Abstract
Background
Patients with ischemic stroke and atrial fibrillation (AF) are at high risk of developing hemorrhagic transformation (HT). The aim of the current study is to evaluate the incidence of hemorrhagic transformation and associated risk factors in a hospital-based sample with ischemic stroke and AF patients. A prospective study with a total of 234 stroke patients with AF was consecutively recruited. HT incidence was determined by computed tomography (CT) or magnetic resonance imaging (MRI). Risk factors associated with HT was identified by comparing patients with and without HT.
Results
The incidence of HT in ischemic stroke with AF was 22.6%. Univariate analysis established that old age, hypertension, diabetes mellitus, anticoagulant medications, NIHSS, cerebral microbleeds (CMB), superficial siderosis (SS) and size of infarction were significantly more frequent with HT. Multivariable logistic regression analysis demonstrated that old age [odds ratio (OR): 1.05, confidence interval (CI) 1.01–1.09], size of infarction (OR: 2.57, CI 1.06–6.27) and CMB ≥ 10 (OR: 4.68, CI 1.71–12.84) were significantly associated with the risk of HT.
Conclusions
Older age, larger infarction size, and CMB ≥ 10 were risk factors significantly associated with HT.
Similar content being viewed by others
Background
Patients with ischemic stroke and atrial fibrillation (AF) were associated with an increased risk of developing hemorrhagic transformation (HT) [1, 2]. Therefore, early recognition of HT is essential for the appropriate management and better prognosis.
Following acute stroke, breakdown of the blood brain barrier (BBB) results in friable intracranial vasculature. This breakdown increases the risk of HT and intracerebral bleeding, specifically into the area of ischemia. AF was reported to be an independent predictor of HT [3]. In fact, HT risk was nearly fivefold higher in patients with AF than in those without AF [4].
Several studies have identified risk factors for HT in patients with cerebral infarction including vascular risk factors, laboratory and radiological findings [5,6,7], but most of these studies were retrospective [5, 7]. The increasing use of heme-sensitive T2* Gradient-Recall Echo (GRE-T2) and susceptibility-weighted (SWI) sequences in magnetic resonance imaging (MRI) of the brain had led to detection of many predicting radiological markers [8, 9]. The loss of integrity of the endothelial basal lamina seems to be the primary cause of hemorrhage after focal cerebral ischemia [10]. Cerebral microbleeds (CMB) are a promising radiological marker of the cerebral small vessel diseases that are prone to bleeding and cause spontaneous ICH [11]. Most currently available bleeding risk scores, which include clinical risk factors but not neuroimaging markers, show only modest predictive value [12].
The aim of this study is to evaluate the incidence of hemorrhagic transformation and associated risk factors in ischemic stroke with AF patients.
Methods
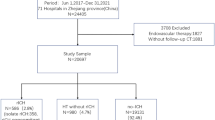
The present study is a prospective hospital-based study and included 250 ischemic stroke patients with AF that were recruited from stroke and intensive care units of Neurology Department from June 2019 to March 2021. Inclusion criteria included any ischemic stroke patients with AF and aged ≥ 18 years. Exclusion criteria included patients with hepatic, renal or hematological diseases. Informed consent was obtained from participating patients or their relatives. Ethical approval was obtained from the ethics committee of the neurology department.
All patients were subjected to detailed history taking with paying special attention to medical history of stroke risk factors. Medical data were obtained from the patients and/or their relatives. Complete general and neurological examinations including Glasgow Coma Scale (GCS) were performed. National Institutes of Health Stroke Scale (NIHSS) was conducted to evaluate the severity of the stroke. Electrocardiogram (ECG) was performed for all patients.
Full laboratory tests were done including complete blood count (CBC), lipid profile (total cholesterol, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C) and triglycerides), blood glucose level, liver and renal function tests, platelet count, international normalized ratios, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).
At admission, all patients had performed conventional MRI, GRET2WI (T2*WI) and diffusion-weighted image using 1.5 T MR Scanner (Achieva, Philips Medical System) and section thickness was set at 5 mm and gap was set at 1 mm. Follow-up computed tomography (CT) or MRI scans after one week of admission or immediately in case of clinical worsening was done for detection of HT. Patients were divided into two groups according to presence of the HT.
CMBs were identified as hypointense or black (signal loss) rounded regions with < 10 mm in diameter on T2*WI and validated Brain Observer Microbleed Scale was used for rating the CMBs [13].
Superficial siderosis was identified as hypointensity gyriform without matching hyperintense signal on T1-weighted sequences or FLAIR [14]. Fazekas scale was used to assess deep white matter lesions in FLAIR sequences [15]. The reference template of the brain at older age was used to evaluate cerebral atrophy [16]. Data were analyzed by two radiologists who were blind to the study design.
Infarct size was measured during a second CT or MRI scan and was determined by the largest diameter of the lesion [17]. The size of infarct was ranked as follows: small size (< 1 cm), medium size (1–3 cm) and large size (> 3 cm) [17].
Statistical analysis was performed using SPSS statistics (V.20.0, 2010; IBM Corp., NY, USA) [18]. Qualitative variables were presented as numbers and percentages, whereas quantitative variables were expressed as mean (± SD) or median (IQR) for normally and non-normally distributed data, respectively. Comparison between groups was performed using the χ2, t-test or Mann–Whitney U nonparametric test as appropriate. The Alpha level of significance was set at p-value of 0.05 or less. Association of risk factors identified in univariate analysis with HT was assessed using multivariable logistic regression analysis. Level of association between HT and risk factors was presented as adjusted odds ratios.
Results
A total of 234 from 250 ischemic stroke patients with AF completed the study. Five patients refused to continue the study, 7 patients had missing data and 4 patients were discharged before having the second neuroimaging had been done. Demographic and clinical characteristics of the studied AF ischemic stroke patients are presented in Table 1. There were 119 (50.9%) male and 115 (49.1%) female patients with the mean age (± SD) of patients was 64.04 (± 9.58) years. The incidence of HT was 22.6% as detected in 53 patients. The prevalence of CMB was 40.6%, 41.5% had WML, superficial siderosis was 16.2% and cortical atrophy was detected in 38.5% of the patients. Stroke severity, as assessed using NIHSS scale on admission, was 13.77 (± 6.27).
As regard site of infarction, there were 172 patients (73.5%) that had anterior circulation infarction in which 68% had middle cerebral artery and 5.5% had anterior cerebral artery. Forty-eight patients (20.5%) had posterior circulation infarction and the remaining of patients (14 patients) had multiple infarction sites. Regarding the site of microbleed, CMBs were found more often in lobar areas than deep areas.
The patients were classified into 2 groups based on the presence of HT (group without HT and group with HT) (Table 2). Patients with HT were more likely to be older in age, hypertensive, diabetic, on anticoagulant medications and had higher NIHSS score at admission. Analysis of neuroimaging data showed statistically significant difference between both groups regarding the presence of CMB, SS and size of infarction. CMB was significantly higher in group with HT (64.4%) than in group without HT (33.2%) and the presence of ≥ 10 CMB was related to higher risk of HT. Patients in the group with HT had larger infarction size (49.1%) compared to the group without HT (28.7%) (Table 2). HT was detected in 39 patients that had anterior circulation infarction, 10 patients had posterior circulation infarction and 4 patients had multiple infarction sites. However, most of HT cases took place in the anterior circulation, there was no statistically significant difference regarding the site (χ2 = 0.298, p = 0.862).
Multivariable logistic regression analysis was used to evaluate the association of significant factors in univariate analysis with risk of HT. Age [odds ratio (OR): 1.05, confidence interval (CI) 1.01–1.09], size of infarction (OR: 2.57, CI 1.06–6.27) and CMB ≥ 10 (OR: 4.68, CI 1.71–12.84) were found to be a significant risk factors of HT (Table 3).
Discussion
The frequency of HT was 22.6% in the present prospective study. This frequency was slightly higher than the frequency of HT (19.2%) we previously reported in ischemic stroke patients without AF [19] and that could be due to the inclusion of higher risk group of ischemic stroke patients. Our frequency of HT was similar with the result of D’Anna and colleagues who reported a frequency of 20.6% HT in patients with AF [20]. Similar to stroke patients without AF, large infarction size and CMB were significantly associated with incidence of HT [19]. However, NIHSS was not statistically significant using logistic regression analysis. This could be explained by the nature of stoke in AF patients that is characterized by high NIHSS [21].
The present study has shown that old age, size of infarction, and CMB ≥ 10 were independently associated with increased risk for HT in ischemic stroke patients with AF. Our results suggest that large infarction size independently predicts HT in ischemic stroke patients with AF. This was consistent with the results of several studies in patients of other ethnicities [6, 7, 19,20,21,22,23,24]. According to the current European guidelines on AF, it was recommended to consider the size of brain infarction and HT before starting anticoagulation. In addition delaying oral anticoagulation was suggested with moderate or large infarction size [25]. It has been reported that HT risk increases by fourfold by increasing the of infarct size from one level to the next [4].
In the current study, we investigated the effect of different grades of CMBs that could predict the risk of future HT. Our data provide some evidence to suggest that CMBs ≥ 10 could be a predictor for HT. This was supported by a recent study demonstrated that the presence of CMB ≥ 5 on MRI might identify subgroups of AF ischemic stroke patients with high ICH risk [26]. Consistent with our result, several studies demonstrated increased HT in patients with CMB [19, 27,28,29,30]. CMB could be an indicator for the severity of cerebral microangiopathy that could explain the abnormal permeability of the arteriolar BBB leading to extravasations of blood components [28, 31]. Therefore, it was suggested that these lesions might be indicative of a bleeding prone state in HT after acute ischemic stroke.
Regarding age in the present study, age was a statistically significant independent predictor for HT. This was in agreement with the result of other studies that identified age as one of the most risk factor for HT [32,33,34]. Moreover, a recent met-analysis included 15 studies with 3480 ischemic stroke patients documented the significant association of HT and age [5].
Our results led to the identification of predictive factors in AF stroke patients who are clinically relevant high-risk for developing HT. Our findings suggest that caution should be considered when prescribing antithrombotic therapies to patients with old age, large cerebral infarction and CMB. Incorporation of MRI screening into clinical practice for patients with AF will lead to considerable improvements in stroke prevention and predictive ability for identification of risk factors for HT. Our study was limited by the study design as the study involves a sample of patients at a single hospital and not a multicenter study, although it is a large hospital in Egypt that serves a wide neighboring area. In addition, we did not study association of HT incidence with the type of AF because a large number of our patients were accidentally diagnosed after admission. Therefore, we could not detect its exact type since persistent and permanent diagnosis would require a much longer time scale than our study. Even with these limitations, our data strongly suggest that age, size of infarction, and CMB are important predictors of HT in ischemic stroke patients with AF. Future risk scores should include CMB as a neuroimaging marker in addition to clinical parameters to identify patients with stroke at risk of HT and further studies are urgently needed to determine how neuroimaging markers may contribute to improvement of risk prediction of HT.
Availability of data and materials
The data results generated or analyzed during this study are included in this published article.
Abbreviations
- AF:
-
Atrial fibrillation
- HT:
-
Hemorrhagic transformation
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- CMB:
-
Cerebral microbleed
- SS:
-
Superficial siderosis
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- BBB:
-
Blood brain barrier
- GCS:
-
Glasgow Coma Scale
- NIHSS:
-
National Institutes of Health Stroke Scale
- ECG:
-
Electrocardiogram
- CBC:
-
Complete blood count
- HDL-C:
-
High-density lipoprotein-cholesterol
- LDL-C:
-
Low-density lipoprotein-cholesterol
- ESR:
-
Erythrocyte sedimentation rate
- CRP:
-
C-reactive protein
References
Chen G, Wang A, Zhao X, Wang C, Liu L, Zheng H, et al. Frequency and risk factors of spontaneous hemorrhagic transformation following ischemic stroke on the initial brain CT or MRI: data from the China National Stroke Registry (CNSR). Neurol Res. 2016;38:538–44.
Tu HT, Campbell BC, Christensen S, Desmond PM, De Silva DA, Parsons MW, EPITHET-DEFUSE Investigators, et al. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation. Int J Stroke. 2015;10:534–40.
Jickling GC, Liu DZ, Stamova B, Ander BP, Zhan XH, Lu AG, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–99.
Tan S, Wang D, Liu M, Zhang S, Wu B, Liu B. Frequency and predictors of spontaneous hemorrhagic transformation in ischemic stroke and its association with prognosis. J Neurol. 2014;261:905–12.
Honig A, Percy J, Sepehry AA, Gomez AG, Field TS, Benavente OR. Hemorrhagic transformation in acute ischemic stroke: a quantitative systematic review. J Clin Med. 2022;11:1162.
Paciaroni M, Agnelli G, Corea F, Ageno W, Alberti A, Lanari A, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke. 2008;39:2249–56.
Zhang K, Luan J, Li C, Chen M. Nomogram to predict hemorrhagic transformation for acute ischemic stroke in Western China: a retrospective analysis. BMC Neurol. 2022;22:156.
Karayiannis C, Soufan C, Chandra RV, Chandra RV, Phan TG, Wong K, et al. Prevalence of brain MRI markers of hemorrhagic risk in patients with stroke and atrial fibrillation. Front Neurol. 2016;7:151.
Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003.
Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/reperfusion. J Cereb Blood Flow Metab. 1996;16:1373–8.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38.
Chao TF, Lip GYH, Lin YJ, Chang SL, Lo LW, Hu YF, et al. Major bleeding and intracranial hemorrhage risk prediction in patients with atrial fibrillation: attention to modifiable bleeding risk factors or use of a bleeding risk stratification score? A nationwide cohort study. Int J Cardiol. 2018;254:157–61.
Cordonnier C, Potter GM, Jackson CA, Doubal F, Keir S, Sudlow CLM, et al. Improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke. 2009;40:94–9.
Zonneveld HI, Goos JD, Wattjes MP, Prins ND, Scheltens P, van der Flier WM, et al. Prevalence of cortical superficial siderosis in a memory clinic population. Neurology. 2014;82:698–704.
Wahlund LO, Barkhof F, Fazekas F. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–22.
Farrell C, Chappell F, Armitage PA, Keston P, Maclullich A, Shenkin S, et al. Development and initial testing of normal reference MR images for the brain at ages 65–70 and 75–80 years. Eur Radiol. 2009;19:177–83.
Pan SL, Wu SC, Wu TH, Lee TK, Chen TH. Location and size of infarct on functional outcome of non cardioembolic ischemic stroke. Disabil Rehabil. 2006;28:977–83.
Corp IBM. IBM SPSS statistics for windows, version 20. Armonk: IBM Corp; 2010.
Elsaid AF, Fahmi RM, Shehta N, Ramadan BM. Machine learning approach for hemorrhagic transformation prediction: capturing predictors’ interaction. Front Neurol. 2022;13:951401.
D’Anna L, Filippidis FT, Harvey K, Marinescu M, Bentley P, Korompoki E, Veltkamp R. Extent of white matter lesion is associated with early hemorrhagic transformation in acute ischemic stroke related to atrial fibrillation. Brain Behav. 2021;11:e2250.
Paciaroni M, Bandini F, Agnelli G, Tsivgoulis G, Yaghi S, Furie KL, et al. Hemorrhagic transformation in patients with acute ischemic stroke and atrial fibrillation: time to initiation of oral anticoagulant therapy and outcomes. J Am Heart Assoc. 2018;7:e010133.
Lee JH, Park KY, Shin JH, Cha JK, Kim HY, Kwon JH, et al. Symptomatic hemorrhagic transformation and its predictors in acute ischemic stroke with atrial fibrillation. Eur Neurol. 2010;64:193–200.
Marsh EB, Llinas RH, Schneider AL, Hillis AE, Lawrence E, Dziedzic P, et al. Predicting hemorrhagic transformation of acute ischemic stroke: prospective validation of the HeRS Score. Medicine. 2016;95:e2430.
Muscari A, Faccioli L, Lega MV, Lorusso A, Masetti M, Trossello MP, et al. Predicting hemorrhagic transformation and its timing from maximum cerebral lesion diameter in non lacunar ischemic strokes. Brain Behav. 2020;10:e01497.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893.
Charidimou A, Karayiannis C, Song TJ, Orken DN, Thijs V, Lemmens R, International META-MICROBLEEDS Initiative, et al. Brain microbleeds, anticoagulation, and hemorrhage risk: meta-analysis in stroke patients with AF. Neurology. 2017;5(89):2317–26.
Chen J, Duris K, Yang X. Effect of cerebral microbleeds on hemorrhagic transformation and functional prognosis after intravenous thrombolysis of cerebral infarction. Brain Hemorrhages. 2022;3:117–9.
Dar NZ, Ain QU, Nazir R, Ahmad A. Cerebral microbleeds in an acute ischemic stroke as a predictor of hemorrhagic transformation. Cureus. 2018;10:e3308.
Lee SJ, Hwang YH, Hong JM, Choi JW, Park JH, Park B, et al. Influence of cerebral microbleeds on mechanical thrombectomy outcomes. Sci Rep. 2022;12:3637.
Xu CX, Xu H, Yi T, Yi XY, Ma JP. Cerebral microbleed burden in ischemic stroke patients on aspirin: prospective cohort of intracranial hemorrhage. Front Neurol. 2021;12:742899.
Koennecke HC. Cerebral microbleeds on MRI: Prevalence, associations, and potential clinical implications. Neurology. 2006;66:165–71.
Marsh EB, Llinas RH, Hillis AE, Gottesman RF. Hemorrhagic transformation in patients with acute ischaemic stroke and an indication for anticoagulation. Eur J Neurol. 2013;20:962–7.
Chen S, Lu X, Zhang W, Han Z, Yang W, Huang X, et al. Does prior antiplatelet treatment increase the risk of hemorrhagic transformation and unfavorable outcome on day 90 after intravenous thrombolysis in acute ischemic stroke patients? J Stroke Cerebrovasc Dis. 2016;25:1366–70.
Pande SD, Win MM, Khine AA, Zaw EM, Manoharraj N, Lolong L, et al. Haemorrhagic transformation following ischaemic stroke: a retrospective study. Sci Rep. 2020;10:5319.
Acknowledgements
Not applicable.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
RF, TE, HH, and BR were involved in crafting the study topic and design. All authors participated in manuscript drafting. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of the neurology department (January, 2018), Faculty of Medicine, Zagazig University. An informed written consent was obtained from all participants. The participants were informed about the purpose of the study.
Consent for publication
Is not applicable in this section.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fahmi, R.M., Elkhatib, T.H.M., Hafez, H.A.F. et al. Factors influencing hemorrhagic transformation in ischemic stroke patients with atrial fibrillation: a hospital based-study. Egypt J Neurol Psychiatry Neurosurg 59, 138 (2023). https://doi.org/10.1186/s41983-023-00739-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00739-y