Abstract
Background
The use of the pipeline embolization device (PED) with single or multiple coverage in cases of intracranial aneurysms is still not well defined. We aimed to compare rates of aneurysm occlusion and complications in patients covered with single versus multiple PEDs.
Methods
For this systematic review, we searched PubMed and SpringerLink databases, and citations for studies on September 2022. All peer-reviewed studies of adult patients diagnosed with intracranial aneurysm covered with single and multiple PEDs were assessed, and the rates of aneurysm occlusion and complications were collected, and have been published between April 20, 2011, and September 30, 2022. The risk of bias assessment was scored using the Newcastle–Ottawa Quality Assessment Scale for cohort studies. Evidence from studies was synthesized as narrative synthesis.
Results
A total of 5 studies with 772 patients and 795 aneurysms were included. A total of 531 (68.8%) patients were covered with a single PED, while 241 (31.2%) with multiple PEDs. The aneurysms are mostly located in the anterior circulation, with 93.84% in the single PED versus 86.08% in the multiple PEDs group. A total of 525 (92.58%) saccular types of aneurysms were covered in a single PED versus 222 (86.98%) in the multiple PEDs group. The overall aneurysms occlusion rates in approximately midterm follow-up were 72.34% in the single PED versus 87.04% in the multiple PEDs group. The overall complication rates among studies were 6.54% in a single PED versus 8.24% in the multiple PEDs group.
Conclusions
There is no significant difference in overall intracranial aneurysm occlusion rates when comparing single versus multiple PEDs coverage for treatment of aneurysms, primarily with longer follow-up times, with low and no significantly different complication rates between groups.
Similar content being viewed by others
Introduction
The Pipeline embolization device from Medtronic, United States, is a braided, multi-alloy, small cylindrical mesh woven, high metal surface area flow diverter which is used to cover the artery walls, diverting blood to flow as usual past the aneurysm rather than into it, and deprived of its blood supply to the aneurysms by altering the blood flow dynamics, reduce blood flow into aneurysm, make it shrink and disappears [1]. Treatment with PED is an effective solution for patients with intracranial aneurysms (IAs). A flow diverter (FD) such as PED is indicated for treatment of wide-necked and large aneurysms [2, 3]. However, large and wide neck aneurysm account for only a small proportion of all IAs, where approximately 80% of all unruptured IAs are small and medium, and most ruptured IAs are smaller than 10 mm [4]. Several studies have examined the efficacy of PED for small and medium IAs, and shown high occlusion rates with low complications rates [5, 6].
Several treatment strategies are applied to increase IA’s occlusion with low complication rates. This includes the coverage of multiple PEDs to increase the metal coverage area across the aneurysm neck [7]. However, the use of a single PED to cover the neck of the aneurysm or multiple PEDs to increase total metal coverage has been controversial. The objectives of the review were to focus on the use of a single PED compared to multiple PEDs in treating patients with IA, then summarize the rates of IA occlusion and periprocedural complications in patients covered with a single PED versus multiple PEDs.
Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Review (PRISMA) guidelines.
Eligibility criteria
Studies were eligible for inclusion if they met the following criteria based on population, intervention-comparison and outcome (PICO):
-
1.
Population: We included adult patients diagnosed with IA of several types (saccular, fusiform, dissecting, or blister-like aneurysm), sizes (small, medium, or large), locations (anterior or posterior circulation), ruptured or unruptured, narrow-neck or wide-neck aneurysm.
-
2.
Intervention: Intervention based on the use of a single/one coverage of PED.
-
3.
Comparison: The use of multiple/more than one coverage of PEDs.
-
4.
Outcome: Outcome had to assess the rates of IA occlusion and complications on clinical and radiographic follow-up.
-
5.
Study design: Peer-reviewed studies.
-
6.
Time: Articles considered include those published between April 20, 2011, and September 30, 2022.
-
7.
Language: Only in English.
Studies were excluded from the review if the studies published before April 20, 2011, because that was the year the device was first approved by the Food and Drug Administration (FDA); not written in English, grey literature and other non-peer-reviewed studies, and studies that not included both outcomes of IA’s occlusion and complications rates.
Information sources and search strategy
The collection of study articles was conducted in September 2022, by searching through the database of PubMed and SpringerLink, and manually searching for bibliographies as additional references from April 20, 2011, and September 30, 2022. The final keywords utilized in PubMed include: “aneurysm” OR “intracranial aneurysms” AND “Pipeline embolization device” OR “Pipeline embolization devices” OR “single Pipeline embolization device” OR “multiple Pipeline embolization device” OR “number of Pipeline embolization devices” AND “occlusion”. The additional filters include dates published between April 20, 2011, and September 30, 2022, and language only in English. The keyword in SpringerLink is “Pipeline embolization device”, with additional filtering in the content type of Article, language only in English, and date published between 2011 – 2023.
Selection process
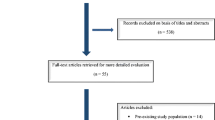
Two researchers (RCK, AG) independently screened titles and abstracts of all articles retrieved. In case of disagreement, consensus on which articles to screen full-text was reached by discussion. If necessary, the third researcher was consulted to make the final decision. Next, two researchers (AB, RCK) independently screened full-text articles for inclusion. Again, in case of disagreement, consensus was reached on inclusion or exclusion by discussion and, if necessary, the third researcher (JP) was consulted. We included studies that reported a comparison of the use of a single versus multiple PEDs coverage in treating patients with IA, with described outcomes of IA’s occlusion and complication rates. The study selection process is depicted in Fig. 1.
Data collection process and data items
Data extraction was performed independently by 2 review authors (AB and RCK) from eligible studies. Extracted data were compared, with any discrepancies being resolved through discussion. RCK entered data into the extraction data form. Data is extracted and documented based on predetermined criteria to identify relevant information. Data collected from each reference included author’s last name, year of publication, study period, study design, country, setting, sample size, mean age, sex (female), interventions (included pre-procedural medications, aneurysm, devices, post-procedural medications) and outcomes (occlusion/obliteration and complications rates).
Risk of bias assessment
The risk of bias assessment with each study was scored using the Newcastle–Ottawa Quality Assessment Scale (NOS) for cohort studies. The NOS addresses three specific domains: (1) Selection; (2) Comparability; and (3) Outcome. Two review authors independently applied the tool to each included study, and recorded supporting information and justifications for judgments of risk of bias for each domain (Good quality; fair quality; poor quality). Any discrepancies in judgments of risk of bias or justifications for judgments were resolved by discussion to reach consensus between the two review authors, with a third review author acting as an arbiter if necessary. Score results from NOS are then visualized in the traffic-light plot and summary plot using Robvis.
Synthesis methods
Structured summaries are used as narrative synthesis with guidance by Synthesis Without Meta-analysis (SWiM) reporting guidelines [8].
Results
Study selection
A total of 535 records were identified using the search strategy. After removal of 16 duplicates, we screened 519 records. After screening based on title and abstract, 7 articles were retrieved for full-text review. From 7 full text-text articles, 2 articles were excluded. Ultimately, 5 articles met all inclusion criteria and were included in the systematic review. Full details of studies are shown in Fig. 1.
We excluded 2 studies from our review [9, 10]. We excluded studies because Tan 2014 only reports about aneurysm occlusion rates, and Cler 2022 only reports about complications.
Study characteristics
In a review examining the comparison between a single PED versus multiple PEDs in patients with IA, the authors included a table presenting for each included study: the citation, study period, study design, country, setting, sample size, mean age, sex (female), interventions, and outcomes.
In total, the data consisted of 772 patients with 795 IAs treated with PED. A total of 531 (68.8%) patients were covered with a single PED, while 241 (31.2%) patients were covered with multiple PEDs. Based on available information, the mean age of the participants across 5 studies was 55 years (SD = 12.8) for patients in a single PED group and 56.9 years (SD = 12.2) for patients in a multiple PEDs group, ranging from 42 to 69 years across all treatments. A total of 477 (87.44%) female patients were covered with a single PED, while 208 (80.92%) female patients with multiple PEDs. For details, see Table 1.
Results of individual studies
For details, see Table 2.
Intracranial aneurysm characteristics
Aneurysm characteristics included in the studies were compared. The IAs treated with PED are mostly located in the anterior circulation, with 93.84% in the single PED group and 86.08% in the multiple PEDs group. One study covered whole PEDs in anterior circulation and excluded posterior circulation [1]. Three other studies showed no difference between a single PED group versus the multiple PEDs group in anterior circulation (P = 0.6, P = 0.08, P = 0.19, respectively) [7, 11, 12]. Two studies only included saccular types of aneurysm [7, 12], while other studies also included fusiform, dissecting and blister-like aneurysm, and reported that no difference between them in a single PED group versus multiple PEDs groups [1, 11, 13]. We counted a total of 525 (92.58%) saccular types of aneurysms in a single PED and 222 (86.98%) saccular aneurysms in multiple PEDs groups. The mean size was reported in 4 studies (except by Vranic and colleagues, it is not stated), with the average aneurysm size was 7.2 mm in a single PED and 9.3 mm in multiple PEDs groups. Three studies stated that the mean size of aneurysms is larger in multiple PEDs groups rather than a single PED group [11,12,13], while 2 other studies show there were no significant differences in size between groups [1, 7].
Intervention characteristics
All patients in studies received DAPT several days before the procedure, mostly with aspirin and clopidogrel. Four studies performed platelet function tests, except a study by Vranic and colleagues. [7]. Poor non-responders to clopidogrel were then switched to prasugrel or ticagrelor as an alternative. Then, 4 studies stated that post-procedural DAPT was administered for several months, except a study by Waqas and colleagues. [12].
We compared the number of devices used in patients treated with PED. These are covered using a single PED versus multiple PEDs. A total of 531 (68.8%) patients were covered with a single PED, while 241 (31.2%) patients were covered with multiple PEDs. Three studies reported that patients in a single PED group were higher than multiple PEDs groups [7, 11, 12], while one study showed patients higher in using multiple PEDs [1], and the remaining study stated no difference between single versus multiple PEDs [13].
Aneurysm occlusion rates
Chalouhi and colleagues reported no difference in the rate of complete or near-complete occlusion, with 84% occlusion rate in a single PED and 87% in multiple PEDs groups (P = 0.8). However, the mean of follow-up in a single PED is longer than multiple PEDs groups (7 months versus 8.9 months, P = 0.01). Retreatment was necessary in 6% of patients in a single PED and 7.5% of patients in multiple PEDs, with no difference in proportion (P = 0.8) [11]. Kabbasch and colleagues reported midterm follow-up (median 7 months) with 70% favorable occlusion in the single PED group and much higher (100%) in multiple PEDs groups (P = 0.03). Complete occlusion counted 60% in a single PED group and 93% higher in multiple PEDs (P = 0.05). Retreatment was necessary in 15% of patients in a single PED and no retreatment in multiple PEDs [13]. Waqas and colleagues reported a 6-month follow-up that was much higher in the multiple PEDs group rather than the single PEDs group (90% versus 67.1%, P = 0.028). The rates of aneurysm occlusion increased in the 12-month follow-up, with multiple PEDs higher than the single PED groups (74.7% versus 91.7%, P = 0.04). On the latest follow-up ≥ 12 months, favorable and complete occlusion reached 100% in multiple PEDs groups and 86.7% in a single PED group (P = 0.057). The rate of retreatment was necessary in 16.2% of single PED patients, versus no retreatment in multiple PEDs (P = 0.01) [12]. Link and colleagues reported 6- and 12-months follow-up with rates of occlusion much higher in multiple PEDs than in a single PED group (75.6% versus 92.9%, P = 0.017 and 81.1% versus 98.4%, P = 0.014, respectively). However, in the longest follow-up showed no difference in obliteration rates between single versus multiple PEDs (92.5% versus 100%, P = 0.083). Retreatment was necessary between groups without significant difference (9.3% versus 3.2%, P = 0.212) [1]. Vranic and colleagues reported 6- and 12-months, and the latest follow-up with no significant difference between groups (70% versus 68.8%; 81.2% versus 83.4%; and 83.6% versus 83.4%, P = 0.65 respectively). Retreatment is needed in 8% of the single PED and 10.4% in multiple PEDs groups without significant difference (P = 0.58) [7]. The overall IAs occlusion rates in approximately midterm follow-up were 72.34% in the single PED and 87.04% in the multiple PEDs group.
Complications rates
Chalohi and colleagues reported higher total complications in patients with multiple PEDs than a single PED (15% versus 5%, P = 0.03). In a single PED group, there were 4 thromboembolic, 3 hemorrhagic (1 distal ICH and 2 aneurysms ruptured), and 4 in-stent thrombosis complications, while in multiple PEDs there were 4 thromboembolic, 4 hemorrhage (all distal ICH), and 2 in-stent thrombosis complications. In stent-stenosis noted in the same portion between groups (± 5% respectively, P = 0.95) [11]. Kabbasch and colleagues reported total complications of 5% in a single PED and 5.5% in multiple PEDs, and the complications were 1 in-stent thrombosis, respectively [13]. Waqas and colleagues reported total complications of 5.6% versus 5.6% between groups. Thromboembolic complications were noted at 2.8% respectively. In a single PED, there were 1 TIA and 2 infarcts, while 1 infarct was in multiple PEDs groups (P = 0.49). Three SAHs are noted in a single PED and 1 SAH in multiple PEDs [12]. Link and colleagues reported 9.1% total complications in single PED and 10.9% in multiple PEDs patients. In this study, complications were divided into major and minor complications in less than 30 or more than 30 days. In major < 30 days there were 2.2% versus 1.1% complications among groups, including 1 ICH and delayed rupture of cavernous ICA aneurysm (P = 0.648). In minor < 30 days there were 4.3% versus 5.3% complications, including 4 thromboembolic, 1 occipital ICH, 1 CCF, and 1 stent occlusion (P = 0.800). In major < 30 days, there were 1.1% (1 thromboembolic) complications in multiple PEDs groups and none in a single PED (P = 0.320). In minor > 30 days, there were 2.6% versus 3.4% complications included 3 visual disturbances and 1 blindness (P = 0.821) [1]. Vranic and colleagues reported total intracranial complications of 6% versus 4.2% between groups (P = 0.42). In single PED included 5 ICH, 2 stroke, 5 TIA, 2 in-stent stenosis, and 1 cranial neuropathy. In multiple PEDs, there was 1 stroke and 1 in-stent thrombosis [7]. Overall complication rates among studies were 6.54% in a single PED versus 8.24% in multiple PEDs groups.
Risk of bias in studies
We used the Newcastle–Ottawa Scale Quality Assessment Scale for cohort studies to assess the risk of bias for each of the included studies. A summary of these assessments is provided in Table 3.
Five of the trials resulted in good quality studies. However, 3 studies had a subject lost to follow-up rate of less than 80%. Therefore, the overall outcome domain in these studies was assessed as Some Concerns (see Figs. 2 and 3).
Discussion
A study by Waqas and colleagues and Link and colleagues showed a significant difference in the occlusion rate in the 6- and 12-months follow-up, with multiple PEDs group had higher occlusion rates than single PED, but on the latest follow-up ≥ 12 months showed no significant difference (P = 0.057 and P = 0.083), respectively. Waqas and colleagues stated the number of PEDs as an independent predictor in 12-months occlusion rates (OR 6.3, 95% CI 1.8–22.8, P = 0.005), while Link and colleagues stated multiple PEDs as an independent predictor of aneurysm occlusion rate at 6 months follow-up (P = 0.015) [1, 12]. Chalouhi and colleagues with mean midterm follow-up of 7 and 8.9 months, and Vranic and colleagues with 6, 12 and ≥ 12 months follow-up showed no difference in the occlusion rates [7, 11]. However, a study by Kabbasch and colleagues showed that occlusion rates were higher in multiple PEDs groups on median 7-months follow-up [13]. We suggest that a longer follow-up period of more than 6 months (likely up to 12 months or more) is needed to assess a better aneurysm occlusion rate. This is in line with a study stating that aneurysm occlusion rates with PED increased with time, confirmed with mid to long-term control angiography [14]. A study by Damiano and colleagues also states that compacting a single PED can outperform overlapping 2 PEDs in aneurysmal flow reduction [15]. Thus, we thought that the occlusion rates for single and multiple PEDs would be no different on longer follow-up.
Four studies showed no significant difference in the rate of complications between single versus multiple PEDs group [1, 7, 12, 13]. However, Chalouhi and colleagues revealed much higher total complication rates in the multiple PEDs groups [11]. This could possibly be due to the older patients’ age compared to the single PED group (P = 0.04) and a higher mean size of aneurysm (P = 0.004), despite a study by Kabbasch and colleagues showing no significant difference in complications between groups related to higher mean size of aneurysm in multiple PEDs. One study concluded that increasing age is associated with higher neurological morbidity and mortality after coverage of intracranial aneurysms [16]. The study by Tan and colleagues reported that longer procedures (> 116 min) and multiple PEDs coverage (> 1) were significant risk for symptomatic thromboembolic events (p < 0.01) [9].
This systematic review has several limitations. We included literature only in English. The studies included were limited by its overall retrospective design, and most studies were conducted in a single center. Thus, results may not be entirely generalizable. In addition, due to the small sample size and unequal portion in the number of samples between groups, there is risk of selection bias. Additional limitations include subjects lost to follow-up, and problems in decision-making regarding the number of PEDs used as this is not yet standardized and is operator dependent. The techniques and equipment used for PED coverage and the DAPT regimen used were also operator dependent. The study by Vranic and colleagues did not perform the platelet function test and may potentially influence outcomes. Several studies did not assess occlusion rates at the immediate post-procedural and 12-months follow-up. Validated measurements are not clearly stated in studies, except by Kabbasch and colleagues. However, we also cannot describe the diameter of each PED that overlapped over one and another in multi-PED settings in increasing porosity and to increase the range of coverage values due to lack of information. Lastly, we only described aneurysms located in anterior or posterior circulation, and did not describe each arterial location covered by the PED in whole studies due to lack of data obtained from 1 study.
Conclusions
Our systematic review of 5 studies found that there is similarity in overall IA occlusion rates when comparing single PED versus multiple PEDs for treatment of IAs, primarily with longer follow-up times, with low and no significantly different in complication rates between the single and multiple PEDs group.
Availability of data and materials
All data generated or analyzed during the study are included in this published article.
Abbreviations
- ASA:
-
Aspirin
- CPG:
-
Clopidogrel
- DAPT:
-
Dual antiplatelet
- FD:
-
Flow diverter
- FU:
-
Follow-up
- IA:
-
Intracranial aneurysm
- NOS:
-
Newcastle–Ottawa scale
References
Link TW, Carnevale JA, Goldberg JL, Jones C, Kocharian G, Boddu SR, et al. Multiple pipeline embolization devices improves aneurysm occlusion without increasing morbidity: a single center experience of 140 cases. J Clin Neurosci. 2021;86:129–35.
Kallmes DF, Brinjikji W, Cekirge S, Fiorella D, Hanel RA, Jabbour P, et al. Safety and efficacy of the pipeline embolization device for treatment of intracranial aneurysms: a pooled analysis of 3 large studies. J Neurosurg. 2017;127(4):775–80.
Hanel RA, Kallmes DF, Lopes DK, Nelson PK, Siddiqui A, Jabbour P, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: the PREMIER study 1 year results. J Neurointerv Surg. 2020;12(1):62–6.
Wiebers DO. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–10.
Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. 2015;36(1):108–15.
Lin N, Brouillard AM, Keigher KM, Lopes DK, Binning MJ, Liebman KM, et al. Utilization of Pipeline embolization device for treatment of ruptured intracranial aneurysms: US multicenter experience. J Neurointerv Surg. 2015;7(11):808–15.
Vranic JE, Harker P, Stapleton CJ, Regenhardt RW, Alotaibi NM, Leslie-Mazwi TM, et al. Impact of endoluminal flow diverter number on aneurysm treatment outcomes: a multicenter study. Stroke. 2022;2(3):1–8.
Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;16:1–5.
Tan LA, Keigher KM, Munich SA, Moftakhar R, Lopes DK. Thromboembolic complications with pipeline embolization device placement: impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerv Surg. 2015;7(3):217–21.
Cler SJ, Lauzier DC, Chatterjee AR, Osbun JW, Moran CJ, Kansagra AP. Time line of occlusion for Intracranial aneurysms treated with the pipeline embolization device. World Neurosurg. 2022;166:750–7.
Chalouhi N, Tjoumakaris S, Phillips JLH, Starke RM, Hasan D, Wu C, et al. A single pipeline embolization device is sufficient for treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2014;35(8):1562–6.
Waqas M, Vakharia K, Gong AD, Rai HH, Wack A, Fayyaz N, et al. One and done? the effect of number of pipeline embolization devices on aneurysm treatment outcomes. Interv Neuroradiol. 2020;26(2):147–55.
Kabbasch C, Mpotsaris A, Behme D, Dorn F, Stavrinou P, Liebig T. Pipeline embolization device for treatment of intracranial aneurysms—the more, the better? A single-center retrospective observational study. J Vasc Interv Neurol. 2016;9(2):14–20.
Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol. 2012;33(8):1436–46.
Damiano RJ, Tutino VM, Paliwal N, Ma D, Davies JM, Siddiqui AH, et al. Compacting a single flow diverter versus overlapping flow diverters for intracranial aneurysms: a computational study. AJNR Am J Neuroradiol. 2017;38(3):603–10.
Brinjikji W, Kallmes DF, Cloft HJ, Lanzino G. Age-related outcomes following intracranial aneurysm treatment with the pipeline embolization device: a subgroup analysis of the IntrePED registry. J Neurosurg. 2016;124(6):1726–30.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
AB, RCK, and AG independently involved in the selection process. AB and RCK also independently involved in data collection process. JP was consulted if any discussion needed. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bahar, A., Kohar, R.C., Gunawan, A. et al. Single versus multiple coverage of pipeline embolization device for treatment of intracranial aneurysms: a systematic review. Egypt J Neurol Psychiatry Neurosurg 59, 130 (2023). https://doi.org/10.1186/s41983-023-00713-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00713-8