Abstract
Background
The most prevalent nerve entrapment disorder, known as carpal tunnel syndrome (CTS), is brought on by wrist-based median nerve compression. Focal demyelination progresses to axonal dysfunction as the condition worsens. In order to detect motor unit (MU) loos, this study compares two motor unit number estimation (MUNE) techniques with compound muscle action potential (CMAP) amplitude. The CMAP amplitude and MUNE of the median nerve in 137 hands of 70 neurophysiologically approved CTS patients, aged 40.27 ± 10.06 years were examined. Another 90 hands from 56 healthy volunteers who are age- and gender-matched serve the control group.
Results
In contrast to 192.5 and 248.5 in controls, the median nerve values of incremental and adapted multipoint stimulation (aMPS) MUNE in CTS patients were, respectively, 111 and 133 (p < 0.0001). Patients with severe CTS compared to those with mild CTS using both methods had significantly lower MUNE. MUNE values are the same regardless of gender or hand dominance. In comparison to MUNE methods (cutoff values of 106.5 and 203, respectively), CMAP amplitude had a sensitivity and specificity of more than 60% in detecting MU loss (cutoff value of 6.85 mV). The CTS grading had no effect on the CMAP amplitude. MUNE values had positive with CMAP amplitude and negative with CTS grading and Phalen test positivity.
Conclusions
When identifying motor nerve involvement in CTS patients, the MUNE technique is more accurate than a standard motor nerve conduction study (NCS). It was emphasized that MUNE evaluation in determining MU loss in the early stages of CTS may be helpful in diagnosis and treatment. There was no correlation between handedness and the number of MUs as determined by MUNE techniques. Both methods almost equally identify MU loss and have the same sensitivity and specificity.
Similar content being viewed by others
Introduction
A common clinical condition of focal peripheral neuropathy known as carpal tunnel syndrome (CTS) is characterized by nerve entrapment of the median nerve at the level of the carpal tunnel [1, 2]. Numbness, paresthesia, tingling, and pain are common in the median nerve distribution in CTS patients. These symptoms are linked to significant impairments in hand function and varying degrees of disability, such as motor weakness in the thumb and, in more severe cases, wasting of the abductor pollicis brevis (APB) muscle. The loss of motor units (MUs) in the hand muscles is the pathophysiological cause of this functional impairment [3, 4].
The number of distinctive symptoms and aggravating or mitigating factors correlates with the likelihood of a precise clinical diagnosis of CTS [5]. Bedside tests used as part of a clinical examination to elicit symptoms of CTS include the Phalen, Tinel, manual carpal compression, and hand elevation tests. These provocative maneuvers may increase the accuracy of the clinical evaluation’s diagnosis [6].
For confirming the diagnosis of CTS and ruling out other conditions in the differential diagnosis, needle electromyography (EMG) is frequently added to electrodiagnostic testing with nerve conduction studies (NCS) [7]. Results from the NCS may be used to confirm the diagnosis of CTS. Whenever symptoms or NCS findings are moderate to severe, needle EMG is also performed to determine the integrity of MUs to help select patients for surgical treatment [8, 9]. For instance, in some severe cases where sensory and motor responses are absent on NCS, EMG can provide objective evidence of continuing neuronal integrity; similarly, in some cases where NCS abnormalities are mild, EMG can demonstrate evidence of more severe active denervation.
The pathophysiology of CTS is characterized by nerve demyelination in the early stages and axonal degeneration as the disease progresses, which may require surgical decompression. Electrodiagnostic techniques are used to determine the extent of focal demyelination and axonal degeneration [10, 11]. The decrement in the compound muscle action potential (CMAP) amplitude can be brought on by distal demyelination or axonal degeneration, though the latter is less common in CTS [12]. Studies have shown that needle EMG in CTS patients is unreliable and controversial because results like fibrillation and a positive sharp wave cannot be used to assess the severity of motor axon injury [13].
Secondary axonal degeneration or conduction block may result in a decrease in the amplitude of the CMAP and sensory nerve action potential (SNAP) [14]. The best indicator of axonal loss is CMAP amplitude, which measures the number of innervated muscle fibers. In slowly progressive denervating disorders, its values fall only when the extent of denervation exceeds the capacity for reinnervation [15].
Some patients are unable to tolerate this aspect of the test and, in some cases, leave the test with an unfavorable memory because the APB muscle experienced the most intense pain with needle EMG [16]. In order to provide a numerical estimate of the number of innervating axons, another neurophysiological tool called motor unit number estimation (MUNE) was used [17,18,19]. MUNE is based on electrophysiological evaluations of MU characteristics and can represent the number of all MUs innervating a muscle or muscle group. Additionally, MU size can be measured using MUNE techniques, enabling the monitoring of MU losses as well as the compensatory collateral reinnervation phenomenon [20].
The purpose of this study is to compare the sensitivity of CMAP amplitude to two MUNE methods in detecting MU loss, as well as their relationship to the severity of CTS.
Methods
In the course of one calendar year (2018), a case–control study was conducted over four months. The research project received ethical approval from the Institute Review Board of Al-Nahrain University’s College of Medicine (Reference # mmm/19, date 26/12/2017). Each individual participant had to give ethical consent in order to be a part of the study.
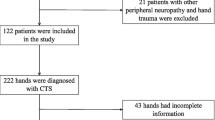
The information was gathered from 70 patients with an average age of 40.27 years (SD = 10.06) who were referred to the Neurophysiology Unit, Al-Imammain Al-Kadhimyian Medical City, from Orthopedics and Neurology clinics, as well as General Practices in Baghdad. CTS was considered for referral if there was paresthesia, pain, swelling in the median nerve distribution area, or digits I–V that was exacerbated by sleep. Another 56 age- and gender-matched volunteers served as the control group in the study.
Participants were not allowed to participate in this study if they had any of the following conditions: cervical root lesions in the symptomatic hand, fracture to the wrist, previous surgery for CTS, pregnancy, severe atrophy of the APB muscle, history of rheumatoid arthritis or wrist arthrosis, known diabetes, thyroid disease, or alcoholism, artificial dialysis, Martin–Gruber anastomosis, or clinical signs of polyneuropathy.
The tests were carried out on a Cadwell VND301 (USA) device by a certified clinical neurophysiologist. Using conventional techniques, the median nerve’s f-wave responses, as well as the peak sensory latency from digit 2, sensory amplitude from digit 2, sensory conduction velocity, motor latency, CMAP amplitude, and motor conduction velocity were examined [21]. Both symptomatic and asymptomatic hands were tested, so 113 hands from 70 patients and 90 hands from controls were employed in this study.
To order to introduce the terms “mild”, “moderate”, and “severe”, a numerical value that could be widely accepted and used to compare with other studies was applied [22]. For mild CTS: presence of prolonged (relative or absolute) median nerve sensory latencies and normal motor studies with no evidence for axon loss. For moderate CTS, presence of abnormal sensory latencies as noted for mild CTS, and (relative or absolute) prolongation of motor distal latency but with no evidence of axon loss. For severe CTS, any of the aforementioned NCS abnormalities with evidence of axon loss as defined by either: (1) an absent or low-amplitude SNAP or mixed NAP; (2) a low-amplitude or absent thenar CMAP; or (3) a needle EMG with fibrillation potentials or MU potential changes (large amplitude, long-duration MU potentials, or excessive polyphasics).
The incremental MUNE was done with a surface active recording electrode was placed over the APB motor point and a reference electrode was placed over the thumb’s metacarpophalangeal joint. At the wrist, the median nerve was stimulated 8 cm away from the active electrode. Before nerve stimulation, the wrist’s precise stimulation site was marked with a pen to ensure accuracy.
The intensity of the stimulation was first increased in order to achieve the maximum CMAP response. Then, in order to help visualize low-amplitude steps in the response envelope, the display’s sensitivity is raised to 100–200 V/div. To activate the first axon, the stimulus intensity is decreased to 3–10 mA, as shown by an all-or-none response. Before the increments in the envelope become indistinguishable by a minimum increment in stimulation intensity, an envelope of responses is obtained with 8–10 discrete steps. To determine the average amplitude of each step, we divide the number of steps by the peak-to-peak amplitude of the envelope. This average value represents the average single MUP and used to determine the MUNE value [23].
For the aMPS MUNE method, the recording electrodes were placed on the APB muscle using the standard belly–tendon method. The median nerve was stimulated five times at three locations: 2 cm proximal to the wrist crease, 4 cm proximal to the wrist crease, and at the cubital fossa. The filter frequencies ranged from 2 to 10 kHz. The ideal stimulus location for each stimulation site was identified by using a submaximal stimulus and moving the stimulator to elicit the greatest response. After that, the amplifier settings were changed to 200 µV/division. Traces were obtained and superimposed using a typical 3-site motor conduction program.
The baseline to negative peak amplitude was measured after the stimulus was gradually intensified until an all-or-none initial response was attained. Three responses of 25 µV amplitude were obtained at each stimulation site. The negative peak amplitude of the third response was measured. The second and third locations received the same stimulation as the first. After gathering the samples, we went through all of the tracings to look for possible repeating motor units (so they would not be included more than once) [24].
Statistical analysis
Microsoft Office Excel 2016 and the statistical package for social sciences (SPSS) version 26 software were used to conduct the statistical analysis. The information was displayed as median and range or mean ± SD. Data from patients and controls were compared using the paired t-test for parametric data and the Mann–Whitney test for non-parametric data. For categorical data, the Chi-square test is used. The Spearmen correlation was employed to examine the relationship between sociodemographic and electrophysiologic data.
The receiver operating characteristic (ROC) curve is used to assess the performance of a diagnostic test by plotting the true-positive (sensitivity) rate against the false-positive (1-specificity) rate at different CMAP amplitude, incremental, and aMPS MUNE threshold settings. A p-value of less than 0.05 was considered significant.
Results
There were no significant differences in age or gender between patients with CTS and controls. The average duration of symptoms was found to be 14.71 ± 14.04 months. The right hand was affected in 19 (27.14%), the left hand was affected in 14 (20%), and both hands were affected in 37 (52.86%) of CTS patients. In terms of the dominant hand, no significant difference was found between the groups. The Phalen test was positive in 48 (68.57%) of the patients in the patient group, as shown in Table 1.
As shown in Table 2, there was a statistically significant difference between the patient and control groups in all electrophysiological measurements of the median nerve motor and sensory parameters (p < 0.001) and MUNE values (p < 0.001).
No significant difference in MUNE values between males and females (p = 0.561 versus p = 0.494), or between dominant and non-dominant hands (p = 0.056 versus p = 0.918) of patients with CTS regardless of whether the method was incremental or aMPS (Table 3).
Table 4 shows that 33 hands had mild CTS, 44 had moderate CTS, and 36 had severe CTS, based on the severity score of CTS. The incremental MUNE was lower in those with severe CTS than in those with mild CTS (p = 0.036), but not in those with moderate CTS. Furthermore, the aMPS MUNE value in those with severe CTS differed significantly from those with mild and moderate CTS (p < 0.001), whereas there was no difference between the two latter groups.
As shown in Table 5 and Fig. 1, the receiver operating characteristic (ROC) curve was used to assess the sensitivity and specificity of median nerve CMAP amplitude as well as MUNE methods in differentiating between patients and controls. Table 6 shows the relationship between MU loss and neurophysiological cutoff values in different grades of CTS. Both MUNE methods, but not the CMAP amplitude, show a significant increase in MU loss with increasing CTS severity (p = 0.009 and p = 0.005, respectively).
There was no correlation found in the Spearman correlation analysis between the incremental and aMPS MUNE values and age, symptom duration, or hand dominancy. As shown in Fig. 2, both MUNE methods were positively related to median nerve CMAP amplitude (r = 0.370; p < 0.001; r = 0.394; p < 0.001, respectively) as shown in Fig. 3. They were also negatively related to CTS grading (r = − 0.236; p = 0.120 and r = − 0.367; p < 0.001, respectively) and Phalen positivity (r = − 0.180; p = 0.023 and r = − 0.244; p = 0.010, respectively).
Discussion
In the present study, median nerve CMAP amplitude, incremental and aMPS MUNE findings, and MU loss in CTS patients were studied in patients with CTS and healthy controls. Additionally, correlations between MUNE values and clinical CTS manifestations have been investigated. Our study showed that CTS patients had lower median nerve CMAP amplitude, which may be related to either axonal degeneration or distal demyelination [25].
The MUNE values of CTS patients of this study were 111 with the incremental method and 133 with the aMPS method. They were significantly less than the 192.5 and 248.5, respectively, of the controls. MUNE values have been identified by Koç and colleagues [26] to be 115.62 ± 31.39 in in a group of CTS patients and 150.47 ± 33.6 in the control group. The mean MUNE values were 48.89 ± 26.30 in the CTS group and 94.33 ± 48.45 in the control group according to Bayrak and colleagues study [27].
In addition, a different study found that the patient group’s MUNE was 81.8 ± 33.9 which was statistically lower than the control’s group MUNE (136.4 ± 22.0) with p < 0.05, a sensitivity of 96.7% and specificity of 85.9% [23]. Yilmaz and colleagues [1], reported that the MUNE technique was sensitive in determining motor nerve involvement in CTS patients with sensory findings, particularly in the early stages of CTS that are seen to be silent or with the slow sensory transmission. In a recent study, the MUNE values for the control group were 134.66 ± 41.00 and 68.72 ± 32.16 for the CTS patients [28]. The variation in patients included in these studies and the use of various techniques account for the discrepancy in MUNE values reported in in the literatures.
Routine NCSs are easy to use, repeatable, and reliable, but their main drawback is that they cannot identify MU loss in its early stages. During the slowly developing clinical picture of CTS, the CMAP amplitude of the relevant median motor nerve is typically found to be within normal limits [1]. The CMAP amplitude does not change significantly until the axons are severely damaged because surviving MU axons reinnervate muscle fibers through collateral sprouting [29].
Since sensory NCS are almost always affected before motor NCS in mild CTS, median nerve motor conduction study is less sensitive than sensory conduction study [30]. As a result, MUNE seems to be the best technique for assessing motor fibers because it can detect MU dysfunction even in the early stages of CTS [23, 27]. In this regard, our study demonstrated that MUNE methods are more sensitive and specific than median nerve CMAP amplitude in identifying MU loss in patients with CTS, as shown in Fig. 1 and Table 5.
Additionally, MUNE values by the two methods were lower than control values across the spectrum of CTS severity, demonstrating that MUNE is more accurate than CMAP amplitude in detecting MU loss even in mild CTS cases, as shown in Tables 4 and 6. In agreement with our findings, Cuturic and Palliyath [31] reported that patients with mild-to-moderate CTS had a clinically silent period of MU loss prior to the onset of clinical signs and symptoms.
In this study, it was discovered that MUNE was negatively correlated with Phalen test positivity and CTS grading and positively correlated with median nerve CMAP amplitude in patients with CTS. Several publications [27, 29] also reported on these findings.
The small sample size of the study, which was caused by patient compliance and the time required to apply the MUNE techniques, as well as the inclusion of only one age group (20–60 years) and the exclusion of endocrine conditions like diabetes mellitus and thyroid function disorders, which frequently contribute to the etiology of CTS, are among the limitations of this study. Additionally, the MUNE method is not frequently used in the follow-up of CTS, so there were not enough studies to compare, which made this investigation difficult.
Conclusion
According to our research, MUNE is a technique that is more capable of detecting motor nerve involvement in CTS patients than traditional motor NCS. The sensitivity and specificity of the incremental and aMPS MUNE methods to detect MU loss are nearly identical. As determined by MUNE techniques, there was no correlation between handedness and the number of MUs.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- APB:
-
Abductor pollicis brevis
- aMPS:
-
Adjusted multipoint stimulation
- CMAP:
-
Compound muscle action potential
- CTS:
-
Carpal tunnel syndrome
- EMG:
-
Electromyography
- MU:
-
Motor unit
- MUAPs:
-
Motor unit action potentials
- MUNE:
-
Motor unit number estimation
- NCS:
-
Nerve conduction study
- ROC:
-
Receiver operating characteristic
- SNAP:
-
Sensory nerve action potential
References
Yilmaz O, Sunter G, Salcini C, Koytak PK, Tanridag T, Us O, et al. Motor-unit number estimation is sensitive in detecting motor nerve involvement in patients with carpal tunnel syndrome. J Clin Neurol. 2016;12(2):166–71.
Wahab KW, Sanya EO, Adebayo PB, Babalola MO, Ibraheem HG. Carpal tunnel syndrome and other entrapment neuropathies. Oman Med J. 2017;32:449–54.
Aboonq MS. Al-hijamah (wet cupping therapy of prophetic medicine) as a novel alternative to surgery for carpal tunnel syndrome. Neurosciences. 2019;24(2):137–41.
Paiva HR, Paiva VDGN, Oliveira EF, Rocha MA. Profile of patients with carpal tunnel syndrome treated at a referral service. Acta Ortop Bras. 2020;28(3):117–20.
Bland JD. Carpal tunnel syndrome. BMJ. 2007;335(7615):343.
Zhang D, Chruscielski CM, Blazar P, Earp BE. Accuracy of provocative tests for carpal tunnel syndrome. J Hand Surg Glob Online. 2020;2(3):121–5.
Rosario NB, De Jesus O. Electrodiagnostic Evaluation of Carpal Tunnel Syndrome. 2023 Aug 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
Raizman NM, Blazar PE. AAOS appropriate use criteria: management of carpal tunnel syndrome. J Am Acad Orthop Surg. 2018;26(6): e131.
Shetty KD, Robbins M, Aragaki D, Basu A, Conlon C, Dworsky M, et al. The quality of electrodiagnostic tests for carpal tunnel syndrome: implications for surgery, outcomes, and expenditures. Muscle Nerve. 2020;62(1):60.
Alanazy MH. Clinical and electrophysiological evaluation of carpal tunnel syndrome: approach and pitfalls. Neurosciences (Riyadh). 2017;22(3):169–80.
Deng X, Chau LP, Chiu SY, Leung KP, Hu Y, Ip WY. Screening of axonal degeneration in carpal tunnel syndrome using ultrasonography and nerve conduction studies. J Vis Exp. 2019; (143).
Hosseininezhad M, Ghayeghran AR, Nasiri P, Saadat S, Esmaili K, Homaei Rad E, et al. Estimation of median nerve axonal degeneration without needle electromyography. Iran J Neurosurg. 2021;7(1):23–30.
Sonoo M, Menkes DL, Bland JDP, Burke D. Nerve conduction studies and EMG in carpal tunnel syndrome: do they add value? Clin Neurophysiol Pract. 2018;3:78–88.
Deng X, Chau LP, Chiu SY, Leung KP, Hu Y, Ip WY. Screening of axonal degeneration in carpal tunnel syndrome using ultrasonography and nerve conduction studies. J Vis Exp. 2019; 143.
El-Emary WS, Hassan MM. Needle electromyography in carpal tunnel syndrome: is it valuable or predictable? Egypt Rheumatol Rehabil. 2016;43(1):41–6.
London ZN. Safety and pain in electrodiagnostic studies. Muscle Nerve. 2017;55(2):149–59.
Paramanathan S, Tankisi H, Andersen H, Fuglsang-Frederiksen A. Axonal loss in patients with inflammatory demyelinating polyneuropathy as determined by motor unit number estimation and MUNIX. Clin Neurophysiol. 2016;127:898–904.
Cirillo G, Todisco V, Ricciardi D, Tedeschi G. Clinical-neurophysiological correlations in chronic inflammatory demyelinating polyradiculoneuropathy patients treated with subcutaneous immunoglobulin. Muscle Nerve. 2019;60:662–7.
Okhovat AA, Advani S, Ziaadini B, Panahi A, Salehizadeh S, Nafissi S, et al. The value of MUNIX as an objective electrophysiological biomarker of disease progression in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2022;65:433–9.
Gooch CL, Doherty TJ, Chan KM, Bromberg MB, Lewis RA, Stashuk DW, et al. Motor unit number estimation: a technology and literature review. Muscle Nerve. 2014;50(6):884–93.
Preston DC, Shapero, BE. Detailed nerve conduction studies routine upper extremity, facial, and phrenic nerve conduction techniques. In: Electromyography and Neuromuscular Disorders: Clinical-electrophysiologic-ultrasound correlations. 4th ed., Section IV, Chap. 10, Philadelphia, PA 19103–2899. Elsevier, Inc. 2021; p. 107–114.
Werner RA, Andary M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve. 2011;44:597–607.
Sohn MK, Jee SJ, Hwang SL, Kim YJ, Shin HD. Motor unit number estimation and motor unit action potential analysis in carpal tunnel syndrome. Ann Rehabil Med. 2011;35(6):816–25.
Jagtap SA, Kuruvilla A, Govind P, Nair MD, Sarada C, Varma RP. Multipoint incremental motor unit number estimation versus amyotrophic lateral sclerosis functional rating scale and the medical research council sum score as an outcome measure in amyotrophic lateral sclerosis. Ann Indian Acad Neurol. 2014;17(3):336–9.
Wang L. Electrodiagnosis of carpal tunnel syndrome. Phys Med Rehabil Clin N Am. 2013;24:67–77.
Koç F, Yerdelen D, Sarıca Y. Motor unit number estimation in cases with carpal tunnel syndrome. Intern J Neuroscience. 2006;116:1263–70.
Bayrak AO, Tilki HE, Coşkun M. Sympathetic skin response and axon count in carpal tunnel syndrome. J Clin Neurophysiol. 2007;24:70–5.
Berk E, Nacitarhan V. The relationship between median nerve axon count and clinical findings and electrophysiological parameters in patients with carpal tunnel syndrome. Ann Med Res. 2019;26(6):1039–44.
Daube JR. Motor unit number estimates–from A to Z. J Neurol Sci. 2006;242:23–35.
Patel K, Horak HA. Electrodiagnosis of common mononeuropathies: median nerve, ulnar, and fibular (peroneal) neuropathies. Neurol Clin. 2021;39:939–55.
Cuturic M, Palliyath S. Motor unit number estimate (MUNE) testing in male patients with mild to moderate carpal tunnel syndrome. Electromyogr Clin Neurophysiol. 2000;40:67–72.
Acknowledgements
We thank professor Dr. Farqad B. Hamdan from the Department of Physiology, College of Medicine/Al-Nahrain University for helping in statistical analysis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All the authors have directly participated in the preparation of this manuscript and have approved the final version submitted. ‘SA’ and ‘HK’ did the electrodiagnostic tests. ‘SA’ and ‘HK’ drafted the manuscript. ‘SA’ and ‘HK’ conceived the study and participated in its design and interpretation. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institute Review Board (IRB) of Al-Nahrain University’s College of Medicine granted ethical approval for the research project (Reference # mmm/19, date 26/12/2017). Written consent for participation from all subjects was ensured.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdul-Muneem, S.D.AD., Kaddoori, H.G. Value of MUNE versus compound muscle action potential in assessing motor unit loss in patients with carpal tunnel syndrome. Egypt J Neurol Psychiatry Neurosurg 60, 22 (2024). https://doi.org/10.1186/s41983-024-00796-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-024-00796-x