Abstract
Alzheimer’s disease (AD) is a neurodegenerative condition that causes cognitive decline, memory loss, and reduced personal autonomy. The pathology of AD involves the aggregation of abnormal brain proteins, specifically beta-amyloid plaques and tau tangles, disrupting neuronal communication and leading to the loss of brain cells. Aducanumab, a monoclonal antibody, demonstrates promise in clinical trials by selectively binding to aggregated amyloid-beta, leading to a notable decrease in plaque burden and potential cognitive benefits. However, regulatory approval for aducanumab remains controversial. Lecanemab and donanemab are recent additions to the AD’s treatment landscape, both targeting aggregated amyloid-beta. Lecanemab shares similarities with aducanumab in its mechanism of action, while donanemab employs a distinct approach by binding to a specific truncated form of amyloid-beta. Positive outcomes have been observed in early-stage clinical trials for both drugs, demonstrating a reduction in amyloid-beta plaques. While aducanumab’s approval offers hope for AD’s treatment, ongoing studies on lecanemab and donanemab are imperative for a comprehensive understanding of their potential in disease modification. Here, we show in this review the potential AD treatments, with a focus on their primary action targeting the reduction of amyloid-beta plaques ultimately giving a broader insight on the topic. The review emphasizes the necessity for long-term efficacy and safety data to assess the overall impact of these drugs on cognitive decline and functional outcomes for future researchers to endeavor. In conclusion, the development of amyloid-beta targeting monoclonal antibodies represents a significant stride in AD’s treatment, demanding further investigation to ascertain their true potential and role in the therapeutic arsenal for this challenging condition.
Similar content being viewed by others
Understanding Alzheimer’s disease: advances and approaches in treatment
Alzheimer’s disease (AD), a profound neurodegenerative condition, leads to memory impairment, cognitive regression, and a marked erosion of personal autonomy. Alzheimer’s disease (AD) and Parkinson’s disease (PD) stand as the predominant neurodegenerative conditions. AD accounts for roughly 60–70% of the approximately 50 million individuals globally afflicted by dementia, a significant contributor to disability and dependency among older adults. PD affects over 10 million people worldwide, with its occurrence becoming more common as individuals age [1]. Multiple sclerosis (MS), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) are among the other conditions that can lead to neurodegeneration. Alzheimer’s disease (AD) is a neurological condition associated primarily with aging and is marked by a gradual decline in cognitive abilities. Dementia, a broader term, encompasses various conditions resulting from illnesses or brain injuries that affect memory, thinking, and behavior, disrupting daily life. The progression of AD occurs as brain cells deteriorate and brain function slows down. When identified early, it may be referred to as early-onset AD. While there are medications available to slow the progression of AD, no cure has been found for the disease [2]. It places a substantial burden on society and families, making it a prominent focal point of extensive research aiming to understand its causes, clinical manifestations, underlying mechanisms, prevalence, and therapeutic options.
AD stands as the predominant form of dementia, a term that refers to significant reduction in cognitive activity to the extent that it hinders an individual’s ability to perform everyday tasks, constituting a minimum of two-thirds of dementia cases in individuals aged 65 and above. Early onset, occurring before 65 years of age, is uncommon and observed in less than 10% of AD patients [3].
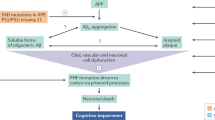
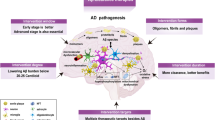
While the precise pathogenesis of AD is not fully understood, it is associated with the aggregation of aberrant brain proteins, particularly beta-amyloid plaques and tau tangles, disrupting neuronal communication and causing loss of brain cells which ultimately results in the characteristic cognitive decline observed in the disease [4]. AD a predominant chronic neurodegenerative condition, characterized by a subtle onset of progressive cytokine dysfunctions, primarily affecting memory initially. However, as the disease advances, it can lead to motor, sensory, and autonomic dysfunctions in later stages [5]. The decline in processing contextual information and the regulation of responses to threat observed with aging are linked to changes in structure and function within the prefrontal cortex (PFC) and the medial temporal lobe [6]. Positron emission tomography using 2-deoxy-2-[fluorine-18] fluoro-D-glucose has revealed a deficiency of central nodes within the theory of mind network in individuals diagnosed with mild cognitive impairment attributable to Alzheimer’s disease [7]. Brain autopsies and imaging examinations demonstrate brain atrophy affecting regions, such as the frontal, temporal, and parietal cortices, along with the entorhinal cortices, amygdala, and hippocampus [8]. The presence of amyloid beta (Aβ) peptide and tau protein deposition is a notable feature observed not only in Alzheimer’s disease but also in other conditions [9].
Alzheimer’s disease (AD) is now understood as a neurodegenerative disorder characterized by a prolonged trajectory that commences silently many years before symptoms manifest. Its progression is gradual and consistent, eventually leading to the impairment of cognitive function. AD predominantly affects older individuals [10]. Neuropsychiatric symptoms (NPS) are prevalent among individuals with Alzheimer’s disease (AD), impacting as many as 97% of patients and affecting approximately 80% of current AD cases. These symptoms encompass a range of issues, including depression, anxiety, agitation, aggression, and apathy. The severity of these symptoms in AD is often correlated with the advancement of the disease and the degree of cognitive deterioration experienced by the individual [11].
Numerous changes and alterations have been observed in different regions of the brain, including enlarged ventricles, shrinkage of the cerebral cortex and hippocampus, and the formation of NFTs and Aβ plaques. Figure 1 illustrates these changes in the Alzheimer’s brain. These alterations involve variations in the hippocampal area, cerebral cortex, ventricles, and neuronal structures.
Presently, there are roughly 50 million individuals worldwide grappling with AD, and forecasts suggest that this figure will multiply every 5 years, reaching an estimated 152 million by 2050. The repercussions of AD extend to individuals, their families, and the economy, with global costs estimated at around US$1 trillion annually. As of now, a remedy for AD remains elusive, although there are available interventions designed to enhance symptoms [12, 13].
Traditionally, drugs such as galantamine, rivastigmine, and donepezil have been mainstays in Alzheimer’s disease (AD) treatment, primarily addressing symptomatic relief. However, recent advancements in AD therapy have shifted towards monoclonal antibodies, offering potential benefits. Given the intricate nature of AD’s development, there is a growing understanding that medications targeting diverse molecular pathways and disease-modifying therapies may offer superior treatment outcomes. Notably, Aducanumab, an amyloid-beta-directed monoclonal antibody, has received FDA approval for treating moderate phases of AD and mild cognitive impairment (MCI). However, caution must be exercised, as it is contraindicated for individuals with varying stages of AD, vascular dementia, and Lewy body dementia [14]. While disease-modifying treatments for AD are unavailable, early diagnosis and effective management can significantly improve patients’ and caregivers’ quality of life. Approved drugs primarily target cognitive, behavioral, and functional symptoms, such as cholinesterase inhibitors (ChEIs) and N-methyl-D-aspartate receptor antagonist memantine. Typically, mild-to-moderate AD patients start with a ChEI, possibly adding memantine for progressing symptoms. Recent efforts aim to develop therapies to slow AD’s progression. However, many treatments failed in advanced AD trials, suggesting the need for disease-modifying agents earlier in the disease stage [15]. Aducanumab is an amyloid-beta-directed monoclonal antibody, while Lecanemab is the first fully approved drug to slow down AD. For moderate phases of AD and mild cognitive impairment (MCI), aducanumab has FDA approval. It should not be given to those with mild, moderate, or severe stages of Alzheimer’s disease (AD), as well as those with vascular dementia and Lewy body dementia [14]. Aducanumab targets and reduces beta-amyloid plaques in the brain. Aducanumab is currently only prescribed to individuals in the initial phases of the disease, specifically those who display mild neurological symptoms [16].
Other than Aducanumab, newly developed medications, such as Lecanemab and Donanemab, are also being investigated as potential therapies for AD.
Mechanism underlying Alzheimer’s disease
The distinctive neuropathological features of AD include presence of amyloid plaques and cerebral amyloid angiopathy, neurofibrillary tangles, as well as glial reactions, loss of neurons and synapses [17]. The amyloid cascade hypothesis, which suggests an imbalance between the production and clearance of proteins, is widely accepted in AD research [18].
Amyloid precursor protein (APP), in normal circumstances, when cleaved by β-secretase and γ-secretase, leads to the production of beta-amyloid (Aβ) fragments and amyloidogenic processes [19]. An abnormal precursor processing leads to the formation of extracellular senile plaque lesions due to the accumulation of Aβ40 and Aβ42. Aβ42 is found in higher concentrations as compared to Aβ40 [18]. At the cellular level within the brain affected by AD, a coordinated interplay of factors, including mitochondrial dysfunction, oxidative stress, aberrant buildup of proteins, such as Aβ42 and tau leading to toxic effects, and the inflammatory response initiated by microglia, collectively contribute to the pathogenesis of AD [20].
Diagnostic precision by biomarkers in AD
Amyloid beta peptides: the peptide amyloid-beta (Aβ) in cerebrospinal fluid (CSF) is increasingly used as a biomarker for amyloid pathology in clinical trials and in recently proposed revised clinical criteria for AD disease [21]. Total tau protein: CSF t-tau seems a more dynamic biomarker, which reflects the intensity of both acute neuronal damage and chronic neuronal degeneration in the brain, with high levels of CSF t-tau associated with faster progression from MCI to AD, more rapid cognitive decline and higher mortality rate among AD patients [22]. Both t-tau and Aβ42 are significantly altered in MCI subjects who are at increased risk of AD over time [23]. Hyperphosphorylated tau protein: Tau protein in a healthy brain contains 2–3 mol of phosphate per mole of the protein. In AD tau protein is hyperphosphorylated to at least three times the normal amount, and in this altered state, it is aggregated into paired helical filaments forming neurofibrillary tangles, which are a hallmark of the disease. This abnormal hyperphosphorylation of tau is also observed in other neurodegenerative disorders called tauopathies. The density of neurofibrillary tangles in the neocortex is correlated with dementia and is a rational therapeutic target [24]. Figure 2 shows Alzheimer’s biomarkers: Aβ42 (40%) is the most significant, while CSF t-tau and hyperphosphorylated tau each represent 30% of the analysis.
Exploring parameters of aducanumab: a promising advancement in Alzheimer’s disease treatment
It is a human monoclonal antibody designed to selectively target Amyloid β aggregates [25]. Aducanumab infusions are done monthly and require approximately one hour to complete. Infusions should be at least 21 days apart [16]. In a study involving the escalation of aducanumab doses for individuals with mild-to-moderate AD, conducted under placebo control, symptomatic ARIA-E manifested in three out of 53 participants [26]. The maximum dose of 60 mg/kg aducanumab was administered to all three individuals, with one of them being ApoE4 homozygous. Symptomatic ARIA-E occurred in these cases, while no ARIA was observed at doses below 30 mg/kg. The onset of ARIA was first identified through MRI or clinical assessments between days 22 and 31, and in two instances, resolution on MRI occurred within 28 days. However, in a more severe case, encompassing both ARIA-E and ARIA-H, resolution took 84 days. In a separate investigation involving a single patient experiencing severe symptomatic ARIA-E and ARIA-H, symptoms were linked to malignant hypertension and epileptiform activity. This patient showed improvement following intravenous methylprednisolone (pulse) and levetiracetam treatment [27]. There are several adverse effects, including ARIA edema, ARIA-H deposition microhemorrhage, ARIA-H superficial siderosis, headache, falls, diarrhea, confusion/delirium/altered mental status disorientation, and hypersensitivity reactions [28]. Aducanumab was subjected to subsequent trials (EMERGE and ENGAGE), and while ENGAGE did not reveal a positive effect in comparison with the placebo, EMERGE revealed amyloid reduction and was supported by an ad hoc analysis [29] Favorable pharmacokinetic parameters have been identified for aducanumab.
Mechanism of action of aducanumab
Aducanumab, as an anti-Aβ monoclonal antibody, reduces plaque deposition by specifically targeting both aggregated insoluble and soluble forms of Aβ.[30] It attaches to Aβ oligomers (ABO) and amyloid plaques in the brain even at low concentrations, triggering microglia activation for the removal of amyloid species. Throughout phase 1b (PRIME) and phase 3 studies, aducanumab has consistently shown a notable decrease in Aβ plaques, with the reduction correlating with the treatment dose and duration [16, 31]. The effectiveness of aducanumab was assessed across three distinct studies involving a total of 3482 participants. These trials encompassed dose-ranging investigations in individuals with AD, featuring placebo control, double-blind methodologies, and randomization [32].
Previous monoclonal antibodies (mABs), such as solanezumab, crenazumab, and bapinuezemab, did not achieve the primary efficacy goals in studies focused on symptomatic AD [33, 34].
Aducanumab is the inaugural monoclonal antibody to establish a direct correlation between the removal of amyloid and a deceleration in the progression of cognitive impairment [35].
Exploring aducanumab (an in-depth analysis of clinical trials)
The approval of the initial disease-targeted anti-amyloid β-drug has the potential to raise the expectations of individuals dealing with AD. The FDA’s determination regarding the endorsement of aducanumab holds significant weight for Alzheimer’s patients and their families, potentially intensifying the expectation for healthcare providers to prescribe this medication. Consequently, it becomes crucial to pinpoint the appropriate candidates who are more likely to experience benefits from this drug. Ensuring the optimal efficacy and safety of the medication requires a meticulous selection process for patients eligible for long-term monthly infusion. Prior to the initiation of the drug, it is essential to conduct a recent MRI and a PET scan for visualizing the density of β-amyloid aggregates [36].
In preclinical mouse investigations, the aducanumab analog exhibited varying impacts on Aß plaques. Acute administration resulted in a noteworthy ~ 48% decrease, surpassing the ~ 14% reduction observed in control mice (P < 0.0001) [37]. The analog also exhibited promise in reinstating neuronal calcium regulation, mitigating calcium overload (P < 0.05), enhancing NMDA receptor calcium permeability (P < 0.05), and normalizing the sarco-endoplasmic reticulum calcium ATPase (SERCA) pump (P < 0.001) [37, 38].
Phase I trials involving 53 participants demonstrated Aducanumab’s safety up to 30 mg/kg, with ARIA-E as the primary side effect [39]. Subsequent Phase II investigations (Prime) disclosed reductions in Aß plaques and cognitive benefits at 12 months, albeit with an escalated ARIA-E risk at higher doses (10 mg/kg) [26].
Phase III trials (Emerge and Engage) encompassing 3290 participants with Mild Cognitive Impairment (MCI) or mild AD yielded positive outcomes. High-dose Aducanumab significantly diminished Aß plaques and ameliorated cognitive and functional metrics [34, 40]. Post hoc analyses validated reductions in Aß plaque size, with both low and high doses manifesting statistically significant drops in PET SUVR [41,42,43].
The Emerge trial underscored Aducanumab’s potential to decelerate cognitive and functional deterioration in mild AD patients, unveiling marked enhancements in neuropsychiatric symptoms and reduced caregiver distress. These findings emphasize Aducanumab’s potential for enhancing cognitive and functional facets in AD patients [44].
Imaging protocol guidance for aducanumab administration
An MRI, including specific sequences, such as T1, T2, FLAIR, T2* GRE (or SWI), and DWI, taken within a year before aducanumab treatment, helps monitor ARIA risk. A 3-Tesla MRI reveals more microhemorrhages, while SWI detects more ARIA compared to GRE images. Consistent MRI devices and protocols are recommended for comparison. CT scans do not provide sufficient data for ARIA risk assessment. Patients’ ineligible for MRI should avoid aducanumab if they have certain medical implants or devices [44].
Table 1 outlines the criteria for Aducanumab use in Alzheimer’s disease treatment, including patient age, diagnosis, mental status, amyloid pathology, genetics, and medical history. These guidelines help clinicians select candidates for therapy, aiming to improve outcomes and reduce risks. The table underscores the importance of detailed assessments in clinical decision-making.
Dosage and administration of aducanumab
Aducanumab is available in the pharmaceutical market as transparent, opalescent, and a colorless to yellow solution, in the form of single-dose vials designed to be administered intravenously. Appropriate dosage of aducanumab is subjected to the patient’s body weight, with the recommendation of using 100 mL of 0.9% sodium chloride injection to dilute the drug before commencing of infusion. Moreover, it is suggested to let the diluted medication equate with room temperature prior to initiating the therapy with prompt administration and avoiding unnecessary delays. Any remaining diluted portions of the medication following the administration process should be disposed of properly.
Table 2 displays the dosages for Aducanumab single dose vials in mg/mL, supporting flexible administration tailored to patient body weight and treatment stage. It serves as a reference for clinicians to prepare and administer Aducanumab infusions for Alzheimer’s disease, ensuring dosage accuracy and protocol adherence.
Table 3 details the Aducanumab infusion dosing regimen for Alzheimer’s disease, showing dose escalation from the first to the seventh infusion. Doses are expressed in mg/kg, adjusted for patient weight, to balance therapeutic benefits with monitoring for adverse effects such as amyloid-related imaging abnormalities (ARIA), essential for safe and effective treatment.
Dose-missed scenarios
In instances where patients have experienced interruptions in their aducanumab dosing, it is suggested to promptly resume treatment with the next scheduled dose, observing the prior infusion’s dosage level. In circumstances where subjects have missed three or more consecutive infusions and intend to continue aducanumab therapy, it is advisable to recommence the infusion regimen at a dose one level lower than the previous one administered [46].
Adverse effects
Therapeutic effects of Aducanumab can be associated with several adverse effects. These include ARIA edema, ARIA-H microhemorrhage (19%), ARIA-H superficial siderosis (15%), headache (21%), falls (15%), diarrhea (9%), confusion/delirium/altered mental status/disorientation (8%), hypersensitivity reactions (angioedema, urticaria) (< 1%), and immunogenicity (< 1%) [26, 28].
Toxicity
ARIA-E can manifest as brain edema or sulcal effusions, while ARIA-H can be observed as microhemorrhage and superficial siderosis on brain imaging. Clinical symptoms of ARIA were present in about 24% of subjects during clinical studies, with headache being the most common manifestation (13%) [47].
Hypersensitivity
Prompt discontinuation of aducanumab and appropriate management should be initiated in patients with a possibility of hypersensitivity reactions, such as angioedema and urticarial [33].
Concomitant medications
New anti-amyloid antibodies, such as aducanumab and donanemab, reduce Alzheimer’s-related brain amyloid but carry a risk of amyloid-related imaging abnormalities (ARIA), including mortalities due to edema (ARIA-E) and hemorrhage (ARIA-H).
Lecanemab
Lecanemab, a humanized IgG1 iteration of the murine antibody mAb158, specifically addresses soluble Aβ protofibrils and retains efficacy against insoluble fibrils. These Aβ protofibrils, characterized as substantial and soluble aggregates, contribute to neurotoxicity by disrupting the electrophysiological systems crucial for memory function [48]. Although clinical trials have indicated that lecanemab is superior to a placebo in reducing Aβ burden in individuals with early-stage AD, there have been no direct comparisons between lecanemab and other medications for AD [49]. In 21.5% of treated individuals, lecanemab was linked to amyloid-related imaging abnormalities (ARIAs), encompassing both edema and microhemorrhage types [50] According to the findings of a study(phase 2 Study 201), the incidence of ARIA‐E in the lecanemab (10 mg/kg biweekly, intravenous [IV]) occurred at a relatively low rate (< 10%; symptomatic rate: < 3%). ARIA was generally mild‐to‐moderate, occurred early (< 3 months), was higher in apolipoprotein E4 (ApoE4) carriers, and was correlated with maximum lecanemab concentrations at steady state. These findings support lecanemab initiation at 10 mg/kg biweekly without titration [51]. High-dose lecanemab administration was halted for ApoE4 carriers, comprising 70% of the study population, complicating result interpretation and potentially underestimating ARIA risk. Notably, ARIA rates with lecanemab were relatively lower than other antibodies, with a < 10% incidence of asymptomatic ARIA-E, occurring early and generally of mild-to-moderate severity [52].
Donanemab
Donanemab is a humanized antibody that targets the reduction of Aβ plaques in AD patients [53] It aims to clear pGlu-Aβ after formation and/or block aggregation, specifically targeting the N-truncated pyroglutamate amyloid-β peptide at position 3 (pGlu3-Aβ, AβpE3). The antibody has been shown to have strong action with amyloid plaques, particularly cored plaques in the central nervous system [54]. Preliminary evidence suggests that Donanemab may delay cognitive and functional decline in patients with mild-to-moderate AD, the treatment regimen, comprising 700 mg for the first 3 doses and 1,400 mg every 4 weeks for 72 weeks, resulted in a 26.7% ARIA incidence, with 6.1% being symptomatic ARIA-E cases. ARIA-E, generally asymptomatic and self-resolving, showed a higher risk in homozygous ApoE4 individuals. Despite the development of anti-drug antibodies in 90% of subjects, donanemab exhibited improved cognition and daily living activities in early AD patients compared to the placebo at 76 weeks. However, longer and larger trials are imperative for a comprehensive evaluation of donanemab’s efficacy and safety in AD [47].
Discussion
The extensive review of Alzheimer’s disease (AD) and its current therapeutic landscape sets the stage for a robust discussion on the potential of emerging monoclonal antibodies such as aducanumab, lecanemab, and donanemab in AD treatment. These antibodies represent novel approaches aimed at targeting different facets of AD pathology, offering promise but also presenting challenges and limitations that must be carefully considered. The evidence supporting the feasibility of disease modification in drug development programs will pave the way for advancing therapies targeting various aspects of Alzheimer’s disease (AD) pathophysiology. Beyond amyloid, potential therapeutic targets include tau biology, neuroinflammation, neuronal metabolism and energetics, oxidative stress, proteostasis, autophagy, vascular features of AD, epigenetic changes, apolipoprotein E and lipid abnormalities, synaptic plasticity, neuroprotection, and hormonal features of AD. Notably, reductions in plasma and CSF p-tau and total tau following amyloid plaque removal suggest that tau-related changes may play a crucial role in the response to anti-amyloid monoclonal antibodies (MABs), encouraging the exploration of treatments directly targeting tau biology. Moreover, the activation of microglia by anti-amyloid MABs to remove amyloid suggests that targeting these cells could be a viable therapeutic strategy. The success in achieving disease modification by removing amyloid plaques is likely to inspire the development of drugs targeting aggregated proteins in other neurodegenerative diseases, such as tau proteins in tauopathies, alpha-synuclein protein in Parkinson’s disease, and TAR DNA-binding protein 43 (TDP43) in amyotrophic lateral sclerosis and frontotemporal dementia.
The significance of epitope specificity in determining the efficacy of antibodies targeting Aβ is underscored by studies demonstrating that antibodies directed against specific N-terminal epitopes, notably the marginal 11 epitopes, exhibit notable efficacy in inducing plaque clearance, while those targeting a C-terminal epitope prove ineffective in mitigating amyloid burden or neuritic pathology. These findings challenge conventional assumptions, suggesting that capturing soluble Aβ may not be necessary for reducing neuritic pathology. Furthermore, investigations into the interplay between IgG subclass and efficacy reveal that IgG2 antibodies, with a higher affinity for Fcγ receptors, demonstrate superior efficacy in reducing plaque burden compared to IgG1, emphasizing the critical role of Fc receptors in the phagocytic activity of microglia. These insights offer valuable guidance for the development of more targeted and effective therapeutic interventions for Aβ-related conditions, emphasizing the importance of epitope specificity and Fc-mediated plaque clearance in antibody design and evaluation [55]. Figure 3 illustrates understanding of antibody efficacy in Alzheimer’s therapy, emphasizing the need for targeted approaches based on epitope specificity and antibody subclass characteristics.
These antibodies target various aspects of AD pathology, ranging from amyloid-beta plaque reduction to soluble Aβ protofibril clearance, holding promise for disease modification and cognitive preservation. Aducanumab, with its FDA approval for treating early stages of AD and demonstrated efficacy in reducing amyloid plaques, represents a significant advancement in AD therapy. However, challenges related to adverse effects, particularly ARIA, underscore the need for careful risk management strategies and patient selection criteria. Lecanemab, targeting soluble Aβ protofibrils, presents an alternative approach with a potentially lower risk of ARIA compared to aducanumab. Initial studies have shown promise in reducing Aβ burden in early-stage AD, but further research is needed to establish its efficacy and safety profile, especially in individuals with genetic risk factors such as ApoE4. It’s noteworthy that aducanumab has emerged as the initial newly sanctioned medication for prodromal and mild Alzheimer’s disease (AD) by the FDA since memantine’s introduction in 2003 [56, 57].
The development of lecanemab represents a step forward in diversifying AD treatment options and underscores the importance of exploring multiple therapeutic targets. An 18-month observation in a study investigating amyloid levels, a group of individuals received treatment by lecanemab [50]. Following 18 months of treatment, the average amyloid level in this group was found to be 22.99 centiloids. Centiloids are used as a unit of measurement to quantify amyloid levels in the brain.
The observation indicates that the mean amyloid level of 22.99 centiloids in the lecanemab group fell below the threshold for amyloid positivity, which is approximately 30 centiloids. This implies that, on average, the participants who received lecanemab had amyloid levels below the threshold considered indicative of elevated brain amyloid levels. Put simply, the treatment appeared to be effective in reducing or impeding amyloid buildup in the brain among these participants [50, 58,59,60,61,62].
These findings hold significance because lecanemab may hold the potential to decelerate or modify the progression of the disease. However, it is crucial to interpret these results with caution, as they are derived from observations within a specific study. Additional research is typically necessary to validate and gain further insights into these findings.
Donanemab, targeting pGlu3-Aβ, has shown preliminary evidence of cognitive and functional benefits in mild-to-moderate AD patients. In a randomized phase 2 trial conducted on patients with early symptomatic Alzheimer’s disease, the administration of donanemab demonstrated a modest reduction in cognitive and functional decline when compared to a placebo [47]. Although the treatment showed some efficacy, it did not achieve the anticipated goal of slowing disease progression by half, which was initially assumed during the power calculation for the trial. In addition, the use of donanemab led to the emergence of ARIA.
Around 25% of participants in the donanemab group experienced ARIA-E (Amyloid-Related Imaging Abnormalities-Effusions), with 6.1% of them reporting symptomatic ARIA-E. Notably, a higher occurrence of ARIA-E was observed among individuals who carried the APOE ε4 allele, which aligns with findings from other trials involving antibodies that target plaques [16, 33, 47, 63, 64].
Overall, while the phase 2 trial showed promising results in terms of reducing cognitive and functional decline, the inability to achieve the predetermined goal of halving disease progression and the emergence of imaging abnormalities underscore the necessity for further research. Longer and more extensive trials will provide a more comprehensive understanding of donanemab’s efficacy, safety, and overall impact on patients with early symptomatic Alzheimer’s disease.
In the phase 3 trial [64], donanemab showed notable clinical benefits that were considered to be meaningful in terms of slowing clinical progression by more than 20% [65, 66]. These benefits were observed across both the low/medium tau and combined populations, regardless of the statistical model used for analysis. The positive effects of donanemab were evident when assessing patients using the iADRS (Integrated Alzheimer’s Disease Rating Scale) and CDR–SB (Clinical Dementia Rating–Sum of Boxes) scales.
Furthermore, additional evidence supporting the clinical relevance of donanemab was found in the form of a 38.6% reduction in the risk of disease progression, as measured by the CDR-G (Clinical Dementia Rating-Global) score. The study also estimated that participants in the low/medium tau population who received donanemab experienced a time benefit of 4.4 to 7.5 months saved over the 18-month duration of the study.
An interesting finding from the trial was that approximately 47% of participants who received donanemab showed no change in the CDR–SB score at the 1-year mark, indicating no disease progression. In comparison, only 29% of participants who received the placebo demonstrated the same lack of progression.
These findings strongly suggest that donanemab treatment has a clinically meaningful impact in slowing the progression of the disease, as evidenced by improvements on multiple assessment scales and measures. The results indicate a clear benefit in terms of cognitive and functional decline, reduced risk of disease progression, and the potential for prolonged stability in patients receiving donanemab.
The research outcomes concerning monoclonal antibodies such as aducanumab, lecanemab, and donanemab carry significant implications for the field of Alzheimer’s disease (AD) treatment. They emphasize the necessity of personalized medical strategies customized according to the unique genetic and clinical characteristics of each patient. Furthermore, they emphasize the ongoing requirement for refining the criteria used to select patients, optimizing the schedules for administering doses, and investigating combinations of therapies to improve treatment effectiveness. By addressing these challenges and leveraging insights gained from previous studies, this avenue of investigation holds promise for enhancing outcomes for individuals grappling with AD and edging closer to developing impactful disease-modifying therapies.
Despite the promise of monoclonal antibodies, translating these findings into clinical practice poses significant challenges. The high cost of treatment, logistical complexities associated with intravenous infusion, and the need for specialized healthcare infrastructure present formidable hurdles. Furthermore, the complex interplay of genetic, environmental, and lifestyle factors contributing to AD pathogenesis necessitates a comprehensive and multidisciplinary approach to treatment.
While monoclonal antibodies such as aducanumab, lecanemab, and donanemab offer hope for advancing AD therapy, their successful integration into clinical practice requires addressing existing challenges and harnessing the full potential of precision medicine. By overcoming these obstacles and building upon the foundation of previous research, this line of investigation holds promise for improving outcomes for individuals affected by AD and moving closer to the development of effective disease-modifying treatments. Passive immunization stands out as one of the most dynamic domains in Alzheimer’s disease (AD) research, offering considerable potential as a disease-modifying treatment for AD [67].
Conclusion
The emergence of new therapeutic agents such as Aducanumab, Lecanemab, and Donanemab for Alzheimer’s disease (AD) offers promising prospects for managing this debilitating condition. Aducanumab, the first approved disease-targeted anti-amyloid β-drug, has demonstrated efficacy in reducing amyloid plaques and decelerating cognitive decline in mild AD patients. However, its use is associated with adverse effects, particularly ARIA, necessitating careful patient selection and monitoring. Lecanemab and Donanemab also show potential in reducing Aβ burden, but their efficacy in improving cognitive function and safety profiles require further investigation.
The development of these therapeutic agents underscores the importance of understanding the underlying mechanisms of AD, particularly the amyloid cascade hypothesis. It highlights the need for refining theoretical models and methodological approaches to better predict treatment responses and adverse effects. Moreover, the success of these agents in early-stage AD emphasizes the importance of early diagnosis and intervention.
Future research should focus on identifying reliable biomarkers for early detection of AD, developing strategies to mitigate adverse effects, and exploring combination therapies to enhance treatment efficacy. In addition, there is a need to understand the long-term effects of these agents and their impact on disease progression. Addressing these knowledge gaps will contribute to the development of more effective and safer treatments for AD, ultimately improving the quality of life for patients and their families.
Data availability
Data sharing is not applicable to this article as no datasets were generated during the current study.
Abbreviations
- AD:
-
Alzheimer’s disease
- APP:
-
Amyloid precursor protein
- ChEIs:
-
Cholinesterase inhibitors
- Aβ:
-
Beta-amyloid
- ARIA-H:
-
Amyloid-related imaging abnormalities-hemosiderin
- ARIA-E:
-
Amyloid-related imaging abnormalities-edema
- IgG1:
-
Immunoglobulin gamma 1
- NFT:
-
Neurofibrillary tangle
- SERCA:
-
Sarco endoplasmic reticulum calcium ATPase pump
- MCI:
-
Mild cognitive impairment
- PET:
-
Positron emission tomography
- SUVR:
-
Standardized uptake value ratio
- MRI:
-
Magnetic resonance imaging
- FLAIR:
-
Fluid-attenuated inversion recovery
- GRE:
-
Gradient echo
- SWI:
-
Susceptibility-weighted imaging
- DWI:
-
Diffusion-weighted imaging
- CT scans:
-
Computed tomography
- FDA:
-
Food and drug administration
- CSF:
-
Cerebrospinal fluid
- MMSE:
-
Mini Mental State Examination
- ApoE4:
-
Apolipoprotein E4
- pGlu-Aβ:
-
Pyroglutamate Aβ
- AβpE3:
-
Pyroglutamate-modified amyloid-β
References
Chin JH, Vora N. The global burden of neurologic diseases. Neurology. 2014;83(4):349–51.
Cummings J, Lee G, Zhong K, Fonseca J, Taghva K. Alzheimer’s disease drug development pipeline: 2021. Alzheimer’s Dement Transl Res Clin Intervent. 2021;7(1): e12179.
Mendez MF. Early-onset Alzheimer disease. Neurol Clin. 2017;35(2):263–81.
Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82(4):756–71.
Dias FL, Silva RM, Moraes EN, Caramelli P. Clinical and autonomic profile of patients with Alzheimer’s disease and mixed dementia patients. Rev Assoc Med Bras (1992). 2013;59(5):435–41.
Battaglia S, Garofalo S, di Pellegrino G. Context-dependent extinction of threat memories: influences of healthy aging. Sci Rep. 2018;8(1):12592.
Orso B, Lorenzini L, Arnaldi D, Girtler N, Brugnolo A, Doglione E, et al. The role of hub and spoke regions in theory of mind in early Alzheimer’s disease and frontotemporal dementia. Biomedicines. 2022;10(3):544.
DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):32.
Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2017;9(7): a028035.
Lloret A, Esteve D, Lloret MA, Cervera-Ferri A, Lopez B, Nepomuceno M, et al. When does Alzheimer’s disease really start? The role of biomarkers. Focus (Am Psychiatr Publ). 2021;19(3):355–64.
Pless A, Ware D, Saggu S, Rehman H, Morgan J, Wang Q. Understanding neuropsychiatric symptoms in Alzheimer’s disease: challenges and advances in diagnosis and treatment. Front Neurosci. 2023;17:1263771.
Yiannopoulou KG, Papageorgiou SG. Current and future treatments in Alzheimer disease: an update. J Central Nerv Syst Dis. 2020;12:1179573520907397.
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413–46.
Hazegh Fetratjoo D, Kargar A, Noroozian M. Aducanumab: an uprising hope with vague horizons. Egypt J Neurol Psychiatry Neurosurg. 2023;59(1):85.
Grossberg GT, Tong G, Burke AD, Tariot PN. Present algorithms and future treatments for Alzheimer’s disease. J Alzheimers Dis. 2019;67(4):1157–71.
Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–6.
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1): a006189.
Yiannopoulou KG, Papageorgiou SG. Current and future treatments for Alzheimer’s disease. Ther Adv Neurol Disord. 2013;6(1):19–33.
Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discovery. 2004;3(3):205–14.
Goel P, Chakrabarti S, Goel K, Bhutani K, Chopra T, Bali S. Neuronal cell death mechanisms in Alzheimer’s disease: an insight. Front Mol Neurosci. 2022;15: 937133.
Pannee J, Portelius E, Minthon L, Gobom J, Andreasson U, Zetterberg H, et al. Reference measurement procedure for CSF amyloid beta (Aβ) 1–42 and the CSF Aβ1–42/Aβ1–40 ratio–a cross-validation study against amyloid PET. J Neurochem. 2016;139(4):651–8.
Blennow K, Dubois B, Fagan AM, Lewczuk P, De Leon MJ, Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimers Dement. 2015;11(1):58–69.
Hampel H, Teipel SJ, Fuchsberger T, Andreasen N, Wiltfang J, Otto M, et al. Value of CSF β-amyloid1–42 and tau as predictors of Alzheimer’s disease in patients with mild cognitive impairment. Mol Psychiatry. 2004;9(7):705–10.
Wang J-Z, Xia Y-Y, Grundke-Iqbal I, Iqbal K. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimers Dis. 2013;33(s1):S123–39.
Arndt JW, Qian F, Smith BA, Quan C, Kilambi KP, Bush MW, et al. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β. Sci Rep. 2018;8(1):6412.
Ferrero J, Williams L, Stella H, Leitermann K, Mikulskis A, O’Gorman J, et al. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer’s disease. Alzheimer’s Dement Transl Res Clin Intervent. 2016;2(3):169–76.
VandeVrede L, Gibbs DM, Koestler M, La Joie R, Ljubenkov PA, Provost K, et al. Symptomatic amyloid-related imaging abnormalities in an APOE ε4/ε4 patient treated with aducanumab. Alzheimer’s Dement Diagn Assess Dis Monit. 2020;12(1): e12101.
Salloway S, Chalkias S, Barkhof F, Burkett P, Barakos J, Purcell D, et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 2022;79(1):13–21.
Coerver K, Yu MM, D’Abreu A, Wasserman M, Nair KV. Practical considerations in the administration of aducanumab for the neurologist. Neurol Clin Pract. 2022;12(2):169–75.
Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15(2):73–88.
Budd Haeberlein S, Aisen P, Barkhof F, Chalkias S, Chen T, Cohen S, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimer’s Dis. 2022;9(2):197–210.
Canady VA. FDA approves first drug therapy for Alzheimer’s in 18 years. Ment Heal Wkly. 2021;31(23):3–4.
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):322–33.
Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med. 2018;378(4):321–30.
Sabbagh MN, Cummings J. Open Peer Commentary to “Failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE Trials as reported by Biogen December 2019.” Alzheimer’s Dement. 2021;17(4):702.
Hameed S, Fuh J-L, Senanarong V, Ebenezer EGM, Looi I, Dominguez JC, et al. Role of fluid biomarkers and PET imaging in early diagnosis and its clinical implication in the management of Alzheimer’s disease. J Alzheimer’s Dis Rep. 2020;4(1):21–37.
Kastanenka KV, Bussiere T, Shakerdge N, Qian F, Weinreb PH, Rhodes K, et al. Immunotherapy with aducanumab restores calcium homeostasis in Tg2576 mice. J Neurosci. 2016;36(50):12549–58.
Gamage KK, Kumar S. Aducanumab therapy ameliorates calcium overload in a mouse model of Alzheimer’s disease. J Neurosci. 2017;37(17):4430–2.
Wojtunik-Kulesza K, Rudkowska M, Orzeł-Sajdłowska A. Aducanumab—hope or disappointment for Alzheimer’s disease. Int J Mol Sci. 2023;24(5):4367.
Tian Hui Kwan A, Arfaie S, Therriault J, Rosa-Neto P, Gauthier S. Lessons learnt from the second generation of anti-amyloid monoclonal antibodies clinical trials. Dement Geriatr Cogn Disord. 2021;49(4):334–48.
Avgerinos KI, Ferrucci L, Kapogiannis D. Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: a systematic review and meta-analysis of phase III RCTs in Alzheimer’s disease. Ageing Res Rev. 2021;68: 101339.
Howard R, Liu KY. Questions EMERGE as Biogen claims aducanumab turnaround. Nat Rev Neurol. 2020;16(2):63–4.
Knopman DS, Jones DT, Greicius MD. Failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimer’s Dement. 2021;17(4):696–701.
Cummings J, Aisen P, Lemere C, Atri A, Sabbagh M, Salloway S. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimer’s Res Ther. 2021;13(1):98.
Schneider L. A resurrection of aducanumab for Alzheimer’s disease. Lancet Neurol. 2020;19(2):111–2.
Cummings J, Rabinovici G, Atri A, Aisen P, Apostolova L, Hendrix S, et al. Aducanumab: appropriate use recommendations update. J Prev Alzheimer’s Dis. 2022;9(2):221–30.
Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691–704.
Lord A, Gumucio A, Englund H, Sehlin D, Sundquist VS, Söderberg L, et al. An amyloid-β protofibril-selective antibody prevents amyloid formation in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;36(3):425–34.
Chowdhury S, Chowdhury NS. Novel anti-amyloid-beta (Aβ) monoclonal antibody lecanemab for Alzheimer’s disease: a systematic review. Int J Immunopathol Pharmacol. 2023;37:03946320231209839.
Van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21.
Honig LS, Barakos J, Dhadda S, Kanekiyo M, Reyderman L, Irizarry M, et al. ARIA in patients treated with lecanemab (BAN2401) in a phase 2 study in early Alzheimer’s disease. Alzheimer’s Dementia Transl Res Clin Intervent. 2023;9(1): e12377.
Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RY, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res Ther. 2021;13:1–14.
DeMattos RB, Lu J, Tang Y, Racke MM, DeLong CA, Tzaferis JA, et al. A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer’s disease mice. Neuron. 2012;76(5):908–20.
Bayer TA. Pyroglutamate Aβ cascade as drug target in Alzheimer’s disease. Mol Psychiatry. 2022;27(4):1880–5.
Neațu M, Covaliu A, Ioniță I, Jugurt A, Davidescu EI, Popescu BO. Monoclonal antibody therapy in Alzheimer’s disease. Pharmaceutics. 2023;16(1):60.
Song C, Shi J, Zhang P, Zhang Y, Xu J, Zhao L, et al. Immunotherapy for Alzheimer’s disease: targeting β-amyloid and beyond. Transl Neurodegener. 2022;11(1):18.
Haddad HW, Malone GW, Comardelle NJ, Degueure AE, Kaye AM, Kaye AD. Aducanumab, a novel anti-amyloid monoclonal antibody, for the treatment of Alzheimer’s disease: a comprehensive review. Health Psychol Res. 2022;10(1).
Tucker S, Möller C, Tegerstedt K, Lord A, Laudon H, Sjödahl J, et al. The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J Alzheimers Dis. 2015;43(2):575–88.
Magnusson K, Sehlin D, Syvänen S, Svedberg MM, Philipson O, Söderberg L, et al. Specific uptake of an amyloid-β protofibril-binding antibody-tracer in AβPP transgenic mouse brain. J Alzheimers Dis. 2013;37(1):29–40.
Söderberg L, Johannesson M, Nygren P, Laudon H, Eriksson F, Osswald G, et al. Lecanemab, aducanumab, and gantenerumab—binding profiles to different forms of amyloid-beta might explain efficacy and side effects in clinical trials for Alzheimer’s disease. Neurotherapeutics. 2023;20(1):195–206.
Lublin AL, Gandy S. Amyloid-β oligomers: possible roles as key neurotoxins in Alzheimer’s disease. Mount Sinai J Med. 2010;77(1):43–9.
Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–12.
Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11(3):241–9.
Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512–27.
Vellas B, Andrieu S, Sampaio C, Wilcock G. Disease-modifying trials in Alzheimer’s disease: a European task force consensus. The Lancet Neurology. 2007;6(1):56–62.
Abushakra S, Porsteinsson A, Vellas B, Cummings J, Gauthier S, Hey J, et al. Clinical benefits of tramiprosate in Alzheimer’s disease are associated with higher number of APOE4 alleles: the “APOE4 Gene-Dose Effect.” J Prev Alzheimers Dis. 2016;3(4):219–28.
Zhang G, Wang Z, Hu H, Zhao M, Sun L. Microglia in Alzheimer’s disease: a target for therapeutic intervention. Front Cell Neurosci. 2021;15: 749587.
Acknowledgements
Not applicable.
Compliance with instruction to authors
We hereby affirm that this manuscript has been meticulously prepared in strict accordance with all prescribed instructions provided to the authors.
Authorship confirmation and approval
We confirm that the authorship requirements have been diligently met, and the final version of the manuscript has been unanimously approved by all contributing authors.
Publication status
We certify that this manuscript is entirely original and has not been published previously, nor is it currently under consideration by any other journal.
Reporting checklist
We followed the PRISMA Guidelines for this review.
Ethical consideration
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Funding
The authors received no extramural funding for the study.
Author information
Authors and Affiliations
Contributions
TBA did the conceptualization, SNK and AR conducted the literature and drafting, the editing and supervision was performed by KB, SMIA, SMSA. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests and there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ameen, T.B., Kashif, S.N., Abbas, S.M.I. et al. Unraveling Alzheimer’s: the promise of aducanumab, lecanemab, and donanemab. Egypt J Neurol Psychiatry Neurosurg 60, 72 (2024). https://doi.org/10.1186/s41983-024-00845-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-024-00845-5