Abstract
Background
Cerebral venous sinus thrombosis (CVST) is a rare form of stroke that is mainly seen in young women. It is frequently associated with hemorrhagic venous infarction and subarachnoid hemorrhage. There are few reports of CVST associated with chronic non-traumatic subdural hematoma (SDH). The diagnosis of CVST with spontaneous SDH is difficult because of the variability of its clinical features. The management of SDH associated with CVST is controversial and not well-established.
Case presentation
We report a 26-year-old woman with positive COVID-19 serology who presented with superior longitudinal sinus thrombosis associated with chronic spontaneous SDH. She was managed conservatively and treated with anticoagulation and corticosteroids. A follow-up angioscan 1 month after treatment showed regression of the SDH volume with partial repermeabilisation of the thrombosed sinus. Three months later, the follow-up angioscan showed complete resolution of the chronic SDH and superior longitudinal sinus thrombosis with restoration of venous flow.
Conclusions
CVST can also present with spontaneous chronic SDH. The management of SDH concomitant with CVST remains controversial due to the rarity of its presentation and the risks associated with the use of anticoagulation.
Similar content being viewed by others
Introduction
Cerebral venous sinus thrombosis (CVST) is a rare and life-threatening condition [1]. The most common clinical presentations reported in large case registries are intracranial hypertension and hemorrhagic cerebral venous infarction [2,3,4].
Most patients with CVST are under 50 years of age. There are many associated factors that predispose patients to CVST [4]: systemic factors that increase the risk of CVST include genetic conditions, pregnancy, hormonal therapies, malignancies, polycythemia vera and other myeloproliferative disorders, dehydration or trauma. Mechanical factors, which reduce blood flow in the cerebral sinuses and promote thrombosis, include adjacent infections (usually mastoiditis), neoplastic invasion of the sinus, trauma and neurosurgical procedures.
Currently, coronavirus 2019 (COVID-19) infection has been shown to predispose patients to arterial and venous thromboembolic events, such as CVST [5].
The increasing availability of magnetic resonance imaging (MRI), magnetic resonance angiography (MRA) and computed tomography angiography (CTA) has improved the ability to detect other clinical manifestations of CVST, including subdural hematoma (SDH) [6,7,8] and subarachnoid hemorrhage (SAH) [9, 10].
Chronic subdural hematomas are well-defined entities that occur following head injury, coagulopathy or neoplastic disease [11]. SDH secondary to CVST is very rare, and high suspicion should be maintained in high-risk cases. The prognosis in patients with a rapid onset and low neurological score remains poor [12].
The management of CVST is challenging, as the requirements of anticoagulation for the management of CVST are contradictory to the risk of expansion or recurrence of subdural hemorrhage [13]. Here, we present a case of CVST diagnosed following spontaneous SDH.
Case presentation
We report the case of a 26-year-old female patient who presented to the neurosurgical emergency room with severe constrictive headache with cervical radiation, resistant to step-1 analgesics, associated with nausea and tinnitus, which had been present for 2 and a half months after a caesarean section. She had a history of gravid hypertension, with symptoms of COVID-19 1 month before admission (IgM−/IgG + profile in COVID-19 serology). She had been on oral contraceptives since delivery. There was no history of head trauma, neoplasia or coagulopathy.
On examination, the Glasgow Coma Score was 15, and the neurological examination revealed no sensory or motor deficits, no visual disturbances, and no seizures. On fundus examination, there was no papilledema and coagulation parameters were normal.
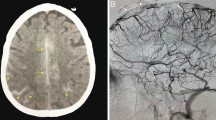
Contrast-enhanced brain MRI revealed cerebral venous thrombosis of the superior longitudinal sinus (empty delta sign) with a 25 mm thick left fronto-parietal chronic subdural hematoma resulting in a 5 mm midline deviation to the right side (Fig. 1).
The patient was managed conservatively, initially treated with parenteral anticoagulation using subcutaneous low molecular weight heparin at a curative dose of 0.8 IU × 2/d, with a hydration regimen and systemic analgesics. After repeated neurological examinations and a new CT scan, brain imaging revealed no further hemorrhage (Fig. 2); she was switched to per os Acenocoumarol combined with methylprednisolone corticosteroids (dose 0.5 mg/kg/day), and prevention of seizures by antiepileptic treatment (Levetiracetam 250 mg × 2/d). The patient was discharged home without neurological deficits.
The evolution was favorable and the patient reported a total disappearance of headaches. A follow-up cerebral angioscanner performed at 1 month after treatment revealed a clear volume regression of the left fronto-parietal SDH (9 mm) compared to the previous examination and the persistence of a partial residual thrombosis of the superior longitudinal sinus achieving a partial repermeabilisation (Fig. 3).
Cerebral angioscan at 3 months showed complete resolution of the chronic SDH and superior longitudinal sinus thrombosis with visible venous sinuses (Fig. 4). His clinical outcome at 3 months was excellent, justifying discontinuation of anticoagulant therapy and corticosteroids by degression.
Discussion
Cerebral venous sinus thrombosis (CVST) accounts for only 0.5–1% of all strokes and usually affects young individuals. It is slightly more common in young women due to pregnancy, puerperium and the use of oral contraceptives. CVST may be due to partially obstructing thrombus or extrinsic compression [12]. Recent reports suggest that there may be an association between COVID-19 infection and the development of CVST [14]. Proposed mechanisms for ischemic events include systemic inflammation, hypercoagulable state and endothelial damage leading to alterations in the normal coagulation cascade [15].
Despite advances in the recognition of CVST in recent years, the diagnosis of CVST is still often overlooked or delayed due to the various underlying risk factors, the wide range of clinical symptoms and the often sub-acute or late onset. It is widely accepted that CVST may present with elevated intracranial pressure (ICP) and hemorrhagic venous infarction on brain computed tomography (CT) scan [4].
Magnetic resonance imaging (MRI) combined with MRI-angiography has largely replaced invasive cerebral angiography and conventional CT for the diagnosis of CVST. Kumral et al., in a study of 220 consecutive patients with CVST noted that the majority of patients (45%) had non-lesional sinus venous thrombosis, 23% had non-hemorrhagic infarction, 20% had hemorrhagic infarction and 12% had intracerebral hemorrhage [16]. There is increasing evidence that it can also cause acute or chronic subdural hematoma (SDH) [6,7,8] and cortical subarachnoid hemorrhage (SAH) [9, 10]. Our case provides further support for the association of CVST and SDH.
CVSTs with spontaneous SDH are rare, and the diagnosis can be easily overlooked [12]. In the International Cerebral Vein and Dural Sinus Thrombosis Study, none of the patients (n = 624) were reported to have SDH [2]. On the other hand, a prospective neurosurgical database identified only three patients diagnosed with CVST manifesting as SDH over a 6-year period [13].
The mechanism that has been proposed by Akins et al. [13] for SDH due to CVST (engorgement and venous hypertension) is analogous to that of SDH due to a dural arteriovenous fistula (AVF) [17, 18]: venous hypertension develops first, then venous bleeding occurs. The bleeding stops when the SDH accumulates and buffers the site of the venous bleed.
Missori et al. [11] performed an evaluation of the intracranial venous system using magnetic resonance angiography in patients with non-traumatic spontaneous SDH, identifying alterations in their venous sinuses. In this study, eight patients with spontaneous SDH who were treated medically or surgically were followed for a period of 7 years. The authors concluded that non-traumatic SDH may occur as a result of venous thrombosis or may be associated with the inability to visualize some of the venous sinuses. Venous flow was restored at long-term follow-up. Increased intravenous pressure is considered to be the pathogenic factor behind non-traumatic SDH [11].
Orthostatic headaches should raise concerns about intracranial hypotension. Clinicians should also look for information that would increase the suspicion of intracranial hypotension, such as recent lumbar puncture, lumbar drainage, spinal surgery, trauma and epidural anesthesia [19,20,21,22,23]. Our patient had a history of epidural anesthesia for a caesarean section, which could raise the suspicion of intracranial hypotension.
Systemic anticoagulation remains the treatment of choice for uncomplicated cerebral venous sinus thrombosis [24], and thrombolytic therapy is reserved for patients, whose condition continues to deteriorate due to thrombus extension despite dose-adjusted heparin. However, the initiation of therapeutic anticoagulation should be carefully weighed against the potential risk of intracranial hemorrhage [25]. The use of anticoagulation for patients with hemorrhagic infarction and CVST is becoming increasingly accepted [4].
Given the mechanism of formation of chronic SDH, which is based on a complex pathway intertwined with angiogenesis, inflammation, recurrent microbleeds, exudates and local coagulopathy [10, 17, 25], it is of interest to use corticosteroids as a therapeutic option, either as monotherapy or as an adjunct to surgery [26]. Surveys of neurosurgeons show that almost a quarter of them use corticosteroids in the conservative treatment of CSDH, and some advocate the use of high doses of corticosteroids [27, 28]. Patients treated with corticosteroids received dexamethasone (initial dose 12–24 mg/day) [29] or methylprednisolone (initial dose 0.5 mg/kg/day) [30] as monotherapy or in addition to surgery.
The diagnosis of CVST in combination with a presentation of SDH is a difficult case to manage. Due to the rarity of this complication, there are currently no fixed guidelines for its management [31].
Khatib et al. propose an algorithm for dealing with this complication by starting anticoagulation immediately if the patient is stable and does not show mass effect or midline shift on neuroimaging [32]. However, if the patient has a neurological deficit with mass effect or midline shift on neuroradiological examination, immediate neurosurgical intervention with supportive care is recommended [32]. They recommend reassessing the patient for the risk of rebleeding two weeks after surgery and to envisage oral anticoagulation [32]. In our case, we chose to apply this algorithm by starting anticoagulation therapy, and considered a satisfactory outcome for 3 months.
There are two previous reports of medically managed SDH cases who were placed on systemic anticoagulation [7, 8], and we have also successfully managed our patient on systemic anticoagulation. Patients should be admitted to an institution with a neurosurgical service and undergo frequent neurological, biological and radiographic monitoring when systemic anticoagulation is initiated. In contrast, early initiation of full anticoagulation (e.g., heparin) is not recommended for patients undergoing neurosurgical intervention for intracranial hemorrhage complicating CVST [7, 8].
There are reports of CVST associated with chronic SDH [6,7,8, 33, 34], in which chronic SDH was evacuated in three patients through drill holes and by performing a craniotomy [6, 33, 34]. In contrast, one patient was treated medically [7], and urokinase or heparin was used to resolve the venous thrombosis [7, 8, 33, 34].
Conclusion
Cerebral venous sinus thrombosis associated with spontaneous subdural hematoma is a rare condition. The risk of CVST in patients with COVID-19 infection should be considered, especially if neurological symptoms develop. MRI angiography should be performed in patients with non-traumatic SDH to detect changes in the venous system.
Accurate and prompt management is necessary to prevent acute and long-term consequences. The management of patients with CVST and SDH is controversial and not well-established due to the rarity of this complication and the limited literature. Due to contraindications to anticoagulation in patients with symptomatic SDH, management is complicated and guidelines need to be developed.
Medical or surgical treatment of subdural hemorrhage appears to resolve the symptoms, and the clinical course appears to be favorable in most patients.
Availability of data and materials
The data sets are available from the corresponding author on reasonable request.
Abbreviations
- CVST:
-
Cerebral venous sinus thrombosis
- SDH:
-
Subdural hematoma
- MRI:
-
Magnetic resonance imaging
- MRA:
-
Magnetic resonance angiography
- CTA:
-
Computed tomography angiography
- SAH:
-
Subarachnoid hemorrhage
- ICP:
-
Intracranial pressure
- AVF:
-
Arteriovenous fistula
References
Zahrani AMA, Sheikh RA. Cerebral venous sinus thrombosis with an intracranial haemorrhage: a case report. Open Access Maced J Med Sci. 2019;7(6):1029–31.
Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F, ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664–70.
Narayan D, Kaul S, Ravishankar K, Suryaprabha T, Bandaru VCSS, Mridula KR, et al. Risk factors, clinical profile, and long-term outcome of 428 patients of cerebral sinus venous thrombosis: insights from Nizam’s Institute Venous Stroke Registry, Hyderabad (India). Neurol India. 2012;60(2):154–9.
Saposnik G, Barinagarrementeria F, Brown RD, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–92.
Dakay K, Cooper J, Bloomfield J, Overby P, Mayer SA, Nuoman R, et al. Cerebral venous sinus thrombosis in COVID-19 infection: a case series and review of the literature. J Stroke Cerebrovasc Dis. 2021;30(1): 105434.
Takamura Y, Morimoto S, Uede T, Yamaki T, Minamida Y, Yamamura A, et al. Cerebral venous sinus thrombosis associated with systemic multiple hemangiomas manifesting as chronic subdural hematoma—case report. Neurol Med Chir (Tokyo). 1996;36(9):650–3.
Singh S, Kumar S, Joseph M, Gnanamuthu C, Alexander M. Cerebral venous sinus thrombosis presenting as subdural haematoma. Australas Radiol. 2005;49(2):101–3.
Takahashi S, Shinoda J, Hayashi T. Cerebral venous sinus thrombosis in an adult patient presenting as headache and acute subdural hematoma. J Stroke Cerebrovasc Dis. 2012;21(4):338–40.
Oppenheim C, Domigo V, Gauvrit JY, Lamy C, Mackowiak-Cordoliani MA, Pruvo JP, et al. Subarachnoid hemorrhage as the initial presentation of dural sinus thrombosis. AJNR Am J Neuroradiol. 2005;26(3):614–7.
Panda S, Prashantha DK, Shankar SR, Nagaraja D. Localized convexity subarachnoid haemorrhage—a sign of early cerebral venous sinus thrombosis. Eur J Neurol. 2010;17(10):1249–58.
Missori P, Domenicucci M, Sassun TE, Tarantino R, Peschillo S. Alterations in the intracranial venous sinuses in spontaneous nontraumatic chronic subdural hematomas. J Clin Neurosci. 2013;20(3):389–93.
Bansal H, Chaudhary A, Mahajan A, Paul B. Acute subdural hematoma secondary to cerebral venous sinus thrombosis: case report and review of literature. Asian J Neurosurg. 2016;11(02):177–177.
Akins P, Axelrod Y, Ji C, Ciporen J, Arshad S, Hawk M, et al. Cerebral venous sinus thrombosis complicated by subdural hematomas: case series and literature review. Surg Neurol Int. 2013;4(1):85.
Abdalkader M, Shaikh SP, Siegler JE, Cervantes-Arslanian AM, Tiu C, Radu RA, et al. Cerebral venous sinus thrombosis in COVID-19 patients: a multicenter study and review of literature. J Stroke Cerebrovasc Dis. 2021;30(6): 105733.
Khan IH, Savarimuthu S, Leung MST, Harky A. The need to manage the risk of thromboembolism in COVID-19 patients. J Vasc Surg. 2020;72(3):799–804.
Kumral E, Polat F, Uzunköprü C, Callı C, Kitiş Ö. The clinical spectrum of intracerebral hematoma, hemorrhagic infarct, non-hemorrhagic infarct, and non-lesional venous stroke in patients with cerebral sinus-venous thrombosis. Eur J Neurol. 2012;19(4):537–43.
Maiuri F, Iaconetta G, Sardo L, Briganti F. Dural arteriovenous malformation associated with recurrent subdural haematoma and intracranial hypertension. Br J Neurosurg. 2001;15(3):273–6.
Ogawa K, Oishi M, Mizutani T, Maejima S, Mori T. Dural arteriovenous fistula on the convexity presenting with pure acute subdural hematoma. Acta Neurol Belg. 2010;110(2):190–2.
Mao YT, Dong Q, Fu JH. Delayed subdural hematoma and cerebral venous thrombosis in a patient with spontaneous intracranial hypotension. Neurol Sci. 2011;32(5):981–3.
Nardone R, Caleri F, Golaszewski S, Ladurner G, Tezzon F, Bailey A, et al. Subdural hematoma in a patient with spontaneous intracranial hypotension and cerebral venous thrombosis. Neurol Sci. 2010;31(5):669–72.
Savoiardo M, Armenise S, Spagnolo P, De Simone T, Mandelli ML, Marcone A, et al. Dural sinus thrombosis in spontaneous intracranial hypotension: hypotheses on possible mechanisms. J Neurol. 2006;253(9):1197–202.
Schievink WI, Maya MM, Pikul BK, Louy C. Spontaneous spinal cerebrospinal fluid leaks as the cause of subdural hematomas in elderly patients on anticoagulation. J Neurosurg. 2010;112(2):295–9.
Yoon KW, Cho MK, Kim YJ, Cho CS, Lee SK. Sinus thrombosis in a patient with intracranial hypotension: a suggested hypothesis of venous stasis. Interv Neuroradiol. 2011;17(2):248–51.
Sahoo RK, Tripathy P, Praharaj HN. Cerebral venous sinus thrombosis with nontraumatic subdural hematoma. Int J Crit Illn Inj Sci. 2015;5(1):59.
Zhang D, Wang J, Zhang Q, He F, Hu X. Cerebral venous thrombosis in spontaneous intracranial hypotension: a report on 4 cases and a review of the literature. Headache. 2018;58(8):1244–55.
Holl DC, Volovici V, Dirven CMF, van Kooten F, Miah IP, Jellema K, et al. Corticosteroid treatment compared with surgery in chronic subdural hematoma: a systematic review and meta-analysis. Acta Neurochir (Wien). 2019;161(6):1231–42.
Baschera D, Tosic L, Westermann L, Oberle J, Alfieri A. Treatment standards for chronic subdural hematoma: results from a survey in Austrian, German, and Swiss neurosurgical units. World Neurosurg. 2018;116:e983–95.
Berghauser Pont LME, Dippel DWJ, Verweij BH, Dirven CMF, Dammers R. Ambivalence among neurologists and neurosurgeons on the treatment of chronic subdural hematoma: a national survey. Acta Neurol Belg. 2013;113(1):55–9.
Fountas K, Kotlia P, Panagiotopoulos V, Fotakopoulos G. The outcome after surgical vs nonsurgical treatment of chronic subdural hematoma with dexamethasone. Interdiscip Neurosurg. 2019;16:70–4.
Dran G, Berthier F, Fontaine D, Rasenrarijao D, Paquis P. Efficacité de la corticothérapie dans le traitement adjuvant des hématomes sous-duraux chroniques. Étude rétrospective sur 198 cas. Neurochirurgie. 2007;53(6):477–82.
Alharshan R, Qureshi HU, AlHada A, Shaikh M, Khalil A. Cerebral venous sinus thrombosis manifesting as a recurrent spontaneous subdural hematoma: a case report. Int J Surg Case Rep. 2020;67:223–6.
Khatib KI, Baviskar AS. Treatment of cerebral venous sinus thrombosis with subdural hematoma and subarachnoid hemorrhage. J Emerg Trauma Shock. 2016;9(4):155–6.
Matsuda M, Matsuda I, Sato M, Handa J. Superior sagittal sinus thrombosis followed by subdural hematoma. Surg Neurol. 1982;18(3):206–11.
Marquardt G, Weidauer S, Lanfermann H, Seifert V. Cerebral venous sinus thrombosis manifesting as bilateral subdural effusion. Acta Neurol Scand. 2004;109(6):425–8.
Acknowledgements
This work would not have been possible without the support of our master Professor Lakhdar Arrouf.
Funding
Funding information is not applicable/no funding was received.
Author information
Authors and Affiliations
Contributions
All authors collected articles and references, discussed and organized the content in the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval is not applicable in this article.
Consent for publication
Patient signed informed consent regarding publishing their data and photographs.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Laouar, F., Brahmia, Y. & Boublata, L. Cerebral venous sinus thrombosis manifesting as chronic spontaneous subdural hematoma: case report and review of the literature. Egypt J Neurol Psychiatry Neurosurg 60, 8 (2024). https://doi.org/10.1186/s41983-023-00778-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00778-5