Abstract
Background
Critical illness myopathy (CIM) has negative impact on patient outcomes. We aimed to explore the diagnostic value of bedside ultrasonography for early identification of CIM in septic patients and its correlation with other diagnostic methods. This prospective observational study included 40 ICU patients diagnosed with sepsis on admission or within 48 h later according to the third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). They were evaluated using muscle ultrasound, electrodiagnostic and clinical muscle assessment (Medical Research Council, MRC) at two time points, the first was between days 2 and 5 and the second was between days 10 and 15.
Results
There was significant deterioration of neuromuscular function between the two evaluation points demonstrated by decline in MRC, abnormal nerve conduction and electromyography (EMG) and increased muscle echogenicity on ultrasonography (P ≤ 0.001). Sepsis-Related Organ Failure Assessment (SOFA) score significantly correlated with different neuromuscular assessment tools. MRC had significant correlation with myopathic EMG (P ≤ 0.001, r = − 0.869) and increased muscle echogenicity (P ≤ 0.001, r = − 0.715). Abnormal ultrasonographic muscle architecture had sensitivity of 100%, specificity of 75% and positive likelihood ratio of 4 in detecting muscle dysfunction compared to myopathic EMG.
Conclusions
Bedside peripheral muscle ultrasound echogenicity grade could be used as an additional screening test in ICU septic patients for early detection of CIM.
Similar content being viewed by others
Background
Intensive care unit acquired weakness (ICUAW) is a major health problem that have negative impact on outcome and long-term functioning. ICUAW is mostly caused by neuromuscular dysfunction which may presents in different forms: critical illness polyneuropathy (CIP), critical illness myopathy (CIM) or co-occurrence of both entities (critical illness neuromyopathy, CINM) [1].
The incidence of ICUAW was reported in literature to range from about 30 to 80% in ICU critically ill patients [1,2,3]. This wide variation of incidence is related to the presence, number and severity of underlying risk factors, the timing of neuromuscular evaluation and the used diagnostic approach [4].
The most common risk factors associated with ICUAW are sepsis, shock, multiorgan failure, mechanical ventilation, hyperglycemia and myotoxic drugs [4].
CIM could be more common and presents earlier than CIP [5]. It presents with flaccid paresis often involving respiratory muscles and usually associated with atrophy of muscles [6]. There is large controversy regarding the optimum diagnostic and assessment tools with reported several limitations. Clinical examination requires co-operative patients, has large inter-observer variation and is time consuming [1].
Complete electrodiagnostic assessment is expensive, moderately invasive, operator-dependent, limited by edema, coagulopathy, electrical interference and full EMG examination requires alert and co-operative patients [1].
Muscle biopsy is invasive, expensive, painful, requires technical expertise and pathological interpretation and has risk of bleeding and infection [1].
Qualitative and quantitative muscle ultrasound is increasingly used to assess muscle atrophy and architecture changes in critically ill patients, however, needs further research to explore its diagnostic accuracy, functional significance, and its correlation with clinical and electrodiagnostic findings before implementation in routine practice.
Methods
This prospective observational study included 40 patients admitted to the critical care department of Beni-Suef university hospitals during the period from June 2021 till April 2022. The study protocol was approved from the local ethical committee of faculty of Medicine, Beni-Suef university and an informed written consent was obtained from the patient next of kin of all participants before enrolment in the study. The included patients were diagnosed on admission or within 48 h later as having sepsis or septic shock according to the third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [7] without affecting conscious level to allow clinical and EMG assessment. Patients with acute condition like acute myocardial infarction, post-cardiac surgery or with history of neuromuscular disorders were excluded from the study.
Clinical assessment included history taking, complete general and neurological examination, in addition to routine laboratory workup: complete blood count, coagulation profile, arterial blood gases, liver and kidney function tests. Organ dysfunction and severity of illness was evaluated using Sepsis-Related Organ Failure Assessment (SOFA) score [8] at two time points following onset of sepsis or septic shock, the first evaluation (between days 2 and 5) and the second evaluation (between days 10 and 15). SOFA score is a clinical evaluation of different physiologic parameters, each scored from 0 (normal function) to 4 (organ failure). These parameters represent different body functions: Respiration (PaO2:FiO2), coagulation (platelets count), liver (bilirubin concentration), cardiovascular (mean arterial pressure), central nervous system (Glasgow coma score) and renal (creatinine level or urine output). Each parameter is scored individually, after which a total score is derived to reflect severity of illness and organ dysfunction [8]. Medical Research Council (MRC) score was carried out at the same two time points following onset of sepsis or septic shock. MRC is a clinical test to measure muscle strength through evaluation of six movements (upper limbs: wrist flexion, forearm flexion and shoulder abduction, lower limbs: ankle dorsiflexion, knee extension and hip flexion) over both sides. Each movement is scored from 0 (no movement) to 5 (active movement against full resistance). Total score is 60 and ICUAW is defined based on clinical grounds as MRC total sum score < 48 [9].
With regard to electrophysiological assessment, all patients were evaluated at the same two time intervals using portable TruTrace Electromyography device (TruTrace NCS/EMG/EP, Deymed Diagnostic s.r.o., Czech Republic). Electrodiagnostic studies included nerve conduction studies (NCS) using surface recording and stimulating electrodes according to the standard electrode positions and distances mentioned in Preston and Shapiro [10], and with room temperature kept at (28–33C) and ensuring well defined and artifact-free responses. Filter settings were as follows: (10 Hz– kHz for motor studies, 20 Hz–2 kHz for sensory studies).
The studied nerves were bilateral ulnar and median nerves (sensory and motor responses) in upper limbs and bilateral common peroneal, posterior tibial (motor responses), and sural nerves (sensory responses) in lower limbs.
The patient was considered to have abnormal NCS when there are two or more abnormal peripheral nerve motor and sensory responses exceeding lower limit of normal values for amplitudes or conduction velocities according to Preston and Shapiro [10, 11].
Moreover, electromyography (EMG) was performed using disposable concentric EMG needle electrodes (0.45 mm diameter, 50 mm length, Technomed). Filter settings were 20 Hz–5 kHz. The muscles selected for analysis were bilateral deltoid, biceps brachii and extensor digitorum communis in upper limbs, tibialis anterior and quadriceps in lower limbs. The patient was counted to have myopathic EMG when at least two of the tested muscles in upper or lower limbs showed myopathic changes (motor unit action potentials of short duration, small amplitude, highly polyphasic and early recruitment pattern), detected either by visual analysis in clear cut findings or by retrograde automatic analysis in borderline cases (MUAP duration < 5 ms, number of phases > 4) [10, 11].
Ultrasonography assessment was conducted twice at the same time intervals using Mindray Dp 20 machine with a 9 to 13 MHz probe real-time linear array scanner. The initial settings were 10 MHz frequency and 49 gain with variable depth, which may be altered individually to visualize the complete muscle. All ultrasound studies were done by one operator trained in musculo-skeletal ultrasound.
Patients were examined in the supine position with extended arms and legs and relaxed muscles. The muscles selected for analysis were bilateral biceps brachii and forearm extensors in upper limbs, tibialis anterior and quadriceps in lower limbs.
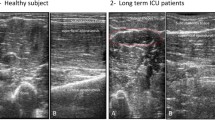
Ultrasonic echogenicity was graded according to Heckmatt and colleagues. This score differentiates ultrasonic echogenicity semi quantitatively into four grades: Grade I: normal, Grade II: increased muscle echo intensity with distinct bone echo, Grade III: marked increased muscle echo with reduced bone echo, Grade IV: very strong muscle echo and complete loss of bone echo. Total echogenicity grade was calculated as the sum of the tested eight muscles grades. The patient was considered to have abnormal U/S when at least two of the tested muscles in upper or lower limb muscles had ultrasonic echogenicity of grades (II–IV) according to the scale of Heckmatt and colleagues [12]
Data were analyzed using SPSS (statistical package for the social science software) Version 25.0. Quantitative variables were expressed by mean and standard deviation or by median and interquartile range (IQR) (as appropriate). Paired data were compared using paired t-test or Wilcoxon test (as appropriate).
Qualitative variables were expressed by number and percent and paired data were compared by McNemar test. Pearson correlation was used to correlate two continuous variables, otherwise Spearman correlation was used. Diagnostic and accuracy tests were computed using MedCalc version (14.8.1). In all tests, P-value was considered significant if less than 0.05.
Results
The patient group included 40 patients with sepsis or septic shock (31 males, their mean age was 57.3 ± 11.4 years).
There was gradual progressive decline of the neuromuscular function assessed by clinical, electrophysiological and ultrasonographic tools (Table 1).
All abnormal nerve conduction values of the patient group were denoting axonal pathology with predominantly small amplitudes and mildly reduced conduction velocities. None of the patients had nerve conduction values (distal latencies and conduction velocities) of demyelinating peripheral nerve disorder (Table 2).
Quadriceps, tibialis anterior and biceps brachii are the most sensitive muscles to show both electrophysiological and ultrasonographic changes in evaluation of myopathy of ICU septic patients (Tables 3, 4, 5 and Fig. 1).
The SOFA score at 1st evaluation was correlated with MRC (P = 0.011, r = − 0.417) and ultrasonographic echogenicity grade (P ≤ 0.001, r = 0.599) at 2nd evaluation (Fig. 2).
The SOFA score at 2nd evaluation was correlated with MRC (P ≤ 0.001, r = − 0.555) and abnormal nerve conduction results (P ≤ 0.001, r = 0.604) at 2nd evaluation.
The total MRC score was correlated with abnormal electrophysiological results and ultrasonographic echogenicity grade at both evaluation time points (Table 6).
Regarding diagnostic accuracy of ultrasonography to detect muscle changes in critically ill patients, it was as sensitive as EMG (Tables 7 and 8).
Discussion
This prospective study was designed to explore the diagnostic accuracy of bedside ultrasonography in assessment of critical illness myopathy in ICU septic patients and to test its correlation with other clinical and electrophysiological diagnostic tools.
The main findings of the current study were gradual and progressive neuromuscular dysfunction that correlated with SOFA score. Moreover, there was significant correlation among different assessment methods and bedside ultrasonography had comparable sensitivity in detecting such dysfunction.
Muscle dysfunction was reported to start early in the first few days during ICU stay with progressive daily structural and functional loss of muscle fibers [6, 13, 14].
Sepsis and septic shock are among the most important risk factors of critical illness myopathy with reported incidence of 100% in these patients compared to relatively lower incidence in other causative factors [15, 16].
This is consistent with our results that showed 60% incidence in the first evaluation and 95% in the second evaluation based on electrophysiological assessment. Despite there being no significant change in SOFA score between the two evaluations in our study, SOFA score at initial evaluation correlated with the decline in neuromuscular function (MRC and US echogenicity). This may indicate that the ICU-acquired neuromuscular dysfunction is multifactorial, however, sepsis had a significant negative impact on that dysfunction.
The pathophysiology underlying septic induced CIM is complex and multifactorial including microcirculatory, cellular, metabolic, and electrical factors [6, 16, 17]. The critical illness and sepsis involve systemic inflammatory responses with resulting overproduction of cytokines, oxygen radicals and nitric oxide that cause microvascular derangement and ischemic hypoxia [6, 18]. Cellular and metabolic changes include mitochondrial dysfunction, depletion of ATP and intracellular antioxidants, increased secretion of stress hormones, and insulin resistance. All these changes cause bioenergetic failure and cytopathic hypoxia [16, 19, 20].
Moreover, electrical muscle membrane inexcitability with slowed conduction velocity within individual muscle fibers caused by reduced or dysfunctional voltage-gated sodium channels was reported in pathophysiology of CIM. This is consistent with the increased relative refractory period of muscle fibers and observed prolonged duration of compound muscle action potential on nerve conduction studies [16, 17, 21].
Critical illness myopathy has major negative impact on both in-hospital and post-discharge outcomes and due to the limited applicability adherent to the gold standard tools of diagnosing such disorder in ICU, these is still unmet need to an easier applicable and non-invasive screening tool for early identification and so implementing intervention and rehabilitation programs at an optimum reversible stage. The current study showed that bedside peripheral muscle ultrasonography using easy applicable echogenicity grading had very good sensitivity in detecting early muscle affection compared to EMG examination and had high correlation with clinical MRC scores.
Previous studies investigated the use of muscle ultrasound in ICU to determine its diagnostic, predictive and prognostic value in patients with ICUAW. Different sonographic measures were used such as diaphragmatic excursion and thickening fraction, peripheral muscle thickening, cross sectional area, pennation angle, echogenicity, and detection of fasciculations [22,23,24].
Le Neindre and colleagues showed that reduced diaphragmatic excursion and thickening fraction had the ability to predict extubation failure with moderate to high specificity [25]. Paolo and colleagues demonstrated the value of quadriceps ultrasound for early detection of ICUAW in long-term critical ill and ventilated patients and concluded that the rate of reduced rectus femoris pennation angle over the first week was the best predictor for later development of ICUAW even before decline of clinical muscle strength detected by MRC score [22].
Other reports highlighted the value of gradual decline of peripheral muscle thickness in critically ill, ventilated and septic patients to predict worse outcomes both in-hospital and post-discharge physical outcome and survival [23, 26]. Moreover, Palakshappa and colleagues showed that rectus femoris cross sectional area was superior to quadriceps muscle thickness in evaluation of ICU sepsis-related weakness and its rate of decline over the first week correlated with clinical muscle strength [27].
Regarding evaluation of peripheral muscle echogenicity, previous studies found that its sequential increase in critically ill and septic patients could predict histological muscle fiber necrosis and clinically detected ICUAW at early stage and recommended its use as an additive tool to gold standard of critical illness neuromyopathy [28,29,30].
Comparable with our findings, Parry and his colleagues reported in ICU patients a strong association of vastus intermedius muscle echogenicity with muscle function and strength assessed by MRC [31].
Regarding diagnostic accuracy of muscle echogenicity in detecting critical illness neuromyopathy, other studies showed similar results that accord with ours. Kelmenson and his colleagues reported 82% sensitivity and 57% specificity of abnormal muscle echogenicity [32]. Moreover, Moubarez and her colleagues reported sensitivity of 94.1% and specificity of 66.7% on day 7 and 100% on day 14 of ICU admission [33].
Conclusions
Bedside peripheral muscle ultrasound echogenicity grade could be used as an additional screening test in ICU septic patients for detection of CIM at an early reversible stage.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CIM:
-
Critical illness myopathy
- CINM:
-
Critical illness neuromyopathy
- CIP:
-
Critical illness polyneuropathy
- EMG:
-
Electromyography
- ICUAW:
-
Intensive care unit acquired weakness
- IQR:
-
Interquartile range
- MRC:
-
Medical Research Council
- NCS:
-
Nerve conduction studies
- Sofa:
-
Sepsis-Related Organ Failure Assessment
- U/S:
-
Ultrasonography
References
Kramer CL. Intensive care unit–acquired weakness. Neurol Clin. 2017;35(4):723–36.
Diaz Ballve LP, Dargains N, Urrutia Inchaustegui JG, Bratos A, Milagros Percaz MDL, Bueno Ardariz C, et al. Weakness acquired in the intensive care unit. Incidence, risk factors and their association with inspiratory weakness. Observational cohort study. Revista Brasileira de terapia intensiva. 2017;29:466–75.
Appleton RTD, Kinsella J, Quasim T. The incidence of intensive care unit-acquired weakness syndromes: a systematic review. J Intensive Care Soc. 2015;16(2):126–36.
García-Martínez MÁ, González JCM, y Mateos AGDL, Teijeira S. Muscle weakness: understanding the principles of myopathy and neuropathy in the critically ill patient and the management options. Clin Nutr. 2020;39(5):1331–44.
Ng K. A new examination of critical illness myopathy. Clin NeuroPhysiol. 2021;132:1332–3.
Rodriguez B, Larsson L, Z’Graggen WJ. Critical illness myopathy: diagnostic approach and resulting therapeutic implications. Curr Treat Options Neurol. 2022;24:1–10.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Vincent J-L, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–10.
Hermans G, Clerckx B, Vanhullebusch T, Segers J, Vanpee G, Robbeets C, et al. Interobserver agreement of Medical Research Council sum-score and handgrip strength in the intensive care unit. Muscle Nerve. 2012;45(1):18–25.
Preston DC, Shapiro BE. Electromyography and neuromuscular disorders e-book: clinical-electrophysiologic correlations (Expert Consult-Online). Elsevier Health Sciences; 2012.
Van Dijk JG. Multiple tests and diagnostic validity. Muscle Nerve. 1995;18(3):353–5.
Heckmatt JZ, Leeman S, Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. J Pediatr. 1982;101(5):656–60.
Lodeserto F, Yende S. Understanding skeletal muscle wasting in critically ill patients. Crit Care. 2014;18(6):617. https://doi.org/10.1186/s13054-014-0617-7.
Dos Santos C, Hussain SNA, Mathur S, Picard M, Herridge M, Correa J, et al. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay. A pilot study. Am J Respir Crit Care Med. 2016;194(7):821–30.
Tennilä A, Salmi T, Pettilä V, Roine RO, Varpula T, Takkunen O. Early signs of critical illness polyneuropathy in ICU patients with systemic inflammatory response syndrome or sepsis. Intensive Care Med. 2000;26(9):1360–3.
Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931–41.
McClafferty B, Umer I, Fye G, Kepko D, Kalayanamitra R, Shahid Z, et al. Approach to critical illness myopathy and polyneuropathy in the older SARS-CoV-2 patients. J Clin Neurosci. 2020;79:241–5.
Schefold JC, Wollersheim T, Grunow JJ, Luedi MM, Z’Graggen WJ, Weber-Carstens S. Muscular weakness and muscle wasting in the critically ill. J Cachexia Sarcopenia Muscle. 2020;11(6):1399–412.
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–23.
Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364(9433):545–8.
Tankisi A, Pedersen TH, Bostock H, Z’Graggen WJ, Larsen LH, Meldgaard M, et al. Early detection of evolving critical illness myopathy with muscle velocity recovery cycles. Clin Neurophysiol. 2021;132(6):1347–57.
Paolo F, Silvia C, Tommaso P, Elena C, Martin D, John JM, et al. The possible predictive value of muscle ultrasound in the diagnosis of ICUAW in long-term critically ill patients. J Crit Care. 2022;71: 154104.
Toledo DO, de Freitas BJ, Dib R, do Amaral Pfeilsticker FJ, Dos Santos DM, Gomes BC, et al. Peripheral muscular ultrasound as outcome assessment tool in critically ill patients on mechanical ventilation: an observational cohort study. Clin Nutr ESPEN. 2021;43:408–14.
Bunnell A, Ney J, Gellhorn A, Hough CL. Quantitative neuromuscular ultrasound in intensive care unit–acquired weakness: a systematic review. Muscle Nerve. 2015;52(5):701–8.
Le Neindre A, Philippart F, Luperto M, Wormser J, Morel-Sapene J, Aho SL, et al. Diagnostic accuracy of diaphragm ultrasound to predict weaning outcome: a systematic review and meta-analysis. Int J Nurs Stud. 2021;117: 103890.
Hadda V, Kumar R, Khilnani GC, Kalaivani M, Madan K, Tiwari P, et al. Trends of loss of peripheral muscle thickness on ultrasonography and its relationship with outcomes among patients with sepsis. J Intensive Care. 2018;6(1):1–10.
Palakshappa JA, Reilly JP, Schweickert WD, Anderson BJ, Khoury V, Shashaty MG, et al. Quantitative peripheral muscle ultrasound in sepsis: muscle area superior to thickness. J Crit Care. 2018;47:324–30.
Puthucheary ZA, Phadke R, Rawal J, McPhail MJ, Sidhu PS, Rowlerson A, et al. Qualitative ultrasound in acute critical illness muscle wasting. Crit Care Med. 2015;43(8):1603–11.
Grimm A, Teschner U, Porzelius C, Ludewig K, Zielske J, Witte OW, et al. Muscle ultrasound for early assessment of critical illness neuromyopathy in severe sepsis. Crit Care. 2013;17(5):1–11.
Cartwright MS, Kwayisi G, Griffin LP, Sarwal A, Walker FO, Harris JM, et al. Quantitative neuromuscular ultrasound in the intensive care unit. Muscle Nerve. 2013;47(2):255–9.
Parry SM, El-Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 2015;30(5):1151.e9-e14.
Kelmenson DA, Quan D, Moss M. What is the diagnostic accuracy of single nerve conduction studies and muscle ultrasound to identify critical illness polyneuromyopathy: a prospective cohort study. Crit Care. 2018;22(1):1–9.
Moubarez DA, Mohamed K, El Din SS, Basheer MA, El Baz A. Muscle ultrasound in assessment of critical illness neuromyopathy in comparison with nerve conduction. J Adv Pharm Educ Res. 2019;9(1):11–6.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SKE pioneered the idea of research. MAO recruited the patients, collected and revised the clinical data. HMS revised the data and participated in study designs and coordination. MME collected and analyzed the electrodiagnostic data, performed statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved from the local ethical committee of faculty of Medicine, Beni-Suef University (Approval No: FMBSUREC/01102019) and in accordance with the principles of Helsinki Declaration and an informed written consent was obtained from the patient next of kin of all participants before enrolment in the study.
Consent for publication
Not applicable.
Competing interests
The author declares that he has no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elkholy, M.M., Osman, M.A., Abd El Basset, A.S. et al. The use of muscle ultrasound to detect critical illness myopathy in patients with sepsis: an observational cohort study. Egypt J Neurol Psychiatry Neurosurg 60, 34 (2024). https://doi.org/10.1186/s41983-024-00808-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-024-00808-w