Abstract
Background
The main aim of surgical intervention for unstable sacral fractures is to obtain a solid construct across the lumbopelvic junction to allow for early mobilization. Both iliosacral screw fixation (ISF) and lumbopelvic fixation (LPF) are widely used surgical techniques used for treatment of unstable sacral fractures. Nevertheless, it is unclear whether one technique provides more favorable postoperative outcomes than the other.
Objective
To compare the three-year outcome of ISF versus LPF in patients with unstable sacral fractures as regard effectiveness and safety of both techniques.
Methods
The study included 54 patients with sacral fractures who underwent sacral fusion using either ISF or LPF at a single institution. Patients were followed up for at least 3 years. Operative and postoperative data were collected and statistically calculated.
Results
Thirty patients were included in the ISF group and 24 patients in the LPF group. The operative time was notably higher in the LPF group (mean 107 min compared to 33 min in the ISF group; p = 0.002). Blood loss was also higher in the LPF group (mean 320 ml compared to 96 ml in the ISF; p = 0.004). Assessment of pelvic fusion was done via Majeed and Matta scores (pelvic fusion outcome scores). The ISF and LPF groups had a comparable Majeed score at the end of the third year of follow-up (excellent rate = 53.3% vs. 58.3%, respectively; p = 0.93). Likewise, ISF and LPF groups had comparable Matta score at the end of the third year of follow-up (excellent rate = 66.7% vs. 70.8%, respectively; p = 0.27). The most commonly reported postoperative complications in the ISF group were screw malposition in 2 cases out of 30 cases (6.6%) and non-union in 2 cases out of 30 cases (6.6%). On the other hand, the most commonly reported postoperative complications in the LPF group were implant prominence in 3 cases out of 24 cases (12.5%) and infection in 2 cases out of 24 cases (8.3%).
Conclusion
LPF and ISF have comparable safety and efficacy in patients with sacral fractures. ISF is an excellent and safe method of fixation, especially in old age to avoid open surgery-related complications. LPF is preferred in young active patients to benefit from rapid weight bearing after surgery and in cases with ambiguous sacral anatomy as sacral dysmorphism.
Similar content being viewed by others
Introduction
The sacrum transfers axial loads from the vertebral column to the pelvis. Below second sacral vertebra, the sacrum is not involved in spinal column support [1].
The sacrum, which serves as the keystone of the pelvic ring, is a common site for pelvic injuries [2, 3].
A significant portion of pelvic fractures are sacral fractures, which can occur alone in 5% of cases or concurrently with other pelvic ring injuries in up to 54% of cases [4]. Given the challenges in radiological assessment and the severity of the accompanying injuries in unconscious poly-traumatized patients, immediate physical examinations may miss more than 50% of sacral fractures [5].
Based on how a fracture affects the neuroforamen, Denis et al. categorized fractures into three zones. Zone I fractures are located lateral to the foramen; zone II fractures traveling through the foramen, and zone III fractures traveling medial to the foramen [6].
Isler classified Denis type-II fractures into subtypes A, B, and C, taking into account the fracture trait through the articular facet of L5–S1 [7].
The Denis zone III fractures are often divided into four types based on the work of Roy-Camille et al. The type I fracture is a flexion fracture with anterior angulation. The type II fracture is also a flexion fracture, with anterior angulation and posterior displacement of the upper fracture segment. Type III fractures are extension fractures with anterior displacement of the upper fracture segment. The type IV fracture is a comminuted fracture of the entire upper segment of the [8, 9].
Finally, fractures involving both sides of the sacrum can be classified as they resemble the letters of the Latin and Greek alphabets (U, T, H, and λ) [9] (Table 1).
The presence or absence of a neurological deficit is the most crucial prognostic factor in the treatment of sacral fractures [10].
Numerous studies recommend conservative treatment for sacral fractures because the surgical management of such fractures is unclear. However, other studies have taken in consideration that sacral fractures should be fixed in poly-trauma patients in order to prevent the systemic side effects and recumbency-related problems [10].
Stabilization procedures such as open lumbopelvic fixation using the classical iliac screws (LPF), local plate osteosynthesis, trans-iliac bars, and iliosacral screw fixation (ISF) have all been discussed in detail [11, 12]. Open lumbopelvic fixation and percutaneous iliosacral fixation are currently the most widely accepted and used techniques for fixing sacral fractures [13,14,15,16]. Biomechanically, LPF appears to be the best method since it transfers vertical loads directly from the lumbar spine to the iliac bone bypassing the sacrum [17, 18]. However, the extensive muscle and soft tissue dissection needed for accurate iliac screw insertion may increase the risk of potential devitalization of these tissues with subsequent increased infection risk [19, 20].
Although posterior open reduction and internal fixation techniques allow for neurovascular decompression and offer clear orientation of the fractured sacrum, they also come with a number of drawbacks, including blood loss and extended operative times. The percutaneous approach avoids these risks and allows for the rapid fixation of posterior pelvic or sacral pathologies in either supine or prone positions, while not interfering with central sacral decompression if indicated [21], but has a lower biomechanical stability when compared with the open LPF method [18, 22].
Minimally invasive percutaneous iliosacral fixation technique is associated with some reported complications like hardware screw failure, iatrogenic neural injury, misplaced screws and incomplete reductions [23].
Safety of ISF method cannot be guaranteed as anatomical screw placement can only be done if the surgeon is familiar with the complex sacral anatomy. As a result, several researchers have described navigation algorithms to improve screw placement accuracy [16, 24].
Few studies have been done on the clinical outcomes or recommended treatment for unstable sacral fractures [20, 25].
This study aims to compare the three-year outcomes of ISF versus LPF for management of patients with unstable sacral fractures.
Methods
The study included 54 patients with sacral fractures who underwent sacral fusion using either ISF or LPF at a single institution. Patients were followed up for at least 3 years. All patients were operated at the Neurosurgery Department of Alexandria University hospital.
Patients with suspected sacral fractures underwent detailed radiological investigations in the form of:
-
(1)
Radiographic examination of the pelvis, including anteroposterior, inlet, and outlet plain-X-ray views.
-
(2)
CT scans with three-dimensional reconstructions to further define the fracture, determine the safe zone for screw insertion in the percutaneous group, and rule out any congenital anomalies that could interfere with safe screw insertion.
-
(3)
MRI lumbosacral spine.
Classification of sacral fractures was done according to Dennis classification and Roy-Camille classification.
Physical examination, including a detailed neurologic evaluation, was done and documented in alert patients after arrival.
All patients provided written informed consent regarding the nature of the fracture and its co-morbidities, the nature of the procedure and type of anesthesia, the suspected length of hospital stay, and the procedure's risks and complications. The patients were given a detailed description of the fixation method, either percutaneous or open lumbopelvic.
Surgical technique was performed according to surgeon’s best knowledge and experience. According to our institute protocols, iliosacral screw fixation technique is preferred in cases with non-comminuted longitudinal fractures, transvers sacral fractures with acceptable closed reduction with a minimal displacement < 1 cm and after exclusion of sacral dysmorphism. On the over hand, lumbopelvic fixation is preferred in cases with severely comminuted fractures with severe neurological deficit, lumbosacral dysmorphism, cases with extended fracture line up to the L5–S1 facet and high transverse bilateral sacral fractures with failed accepted closed reduction.
Exclusion criteria
-
(1)
Osteoporotic sacral stress fractures.
-
(2)
Sacral fractures with unstable anterior pelvic ring disruptions.
-
(3)
Sacral dysmorphism was excluded from percutaneous iliosacral group.
-
(4)
Pathological metastatic sacral fractures.
-
(5)
Sacral fractures presented more than 2 weeks after injury.
-
(6)
Patients with isolated sacroiliac joint dislocations.
The aim was to compare the safety and efficacy of sacral fusion using the open method (LPF) versus the minimally invasive method (ISF).
Outcome measures Clinical outcome was assessed using Majeed pelvic score and Matta score. The Majeed score is a non-validated self-developed pelvic fracture-specific functional assessment instrument with a maximum of 100 points for patients who worked prior to injury and 80 points for those who did not work prior to injury. The score items are pain (30%), return to work (20%), sitting disturbances (10%), sexual impairments (4%) and walking ability (36%). The latter is subdivided into use of walking aids (12%), analysis of unaided gait (12%), and the walking distance (12%). A score of 100 points or 80 points is defined as the best result. Patients who worked before injury are graded as excellent with a score > 85, good with a score of 70–84, fair with a score of 55–69 and poor with a score. Patients who did not work before injury are graded into excellent, good, fair and poor with score values of > 70, 55–69, 45–54 and less than 45, respectively (Table 2) [26, 27].
Plain X-rays and three-dimensional computed tomography (3D-CT) of the lumbopelvic region were used to assess sacral fusion over a three-year period.
Safety of both procedures was assessed via analysis of complications associated with each procedure.
An approval from the research ethics committee of the Faculty of Medicine, Alexandria University (serial number 0305446), was obtained in January 2022. Patients’ consents for participation were obtained according to the institution's protocol.
Surgical technique
According to other comorbidities, the patient was transferred using spinal precautions and positioned supine (only applicable in the percutaneous iliosacral group) or prone (applicable in both groups) on a radiolucent operating table.
In the case of a supine position, one or two folded blankets were placed beneath the patient to elevate the patient from the bed, and the patient was kept at the table’s edge.
This resulted in slight extension and lordosis of the lower spine and pelvis, which aided in the reduction of displaced sacral fractures when combined with lower limb traction to achieve maximum reduction of the displaced sacral fracture. All patients underwent fixation under general anesthesia.
In the percutaneous iliosacral fixation group, a true lateral view of the pelvis, inlet, outlet, and anteroposterior views were ensured and marked on the C-arm, and adequate reduction of the displaced sacral fracture was confirmed (Figs. 1, 2, and 3).
Lateral fluoroscopic view of sacrum in prone position: to confirm starting entry point (A). True lateral view was ensured when two sciatic notches overlap with each other and end plates of S1 vertebra were also overlapped. Iliac cortical density (ICD) needed to be well defined for secure entry point for iliosacral screw fixation (B)
When two sciatic notches and the end plates of the S1 vertebra overlapped, a true lateral view was ensured. Iliac cortical density (ICD) had to be precisely defined in order to provide a secure entry point for iliosacral screw fixation.
When the anterior edges of S1 and S2 overlapped and the vertebral canal was well defined, a true inlet view was obtained. When the superior edge of the symphysis pubis overlapped S2, the true outlet view was confirmed [28].
On lateral view, the entry point for the sacral screw was confirmed. It should be placed beneath and behind ICD. In the case of an S1 iliosacral screw, the guide wire was superior to the S1 foramen in the outlet view, below the L5–S1 intervertebral disk space in the AP view, and within the S1 body in the inlet view. When our osseous trajectory pathway was S2, however, the guide wire was superior to the S2 foramen and inferior to the S1 foramen.
A stab wound was made near the iliac cortical density (ICD). A 6-mm Schanz screw, 4.5-mm cannulated screw driver, bayonet-tipped K-wire, and 4.5-mm cannulated drill bit were required to insert the screw.
A 6-mm Schanz screw was inserted through the stab wound until it came into contact with the outer iliac cortex caudal to the ICD and inferior to the first sacral disk space. The Schanz screw was then tapped for 1 cm with a hammer to form the fixed entry hole in the iliac table. The tip of a 4.5 cannulated screw driver (22.5 cm length) was inserted and settled well in the iliac table hole.
The screw driver was held firmly against this hole without slipping during the transition to the other three pelvic views (AP, outlet and inlet views). The cannulated screw driver was then passed through with a bayonet-tipped K-wire (1.8 mm width—7 cm length) until it reached the iliac cortex.
The three pelvic views guided the drilling of the K-wire. The screw driver was used to change the wire's orientation, allowing for upward and downward tuning in the outlet view and forward and backward tuning in the inlet view.
The wire’s passage was bounded by radiographic markers for the first sacral body (S1 body), which are the S1 foramen inferiorly and the L5–S1 intervertebral disk superiorly in the outlet view, the neural canal posteriorly, and the anterior cortex of the S1 segment anteriorly in the inlet view (Figs. 4 and 5).
The screw driver was removed after the wire had been passed to the desired length, leaving the wire in its track inside the bone. The screw length was determined at this stage by subtracting the portion of the wire outside the bone from the length of an identical wire, and the difference was equal to the screw length.
A 4.5-mm cannulated drill bit was used, and the drilling of the bone was guided by the three pelvic views to ensure that the K-wire was oriented correctly. For fixing, a 7-mm cannulated screw with a washer was used (Figs. 6, 7, 8, and 9).
The iliosacral fixation was performed using 1–3 screws whose lengths were based on morphological assessment.
The LPF was performed using bilateral pedicle screws inserted in lumbar vertebrae 5 (some cases lumbar vertebrae 4 and 5) and S2 alar-iliac screws. Following subperiosteal dissection of the paraspinal muscles, the conventional fluoroscopy-guided technique was used to insert lumbosacral pedicle screws. Further distal dissection along the sacrum showed the entrance location for S2 alar-iliac screws, and it should be lateral at any position between S1 and S2 dorsal foramina or in line with S1 pedicle screws. In order to prevent anterior pelvic wall penetration, the pedicle probe was moved toward the anterior inferior iliac spine while traversing the sacroiliac joint's hard surface. The AP view of the C-arm served as a guidance to prevent breaching into the acetabulum or the sciatic notch. The screw (usually ranges from 70 to 90 mm in adults) was then placed along the required route, and its location was checked by the C arm. Without the use of any side connectors, a rod was inserted to link the S2AI screw to the remaining lumbosacral construct.
All patients received routine postoperative care. Low molecular weight heparin and unfractionated heparin were used for thrombosis prevention. During follow-up visits, patients received a detailed assessment of the functional status and radiological evaluation using Majeed grading system and Matta criteria, respectively [26, 29].
Following the surgeon's judgments and suggestions, weight bearing was permitted. For most cases of percutaneous iliosacral group, three months of prolonged non-weight bearing was recommended. When tolerated, weight bearing was permitted for patients with lumbopelvic fixation. After starting weight bearing, a structured therapy was implemented that focused on conditioning, strengthening, dynamic lumbar stabilization and range of motion.
Data collection and follow-up All patients were assessed during hospital stay and follow-up visits to assess the following: operative time, intraoperative blood loss, intra- and postoperative complications and need for re-operation. Majeed functional scores and clinical grading were used for functional outcome taking into account of pain (30 points), return to work (20 points) sitting (10 points), sexual activity (4 points) and standing (walking aids; 12 points, gait; 12 points and walking distance; 12 points). Score > 85 was considered excellent, 70–84 good, 55–69 fair and < 55 poor [26]. The data records allowed for a three-year follow-up of the patients.
Statistical analysis Data were analyzed using the SPSS V0.25 software for Windows. We used frequencies to summarize categorical data, while continuous data were presented as median and range. The difference in the postoperative functional and radiological outcomes between the ISF and LPF was assessed using Mann–Whitney and Chi-square tests for continuous and categorical data, respectively. A two-sided p-value of less than 5% was considered statistically significant.
Results
Thirty patients were included in the ISF group and 24 patients in the LPF group. The median age of the patients was 36 (18–65) and 39 (18–65) years old in the ISF and LPF groups, respectively. Nearly half of the cases in the ISF group (n = 14; 46.7%) had Denis zone II, compared to 41.7% of the patients in the LPF group. Only 10% of the patients in the ISF group had bilateral vertical fracture and 33.3% had high transverse fracture, compared to 26.7% and 66.7% of the patients in the LPF group, respectively. The operative time (P = 0.002) and blood loss (P = 0.004) were notably higher in the LPF group (Tables 3, 4, and 5).
The pattern and incidence of neurological injuries before and after surgery were classified in both iliosacral group and lumbopelvic fixation group according to Gibbons classification system which demonstrated no significant changes between both groups before and after surgery (Tables 6 and 7).
The ISF and LPF groups had comparable Majeed score at the end of third year of follow-up (excellent rate = 53.3% vs. 58.3%, respectively; p = 0.93). Likewise, ISF and LPF groups had comparable Matta score at the end of third year of follow-up (excellent rate = 66.7% vs. 70.8%, respectively; p = 0.27). The most commonly reported postoperative complications in the ISF group were screw malposition and non-union (6.7% each). On the other hand, most commonly reported postoperative complications in the LPF group were Implant prominence (12.5%) and infection (8.3%), (Tables 8 and 9).
Some illustrative cases included in the study (Table 10)
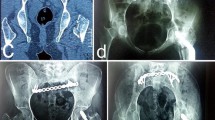
Illustrated case 1 (Fig. 10)
PXR sacrum demonstrating right sacral fracture with distortion of the right sacral foramina (a), CT scan demonstrating the displaced unstable vertical sacral fracture (b), coronal reconstruction CT scan of the sacral fracture (c), fluoroscopic pelvic outlet view after percutaneous fixation of the fracture with three unilateral iliosacral screws with good reduction of the displaced fracture (d), iliosacral screws in pelvic inlet view (e)
-
Thirty-three years aged obese male patient.
-
Mode of trauma is road traffic accident.
-
Zone 2 vertical displaced unstable sacral fracture.
-
Operated by unilateral three percutaneous iliosacral screws.
Illustrated case 2 (Fig. 11)
CT scan demonstrating left bicortical zone 1 unstable vertical sacral fracture (a), coronal reconstruction CT of the left displaced sacral fracture with sparing of the sacral foramina (b), final screw position in pelvic inlet view with good intraosseous trajectory (c), final screw position in pelvic outlet view (d), lateral fluoroscopic view of the inserted iliosacral view in S1 body (e), coronal postoperative CT scans with good reduction and alignment of the fracture (f), axial postoperative CT scans demonstrating the osseous trajectory of the iliosacral screw away from the central canal (g), follow-up postoperative CT scan 3 months postoperative showing good fracture healing with no problem-related screw detected (h), coronal CT scans 3 months postoperative showing good fracture healing with no problem-related screw-detected (i)
-
Twenty-five years aged female patient.
-
Mode of trauma is falling from height.
-
Zone 1 left vertical unstable sacral fracture.
-
Operated by unilateral single percutaneous iliosacral screw.
Illustrated case 3 (Fig. 12)
CT scan showing left displaced zone 1 sacral fracture (a), coronal reconstruction of the sacral fracture demonstrating lateral and upward displacement of the fracture (b), intraoperative pelvic outlet view demonstrating the inserted iliosacral screw in S1 body above S1 sacral foramen and the trajectory of S2 screw during the insertion between S1 and S2 sacral foramina (c), intraoperative pelvic inlet view demonstrating the osseous trajectory of the iliosacral screw inside S1 and S2 body away from the central canal (d), postoperative CT scans with coronal reconstruction showing perfect osseous trajectory of the inserted iliosacral screws and good fracture reduction (e), postoperative CT scans with sagittal reconstruction (f)
-
Forty-two years aged male patient
-
Mode of trauma is road traffic accident.
-
Left Zone 1 vertical sacral fracture.
-
Operated by unilateral double percutaneous iliosacral screws in first and second sacral vertebra.
Illustrated case 4 (Fig. 13)
Preoperative computed tomography demonstrating displaced vertical sacral fracture Denis type 2 (a), intraoperative fluoroscopic scans while introducing 2 guide wires in true pelvic inlet (b), lateral fluoroscopic view demonstrating the truly inserted guide wires below the iliac cortical density (c), fluoroscopic pelvic outlet view while inserting the first iliosacral screw (d)
-
Twenty years aged male patient.
-
Mode of trauma is falling from height.
-
(Zone 2) sacral fracture.
-
Operated by unilateral double iliosacral screws.
Illustrated case 5 (Fig. 14)
Preoperative computed tomography with coronal reconstruction showing bicortical Denis type 1 sacral fracture (a), fluoroscopic pelvic outlet view after introducing the guide wire followed by insertion of the iliosacral screw (b), lateral view of the iliosacral screw in the body of first sacral vertebra below the iliac cortical density (c), final screw position in the pelvic inlet view (d)
-
Fifty years aged male patient.
-
Mode of trauma is motor car accident.
-
(Zone 1) sacral fracture.
-
Operated by unilateral single iliosacral screw.
Illustrated case 6 (Fig. 15)
-
Thirty five years aged female patient.
-
Mode of trauma is falling from height.
-
Denis (type 3) and Roy-Camille (type 2) injury.
-
Operated by standard routine lumbopelvic fixation.
Discussion
Sacral fractures are uncommon sacral traumatic injuries that typically occur following spinal axial loading. Its diagnosis is difficult and is based primarily on clinical suspicion [6, 30].
For these unusual fractures, early reduction and fixation to achieve early mobilization is ideal. External fixation can improve results, but it is not as rigid as internal fixation [31].
Although the open fixation technique, with its posterior wide exposure, allows for sacral decompression of nerve roots and the central sacral canal, it still has a high complication rate. Many disadvantages of posterior open approaches include blood loss, infections, prolonged operating time, and prolonged prone positioning [32].
The percutaneous approach avoids these risks and allows for rapid fixation of posterior pelvic or sacral pathologies in either supine or prone positions, while not interfering with central sacral decompression if indicated [21].
In this study, the age of included patients in both groups was comparable; however, it was much younger than other studies. The mean age of patients in the study of Wenning et al. [33] was 62.2 ± 17.7 and 75.9 ± 14.0 in the LPF and ISF groups, respectively. Similarly, the mean age of patients in the LPF in our study was 38.5 versus 54.5 years in the ISF group [34]. These findings suggest that surgeons tend to less invasive procedures in elderly patients. Hopf et al. [35] evaluated ISF following osteoporotic posterior ring fractures of the pelvis in older patients and demonstrated satisfactory clinical outcomes with decreased intra- and postoperative complications. As a result, patients over the age of 65 who need bilateral sacral fixation may benefit more from ISF therapy if the increased operative time is a concern.
In this study, LPF group's operative time and blood losses were significantly higher, which is expected as ISF is considered a minimally invasive procedure. Likewise, Wenning et al. [33] highlighted that LPF was associated with longer operative time and hospital stay, compared with ISF (p < 0.001). In cases of U/H type sacral fractures, Kelly et al. [34] compared ISF with LPF. Because of the simultaneous decompression of the sacrum, those who had LPF experienced a much longer operative time. However, individuals with a high risk of pneumonia or other pulmonary complications may benefit from rapid weight bearing with LPF, despite the extended surgical time. Regarding blood loss, one of the main advantages of ISF is that it is less invasive and results in less soft tissue stress and blood loss. Nevertheless, patients were still unable to bear their full weight. Percutaneous ISF has been shown to be safe and effective in a number of investigations [36, 37].
The majority of complications associated with the percutaneous iliosacral screw technique result from poor orientation of the pelvic and sacral bony anatomy as well as a lack of understanding of the various pelvic fluoroscopic views. Incorrect screw placement can be dangerous and harmful to many vascular structures, such as gluteal vessels, as well as neural structures, particularly the fifth lumbar root and first sacral root. These risks are increased in cases of altered pelvic or sacral anatomy, such as sacral dysmorphism, and in cases of partially reduced or non-reduced sacral fractures [23].
In our study, screw malposition and non-union were the most frequently reported postoperative complications in the ISF group (6.7% each).
Iatrogenic injury to the lumbar plexus and S1 root as a result of the extra-osseous pathway is the most dangerous complication of the iliosacral screw. This injury was estimated to occur in between 0.5 and 7.7% of cases, while screw mal-positioning under fluoroscopic guidance was reported to occur in 2–15% of cases undergoing percutaneous iliosacral screw fixation [31, 38]. El-Desuoky et al. [39], on the other hand, stated that they had no screw malposition in their series because they began all screw placements by ensuring entry point in true lateral view. Electrophysiological monitoring is extremely beneficial in avoiding iatrogenic neural injury [40]. We could not use electrophysiological monitoring in our series, so we relied on careful and detailed evaluation of preoperative X-rays and CT scans to get a good understanding of the sacral anatomy and rule out any cases with a variant anatomy, such as sacral dysmorphism.
Implant prominence (12.5%) and infections (8.3%) were the most commonly reported postoperative complications in the LPF group. Our infection rates are slightly lower than the existing literature [41, 42]. Infection rates of up to 16% of patients following open LPF were reported by Bellabarba et al. [43], which is double our rate. Wenning et al. [33] showed that the incidence of infection was most common in patients with LPF compared to ISF (13.8% vs. 0%), p = 0.008.
Percutaneous ISF is thought to have lower infection rates than open procedures because it does not expose deep tissue to the outside environment. Routt et al. [23] reported no infections in his series of 177 iliosacral screw fixation operations. In this series, we had no infections in the iliosacral fixation group.
Neural injuries and abnormalities are common complications of sacral fractures, accounting for up to 85% of cases with displaced unstable sacral fractures. Unfortunately, the presence of associated lumbar fractures may augment the neurological injury of these sacral fractures. However, the role of surgical decompression in improving neurological status postoperatively is not well defined [30].
According to some studies, the incidence of neurological recovery is surprisingly similar whether surgical decompression is performed or not [44, 45].
In the study of Schweitzer and his colleagues, 95.6% of the patients who were treated with percutaneous ISF achieved excellent and good functional results [46]. Another study reported 76.2% of excellent and good functional results following ISF [28]. Amin et al. [47] reported 86% good-to-excellent functional results in patients with unstable pelvic ring injuries who were treated with percutaneous ISF. The variation in these findings could be attributed to the patients’ age, BMI, Denis classification, and surgeon skills. Regarding Matta functional scoring, 93.7% of the patients treated with ISF had a good-to-excellent score, compared to 91.6% in the LPF group, with no significant difference (p = 0.27). In a Chinese study, the authors reported 100% good-to-excellent score according to Matta score and 95% according to Majeed score following ISF [48].
We had similar results in our study, the proportion of patients who achieved an excellent and good score on Majeed grading score was 86.6% in the ISF group and 87.2% in the LPF group, with no significant difference (p = 0.93).
Study limitations The small numbers of the cohort group with a relatively short period of follow-up are the main limitations of this study.
Conclusions
LPF and ISF have comparable safety and efficacy in patients with sacral fractures. ISF is an excellent and safe method of fixation especially in old age to avoid open surgery-related complications. LPF is preferred in young active patients to benefit from rapid weight bearing after surgery and in cases with ambiguous sacral anatomy as sacral dysmorphism.
Availability of data and materials
All data used are available from the corresponding author on request.
Abbreviations
- ISF:
-
Iliosacral fixation
- LPF:
-
Lumbopelvic fixation
- ICD:
-
Iliac cortical density
References
Alkadhim M, Zoccali C, Abbasifard S, Avila MJ, Patel AS, Sattarov K, et al. The surgical vascular anatomy of the minimally invasive lateral lumbar interbody approach: a cadaveric and radiographic analysis. Eur Spine J. 2015;24(7):906–11.
Gordon WT, Fleming ME, Johnson AE, Gurney J, Shackelford S, Stockinger ZT. Pelvic fracture care. Mil Med. 2018;183(suppl_2):115–7.
DeRogatis MJ, Breceda AP, Lee P, Issack PS. Sacral fractures with spondylopelvic dissociation. JBJS reviews. 2018;6(5):e3.
Hak DJ, Baran S, Stahel P. Sacral fractures: current strategies in diagnosis and management. Orthopedics. 2009;32(10):752–7.
Hunt N, Jennings A, Smith M. Current management of U-shaped sacral fractures or spino-pelvic dissociation. Injury. 2002;33(2):123–6.
Denis F, Davis S, Comfort T. Sacral fractures: an important problem. Retrospective analysis of 236 cases. Clin Orthop Relat Res. 1988;227:67–81.
Vaccaro AR, Kim DH, Brodke DS, Harris M, Chapman J, Schildhauer T, et al. Diagnosis and management of sacral spine fractures. JBJS. 2004;86(1):166–75.
Benzel EC. Spine surgery 2-Vol set E-book: techniques, complication avoidance, and management (Expert Consult-Online). Elsevier Health Sciences; 2012.
Zoccali C, Skoch J, Patel AS, Walter CM, Avila MJ, Martirosyan NL, et al. The surgical anatomy of the lumbosacroiliac triangle: a cadaveric study. World neurosurgery. 2016;88:36–40.
Fountain S, Hamilton R, Jameson R. Transverse fractures of the sacrum. A report of six cases. J Bone Jt Surg Am Vol. 1977;59(4):486–9.
Griffin DR, Starr AJ, Reinert CM, Jones AL, Whitlock S. Vertically unstable pelvic fractures fixed with percutaneous iliosacral screws: does posterior injury pattern predict fixation failure? J Orthop Trauma. 2006;20(1):S30–6.
Dudda M, Hoffmann M, Schildhauer T. Sacrum fractures and lumbopelvic instabilities in pelvic ring injuries: classification and biomechanical aspects. Unfallchirurg. 2013;116(11):972–8.
Shetty AP, Renjith KR, Perumal R, Anand SV, Kanna RM, Rajasekaran S. Posterior stabilization of unstable sacral fractures: a single-center experience of percutaneous sacroiliac screw and lumbopelvic fixation in 67 cases. Asian Spine J. 2021;15(5):575.
Chen J, Fang Y, Walter MC, Yang Y, Yan X. Anterior subcutaneous internal fixation combined with posterior percutaneous iliosacral screw for treatment of unstable pelvic fractures. Chin J Repar Reconstr Surg. 2020;34(1):21–6.
Abou-Khalil S, Steinmetz S, Mustaki L, Leger B, Thein E, Borens O. Results of open reduction internal fixation versus percutaneous iliosacral screw fixation for unstable pelvic ring injuries: retrospective study of 36 patients. Eur J Orthop Surg Traumatol. 2020;30(5):877–84.
Shaw J, Gary J, Ambrose C, Routt MC. Multidimensional pelvic fluoroscopy: a new and novel technique for assessing safety and accuracy of percutaneous iliosacral screw fixation. J Orthop Trauma. 2020;34(11):572–7.
Schildhauer TA, Ledoux WR, Chapman JR, Henley MB, Tencer AF, Routt MC. Triangular osteosynthesis and iliosacral screw fixation for unstable sacral fractures: a cadaveric and biomechanical evaluation under cyclic loads. J Orthop Trauma. 2003;17(1):22–31.
Lu Y, He Y, Li W, Yang Z, Peng R, Yu L. Comparison of biomechanical performance of five different treatment approaches for fixing posterior pelvic ring injury. J Healthc Eng. 2020;22:2020.
Sagi HC, Militano U, Caron T, Lindvall E. A comprehensive analysis with minimum 1-year follow-up of vertically unstable transforaminal sacral fractures treated with triangular osteosynthesis. J Orthop Trauma. 2009;23(5):313–9.
Jones CB, Sietsema DL, Hoffmann MF. Can lumbopelvic fixation salvage unstable complex sacral fractures? Clin Orthop Relat Res®. 2012;470(8):2132–41.
Keating J. Vertically unstable pelvic fractures-the outcomes of iliosacral screw fixation of the posterior lesion. In Paper presented at the Annual Meeting of the Orthopaedic Association, 1994; 1994.
Tidwell J, Cho R, Reid JS, Boateng H, Copeland C, Sirlin E. Percutaneous sacroiliac screw technique. J Orthop Trauma. 2016;30:S19–20.
Routt MC Jr, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11(8):584–9.
Florio M, Capasso L, Olivi A, Vitiello C, Leone A, Liuzza F. 3D-navigated percutaneous screw fixation of pelvic ring injuries—a pilot study. Injury. 2020;51:S28–33.
Schildhauer TA, Bellabarba C, Nork SE, Barei DP, Routt MLC Jr, Chapman JR. Decompression and lumbopelvic fixation for sacral fracture-dislocations with spino-pelvic dissociation. J Orthop Trauma. 2006;20(7):447–57.
Majeed SA. Grading the outcome of pelvic fractures. J Bone Jt Surg Br Vol. 1989;71(2):304–6.
Matta JM, Saucedo T. Internal fixation of pelvic ring fractures. Clin Orthop Relat Res. 1989;242:83–97.
Shrestha D, Dhoju D, Shrestha R, Sharma V. Percutaneous ilio-sacral screw fixation in supine position under fluoroscopy guidance. Kathmandu Univ Med J. 2015;13(1):56–60.
Matta JM, Tornetta P III. Internal fixation of unstable pelvic ring injuries. Clin Orthop Relat Res (1976–2007). 1996;329:129–40.
Gibbons KJ, Soloniuk DS, Razack N. Neurological injury and patterns of sacral fractures. J Neurosurg. 1990;72(6):889–93.
Tonetti J. Management of recent unstable fractures of the pelvic ring. An update conference supported by the Club Bassin Cotyle (Pelvis-Acetabulum Club). Orthop Traumatol Surg Res. 2013;99(1):S77–86.
Kellam J, McMurtry R, Paley D, Tile M. The unstable pelvic fracture. Operative treatment. Orthop Clin N Am. 1987;18(1):25–41.
Wenning KE, Yilmaz E, Schildhauer TA, Hoffmann MF. Comparison of lumbopelvic fixation and iliosacral screw fixation for the treatment of bilateral sacral fractures. J Orthop Surg Res. 2021;16(1):1–8.
Kelly M, Zhang J, Humphrey CA, Gorczyca JT, Mesfin A. Surgical management of U/H type sacral fractures: outcomes following iliosacral and lumbopelvic fixation. J Spine Surg. 2018;4(2):361.
Hopf JC, Krieglstein CF, Müller LP, Koslowsky TC. Percutaneous iliosacral screw fixation after osteoporotic posterior ring fractures of the pelvis reduces pain significantly in elderly patients. Injury. 2015;46(8):1631–6.
Shuler TE, Boone DC, Gruen GS, Peitzman AB. Percutaneous iliosacral screw fixation: early treatment for unstable posterior pelvic ring disruptions. J Trauma Acute Care Surg. 1995;38(3):453–8.
Chen P-H, Hsu W-H, Li Y-Y, Huang T-W, Huang T-J, Peng K-T. Outcome analysis of unstable posterior ring injury of the pelvis: comparison between percutaneous iliosacral screw fixation and conservative treatment. Biomed J. 2013;36(6).
van den Bosch EW, van Zwienen CMA, van Vugt AB. Fluoroscopic positioning of sacroiliac screws in 88 patients. J Trauma Acute Care Surg. 2002;53(1):44–8.
El-Desouky II, Mohamed MM, Kandil AE. Percutaneous iliosacral screw fixation in vertically unstable pelvic injuries, a refined conventional method. Acta Orthop Belg. 2016;82(1):52–9.
Gardner MJ, Farrell ED, Nork SE, Segina DN, Routt Jr MLC. Percutaneous placement of iliosacral screws without electrodiagnostic monitoring. J Trauma Acute Care Surg. 2009;66(5):1411–5.
König M, Jehan S, Boszczyk A, Boszczyk B. Surgical management of U-shaped sacral fractures: a systematic review of current treatment strategies. Eur Spine J. 2012;21(5):829–36.
Elzohairy M, Salama A. Open reduction internal fixation versus percutaneous iliosacral screw fixation for unstable posterior pelvic ring disruptions. Orthop Traumatol Surg Res. 2017;103(2):223–7.
Bellabarba C, Schildhauer TA, Vaccaro AR, Chapman JR. Complications associated with surgical stabilization of high-grade sacral fracture dislocations with spino-pelvic instability. Spine. 2006;31(11S):S80–8.
Kempen D, Delawi D, Altena M, Kruyt M, van den Bekerom M, Oner F, et al. Neurological outcome after traumatic transverse sacral fractures: a systematic review of 521 patients reported in the literature. JBJS reviews. 2018;6(6):e1.
Kepler CK, Schroeder GD, Hollern DA, Chapman JR, Fehlings MG, Dvorak M, et al. Do formal laminectomy and timing of decompression for patients with sacral fracture and neurologic deficit affect outcome? J Orthop Trauma. 2017;31:S75–80.
Schweitzer D, Zylberberg A, Córdova M, Gonzalez J. Closed reduction and iliosacral percutaneous fixation of unstable pelvic ring fractures. Injury. 2008;39(8):869–74.
Amin MS, Habib MK, Khalid A. Percutaneous ilio-sacral screw fixation for unstable pelvic ring injuries. J Pak Med Assoc. 2016;66(Suppl 3):S112–5.
Li S, Liu Z, Li J, Ren J, Sun T. Treatment of vertical unstable pelvic fracture by percutaneous iliosacral screws fixation. Zhongguo gu Shang China J Orthop Traumatol. 2011;24(2):116–8.
Acknowledgements
Not applicable.
Funding
This study was not funded by any source.
Author information
Authors and Affiliations
Contributions
IS designed the study and wrote the initial manuscript. MAE assisted in the final preparation of the manuscript. AEE has participated in the final revision of the manuscript and added two more cases operated by the same technique. All authors have contributed to this study and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An approval from the research ethics committee of the Faculty of Medicine, Alexandria University (serial number 0305446), was obtained in January 2022. Patients’ consents for participation were obtained according to the institution's protocol.
Consent for publication
Not applicable.
Competing interests
The authors had no competing interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elhabashy, A.M., Fayed, A.A. & Sorour, I. Comparative study between open lumbopelvic fixation and percutaneous iliosacral fixation for management of sacral fractures. Egypt J Neurosurg 38, 41 (2023). https://doi.org/10.1186/s41984-023-00221-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-023-00221-0