deformities of the ventricular system made these previous techniques a real challenge but we could achieve in some cases that the radiology showed few large encystations mainly in one side of the ventricular system.
As regards the resolution of clinical and radiological manifestations after surgery, the success and failure criteria in this very unique population are very vague and unclear. As these cases are very beaten neurologically, and often the skulls close with no relevant fontanelle, imaging coupled with subtle neurological findings are important. The improvement was mainly in the symptoms and signs of increased intracranial pressure which was detected in more than 95% of cases. Many reports from the literature stated that most of these patients are living with cognitive deficits and need daily help in regular activities [24].
For more intraoperative orientation with easy endoscopic manipulation and puncture as many septa as we can some surgeons recommended the use of a navigator or an intraoperative ultrasound. Many other surgeons are criticizing the use of both devices and found them sometimes useless or misleading. In our work, we depended mainly on intraoperative ultrasound and the preoperative planning of the ideal trajectory by studying well the preoperative multiple planes of MRI (coronal, sagittal, and axial). By the previous planes, we were able to choose the ideal trajectory with the ideal site of the burr hole which will lead the tip of the endoscope to reach as many isolated chambers as we can. In very few instances, we used more than one burr hole to achieve the previous target [23, 25, 26].
During the period of the study, there were no significant intraoperative morbidities. Of course, we faced some of the common problems which occur sometimes during these minimally endoscopic procedures. Mild to moderate bleeding was documented in 10 times of our procedures but we could overcome this problem by maintaining the intraventricular irrigation with ringer lactate until the bleeding stopped. In none of the cases of intraoperative bleeding, we were obliged to terminate the procedure because of bleeding. Postoperative complications were managed (CSF leakage, shunt malfunction) and were documented in the literature with similar approaches. We think that our rate of complications is somewhat close to other studies dealing with complex hydrocephalus [2, 11, 27].
Limitations
The major limitation of this study is its retrospective design, which may lead to bias in data selection and outcomes. Although neuronavigation is considered a cornerstone during endoscopic fenestration to localize and target multiple intraventricular loculations, the entire study was performed without a neuronavigation system due to economic concerns.
Conclusions
Post-infection hydrocephalus with multiple intraventricular septations remains a difficult problem in the practice of neurosurgery. It is common in low socioeconomic countries as the infection rate is high.
Neuroendoscopic cyst wall fenestration and opening of the walls of isolated chambers into a single chamber that is drained by a shunt tube, have yielded encouraging results and prognosis in the form of decreasing the number of shunts and surgeries and decreasing the shunt revision rate.
The morbidity and mortality rates of neuroendoscopic surgery and procedures are much lower than traditional procedures and can be considered a valuable option to common multiple shunt implantation surgery and craniotomy in the management of post-infection hydrocephalus with multiple intraventricular septations.
Availability of data and materials
All data related for this study are available for sharing upon request.
Abbreviations
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- ECF:
-
Endoscopic cyst fenestration
- MR:
-
Magnetic resonance
- VPS:
-
Ventriculoperitoneal shunt
- EVD:
-
External ventricular drain
References
Gabbita AC, Raju S. Management of complex hydrocephalus. Neurol India. 2021;69:S2:350-S2:356.
Kalsbeck JE, Desousa AL, Kleiman MB, Goodman JM, Franken EA. Compartmentalization of the cerebral ventricles as a sequela of neonatal meningitis. J Neurosurg. 1980;52:547–52.
Schultz P, Leeds NE. Intraventricular septations complicating neonatal meningitis. J Neurosurg. 1973;38:620–6.
Andresen M, Juhler M. Multiloculated hydrocephalus: a review of current problems in classification and treatment. Childs Nerv Syst. 2012;28:357–62.
Akbari SH, Holekamp TF, Murphy TM, Mercer D, Leonard JR, Smyth MD, Park TS, Limbrick DD Jr. Surgical management of complex multiloculated hydrocephalus in infants and children. Childs Nerv Syst. 2015;31(2):243–9.
David I. Sandberg: endoscopic management of hydrocephalus in pediatric patients: a review of indications, techniques, and outcomes. J Child Neurol. 2008;23(5):550–60.
El-Tantawy MH. Endoscopic fenestration of multiloculated post-infection hydrocephalus. Med J Cairo Univ. 2018;86:2995–3002.
Eshra MA. Endoscopic management of septated, multiloculated hydrocephalus. Alexandria J Med. 2014;50:123–6.
Lewis AI, Keiper GL, Crone KR. Endoscopic treatment of multiloculated hydrocephalus. J Neurosurg. 1995;82:780–5.
Spennato P, Cinalli G, Carannante G, Ruggiero C, Del ML. Multiloculated hydrocephalus. In: Cinalli G, Maixner WJ, Sainte-Rose C, editors. Pediatric Hydrocephalus. Milan: Springer; 2004. p. 219–44.
Berman PH, Bamker BQ. Neonatal meningitis A clinical and pathological study. Pediatrics. 1966;38:6–24.
El-Ghandour NM. Endoscopic cyst fenestration in the treatment of multiloculated hydrocephalus in children. J Neurosurg Pediatr. 2008;1(3):217–22.
Morgenlander JC. Lumbar puncture and CSF examination: answers to three commonly asked questions. Postgrad Med. 1994;95(8):125–31.
Seehusen D, Reeves M, Fomin D. Cerebrospinal fluid analysis. Am Fam Phys. 2003;68(6):1103–8.
Lee YH, Kwon YS, Yang KH. Multiloculated hydrocephalus: open craniotomy or endoscopy. J Korean Neurosurg Soc. 2017;60(3):301–5.
Sandberg DI, Mccomb G, Kreiger MD. Craniotomy for fenestration of multiloculated hydrocephalus in pediatric patients. Neurosurgery. 2005;57(1):100–6.
Lewis AI, Keiper GL, Crone KR. Endoscopic treatment of multiloculated hydrocephalus. J Neurosurg. 1995;82:780–5.
Zuccaro G, Ramos JG. Multiloculated hydrocephalus. Childs Nerv Syst. 2011;27:1609–19.
Oi S, Hidaka M, Honda Y, Togo K, Shinoda M, Shimoda M, Tsugane R, Sato O. Neuroendoscopic surgery for specific forms of hydrocephalus. Child Nerv Syst. 1999;15:56–68.
Teo C, Kadrian D, Hayhurst C. Endoscopic management of complex hydrocephalus. World Neurosurg. 2013;79:S21.e1-S21.e7.
Spennato P, Cinalli G, Ruggiero C, Aliberti F, Trischitta V, Cianciulli E, Maggi G. Neuroendoscopic treatment of multiloculated hydrocephalus in children. J Neurosurg. 2007;106(1 Suppl):29–35.
Peraio S, Amen MM, Ali NM, Zaher A, Mohamed Taha AN, Tamburrini G. Endoscopic Management of pediatric complex hydrocephalus. World Neurosurg. 2018;119:e482–90.
Elkheshin SE, Bebars M. Endoscopic treatment of complex multiloculated hydrocephalus in children, steps that may help to decrease revision rate. Surg Neurol Int. 2021;12:434.
Alojan AA, Alotaibi AR, Alalhareth HN, Alwadei AD, Ammar A. Management and outcome of post-infection multiloculated hydrocephalus: a case series. Saudi J Med Med Sci. 2021;9:261–6.
Kim SA, Letyagin GV, Danilin VE, Sysoeva AA, Rzaev JA, Moisak GI. The benefits of navigated neuro endoscopy in children with multiloculated hydrocephalus. Asian J Neurosurg. 2017;12:483–8.
Schultz P, Leeds NE. Intraventricular septations complicating neonatal meningitis. J Neurosurg. 1973;38:620–6.
Nowosławska E, Polis L, Kaniewska D, Mikołajczyk W, Krawczyk J, Szymański W, Zakrzewski K, Podciechowska J. Effectiveness of neuro endoscopic procedures in the treatment of complex compartmentalized hydrocephalus in children. Childs Nerv Syst. 2003;19(9):659–65.
Acknowledgements
This study was conducted by the authors and no funds, grants, or other support was received.
Funding
No funds or grants were provided for the study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, and read and approved the final manuscript for submission and publication. Contribution to the study was organized according to the: Conceptualization: MMA, MS, Methodology: IA, MS, Formal analysis and investigation: MMA, MS, MB, Writing—original draft preparation: MMA, MS, MB, Writing—review, and editing: MMA, MS, MB, Resources: AFK, IA, Supervision: MMohsen Amen, Mahmoud Saad, Mohamed Badran, Amr Farid Khalil.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This project was approved by the IRB of Mansoura University Faculty of Medicine (R.22.11.1933). This article does not contain any studies with human participants performed by any of the authors.
Consent for publication
All data and records of patients were approved for publications by authors and involved patients.
Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amen, M.M., Badran, M., Zaher, A. et al. The outcome of surgical management of post-infectious hydrocephalus with multiple intraventricular septations. Egypt J Neurosurg 38, 65 (2023). https://doi.org/10.1186/s41984-023-00245-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-023-00245-6