Abstract
Background
Cognitive decline is a common presentation of Parkinson’s disease (PD) and a continued search exists for a reliable biomarker for early identification and management of this clinical problem. The objective of this study is to select the most useful biomarker in assessment of PD-related cognitive decline. This cross-sectional study included 47 patients with PD and 47 matched healthy controls. All participants were assessed by quantitative electroencephalography (QEEG) spectral (relative power and background peak frequency) and connectivity measures (coherence and phase lag degree), in addition to clinical evaluation using Unified Parkinson’s Disease Rating Scale (UPDRS)and Modified Hoehn and Yahr staging scale and neuropsychological assessment of the patients using Montreal Cognitive Assessment (MoCA).
Results
PD patients showed significantly higher relative power in all frequency bands over the right temporal region with no significant changes in peak frequency, coherence and phase lag degree compared to healthy controls. PD patients with impaired cognition (MoCA < 26) had significantly lower global relative power, more marked in alpha and beta frequency bands compared to PD patients with normal cognition. Alpha and beta relative power in frontal and temporal regions showed significant correlation with different cognitive domains of MoCA score.
Conclusions
QEEG measures especially spectral relative power could be used as adjunct to neuropsychological assessment in evaluation of PD-related cognitive decline.
Similar content being viewed by others
Background
Parkinson’s disease is characterized by loss of dopaminergic neurons and its presentation includes motor symptoms (bradykinesia, tremors, and rigidity) as well as non-motor symptoms (cognitive dysfunction, mood, and sleep disorders) [1].
The cognitive impairment profile of PD includes specifically visuospatial and executive dysfunction [2]. Neuropsychological assessment is used to evaluate severe cognitive deterioration, but has limitations related to patient factors like education, personality and intelligence [3].
Quantitative electroencephalography (QEEG) parameters could be ideal functional biomarkers to complement neuropsychological assessment of cognitive deterioration in patients with PD [4].
EEG has the advantages of being simple to acquire, inexpensive, widely available, not dependent on verbal or motor responses and non-invasively measures brain activity directly with high temporal resolution [3, 5]. Moreover, EEG has good test–retest reliability and is not affected by the candidate education and intelligence such as other cognitive tests [4]. All these factors support the suitability of EEG parameters as valuable functional biomarkers.
EEG was used in previous studies to test different clinical domains of PD patients including cognitive decline and demonstrated a leftward shift of the background rhythm frequency with a higher slower frequency power [3, 5,6,7].
QEEG analysis includes many different and complex parameters that can assess variable aspects of brain function. Among these are spectral measures like frequency power analysis and peak frequency, in addition to connectivity parameters which measure functional association among brain regions and quantify brain network disruption [8].
The aim of this study is to test the value of quantitative EEG parameters as functional biomarkers in assessment of patients with PD and to explore their correlation with the cognitive decline in such patients.
Methods
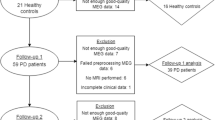
This cross-sectional case control study was conducted on 94 individuals divided into two groups (patients and control) in Beni-Suef University Hospitals during the period from April 2019 till March 2021. The study protocol was approved from the local ethical committee of faculty of Medicine, Beni-Suef University and an informed written consent was obtained from all participants before enrollment in the study.
The patient group included 47 patients fulfilling the criteria for diagnosis of Parkinson’s disease based on British Brain Bank criteria [9].They were recruited from neurology clinic of Beni-Suef University Hospitals, and they should be able to read, write and do simple calculations.
We excluded patients with secondary Parkinsonism, Parkinson Plus syndromes, cerebrovascular stroke, major language disturbance, severe physical, auditory, or visual impairment, and patients with MRI brain findings of multiple infarcts, severe white matter hyperintensity, intracerebral or subdural hemorrhage, tumors, or hydrocephalus. The control group included 47 healthy volunteers age and sex matched with the selected patients and did not include any of the patients’ relatives.
Patients were subjected to the following: clinical assessment including full history taking, complete general and neurological examination. Assessment and staging of Parkinson's disease was carried out using Unified Parkinson’s Disease Rating Scale (UPDRS) [10] and Modified Hoehn and Yahr staging scale [11]. Neuropsychological and cognitive assessment was performed using the Montreal Cognitive Assessment (MoCA) Arabic version with cutoff value < 26. It evaluates seven cognitive domains: visuospatial/executive functions, naming, memory, attention, language, abstraction, and orientation [12, 13]. Magnetic resonance imaging (MRI) of the brain was performed for all patients to exclude any structural lesion, atypical parkinsonism and Parkinson plus syndromes.
All participants (patients and controls) were assessed by quantitative electroencephalogram (QEEG). EEG was recorded using 19 gold disc electrodes were placed on the scalp using electrode paste; according to the international 10/20 system of electrode placement at electrode locations FP1, FP2, F7, F3, FZ, F4, F8, T3, C3, CZ, C4, T4, T5, P3, PZ, P4, T6, O1 and O2 with reference and ground electrodes placed at the forehead. The impedances of the electrodes were always kept below 5 kohms [3].
Raw EEG signals were recorded using Natus, Neurowork EEG system (Nicolet EEG V32 amplifier) with a frequency band of 1–70 Hz. The data were recorded using a sampling rate of 512 Hz. During the session of EEG recording, the subject was supine, relaxed, wakeful and with eyes closed in a quiet environment. An EEG technician followed the recording to ensure the signal quality, minimize eye and muscle artifacts and monitor the wakeful state. EEGs were reviewed by an EEGer with an acceptable experience in EEG interpretation who extracted high quality artifact-free recorded segments using the “Natus Neurowork EEG edit clip and export software tools” with a total duration of extracted segments 3–4 min [14].
The extracted EEG segments of all participants of the two study groups were imported to NeuroGuide software program (NeuroGuide, Deluxe 3.2.1, Applied NeuroScience) in the department of clinical neurophysiology (Neuro-Diagnostic and Research Center), faculty of medicine, Beni-Suef University. The records were re-referenced to linked ear reference, filtered at 1–30 Hz interval, and digitized at sampling rate of 256 Hz. Another manual selection procedure was conducted to obtain 2-s epochs with a minimum total duration of one minute of EEG without any visible artifacts. Split-half reliability and test–retest reliability tests were conducted to ensure high quality selection and only total records with values > 90 and 95%, respectively, were accepted for subsequent spectral analysis[15].
The total selected EEG segments were subjected to power spectral analysis using Fast Fourier Transform (FFT) with a 25% sliding window method of Kaiser and Sterman to eliminate the FFT windowing effects [16]. This yields the average power spectral values for the different frequency bands at each of the 19 recording sites. The used frequency bands were as follows: Delta (1–3.5 Hz), Theta (4–7.5 Hz), Alpha (8-13 Hz) and Beta (14–30 Hz) [8].
Relative band power was calculated by dividing the absolute band power of each frequency band by the total absolute band power from the FFT average per channel. This was calculated separately from six electrodes (F3, F4, T3, T4, O1 and O2). Regional relative band power was averaged as follows: frontal for F3 and F4, temporal for T3 and T4, and occipital for O1 and O2. Global average relative power was averaged from 17 electrodes (FP1 and FP2 were excluded) [3].
The peak frequency within each of the four frequency bands was computed per channel. This was evaluated separately from six electrodes (F3, F4, T3, T4, O1 and O2) and global average peak frequency was averaged from 17 electrodes (FP1 and FP2 were excluded).
Brain connectivity measures (EEG coherence and phase lag degree) were computed using the paired cross-spectrum comparing each single EEG channel to each of the remaining 18 channels yielding pairs of inter-electrode values of connectivity measures in the four specified frequency bands.
EEG coherence and phase lag were assessed at the intra and inter-hemispheric levels as follows: a.intra-hemispheric (fronto-parietal, F3–P3 and F4/-P4 and fronto-temporal, F3–T3 and F4–T4) b.inter-hemispheric (frontal, F3–F4, parietal, P3–P4, and temporal, T3–T4). Global average coherence and phase lag were calculated as the average of all 171 electrode combinations (19 scalp electrodes of the 10–20 system were involved).
Data were analyzed using SPSS (statistical package for the social science software) Version 25.0. Quantitative variables were expressed by mean, standard deviation and 95% confidence interval or by median and interquartile range (IQR) (as appropriate) and were compared using independent t test or Mann–Whitney U test (as appropriate). Qualitative variables were expressed by number and percent and were compared by χ2 test. Pearson correlation was used to correlate two continuous variables, otherwise Spearman correlation was used. In all tests, P-value was considered significant if less than 0.05.
Results
The patient group included 47 patients with Parkinson’s disease (21 males, their mean age was 62.85 ± 8.7 years). The control group included 47 participants (23 males, their mean age was 59.2 ± 8.3 years) matched with the patients in age and sex distribution (P = 0.19 and 0.71, respectively). Other clinical and cognitive characteristics of the patients are demonstrated in (Table 1). Based on the cutoff value of the MoCA score, 32 (68.1%) of the patients had cognitive impairment. All patients included in the study were taking anti-PD medication (levodopa–carbidopa combination).
The PD patients generally had higher relative power in all frequency bands and over all tested regions, however this difference was statistically significant mainly over the right temporal region (Fig. 1).
On the other hand, there was no statistically significant difference between the two study groups regarding the peak frequency in all frequency bands and over all brain regions (Additional file 1: Fig. S1).
The patients and controls showed comparable data with no statistically significant difference of both coherence and phase lag degree in all frequency bands and over all head regions (Additional file 1: Figs. S2, S3).
PD patients with impaired cognition had statistically significant lower global relative power in all frequency bands compared to patients with normal cognition. This difference was more consistent in alpha and beta frequency bands (Figs. 2, 3, 4, 5).
Comparing patient subgroups (normal and impaired cognition) with controls revealed a statistically significant higher relative power of theta, alpha and beta bands in PD patients with normal cognition compared to controls (data are not represented).
Moreover, cognitively impaired patients had limited and focal statistically significant difference in connectivity measures in the left fronto-parietal connections: delta coherence (P = 0.015), alpha coherence (P = 0.021), alpha phase lag degree (P = 0.034) and inter-hemispheric frontal theta coherence (P = 0.023).
Correlation between quantitative EEG parameters and cognitive profile of the patients:
Again, relative power showed the most statistically significant correlations with different cognitive functions specially in the alpha and beta frequency bands, and more at frontal than occipital and temporal regions (Table 2).
Discussion
The present study aimed to explore the value of different quantitative EEG measures as physiological cognitive biomarkers of PD patients.
The PD patients had higher relative power across all frequency bands especially over the right temporal region, while peak frequency, coherence, and phase lag measures did not show any significant changes compared to healthy controls.
By comparing PD patients according to their cognitive profile, the patients with impaired cognition had significantly lower relative power in almost all head regions and across all frequency bands especially alpha and beta spectrum. Moreover, the alpha and beta relative power showed the most statistically significant correlations with different cognitive functions included in MoCA score.
On the other hand, there was focal change in connectivity measures in left fronto-parietal connections of PD patients with impaired cognition.
Several previous reports showed that QEEG is a promising biomarker that correlate with PD-related cognitive deterioration and could be used to early predict conversion from the better states of normal cognition, or mild cognitive impairment to the severe form of cognitive deterioration in PD-dementia (PDD) [17].
These reports included EEG spectral measures that showed a mostly consistent trend of leftward shift of power spectrum and background frequency with higher slower rhythm (delta and theta waves) and lower faster brain activity (alpha and beta) and progressive longitudinal slowing with the more cognitive deterioration [5, 18,19,20,21].
Other studies found that EEG spectral ratio (slow over fast activity) correlated with PD-related cognitive decline [3, 22]. Chaturvedi found that alpha1/theta ratio differentiated PD patients from healthy individuals [23]. Others found that integrative EEG frequency power (beta peak frequency, alpha relative power, and alpha/theta ratio) had the ability to predict progression of PD-MCI to PDD [24].
On the other hand, studies exploring the diagnostic value of functional connectivity were few and demonstrated somewhat conflicting findings.
Some authors showed increased functional connectivity using coherence of theta and beta bands in EEG of PDD [14], and synchronization likelihood of alpha, theta, and beta bands in magnetoencephalography (MEG) of PD patients [25]. Contrary to those, reduced EEG functional connectivity (using phase lag index) of alpha1 band [26], alpha2 band [3] or delta and alpha bands [20] were found in MEG of PDD patients.
The exact pathophysiological mechanisms that underlie the combined cognitive decline and QEEG changes are not precise, however some explanations exist in the literature. Whether EEG changes and cognitive decline occur due to a common mechanism, or result as a compensatory mechanism is unclear [25].
In their pathophysiological explanation, Halliday and McCann described typical PD with initial rapid loss of brainstem and midbrain dopamine neurons, slow neuropathological progression, and neocortical involvement at later stages of PDD [25, 27].
Other authors related the cognitive-neurophysiological association to multiple pathological changes of subcortical–cortical ascending projection systems in addition to subcortical and cortical lesions [14, 19].
Neurotransmitters were also involved in the pathophysiological mechanisms of PD cognitive decline and neurophysiological changes including dopaminergic, cholinergic, noradrenergic, serotoninergic, glutamatergic and monoamine systems [18, 19].
The pathological lesions were also documented by structural MRI of PDD and PD-MCI patients which showed atrophy of cortical and subcortical structures including hippocampal and parietal lobe, disrupted integrity of central white matter tracts and large-scale coordination of brain cognitive networks [28,29,30].
Moreover, functional MRI showed reduced activation of fronto-striatal neural networks during cognitive performance in PD patients. FDG-PET scan showed reduced metabolism in frontal and parietal association cortex and increased activation of cerebellar and dentate nuclei associated with PD cognitive impairment [29, 31].
In the current study, the alpha and beta frequency bands showed the most consistent correlation with cognitive functions which accords with reported value in the literature.
Alpha rhythm is generated by thalamocortical and local cortico-cortical circuits and involved in broad spectrum cognitive functions and attention [32]. Beta activity represents primarily neocortical activity and is involved in in motor and cognitive performance [33]. On the other hand, increased theta and delta activity represents diffuse grey matter dysfunction in both cortical and subcortical areas as well as partial deafferentation of cerebral cortex and these slow rhythms are seen in encephalopathies and late stages of neurodegenerative diseases and dementias [18].
The lack of significant discrimination using EEG background peak frequency, and both connectivity measures in this study could be explained by the heterogenous clinical characteristics of our patient cohort with almost short disease duration (less than 3 years), variable motor disability, most of them had mild cognitive decline and none of them reported to have dementia.
QEEG measures did not show identical correlation or discrimination at the group level which means that each of them reflects different functional aspects. The spectral relative power showed the most significant group-level discrimination and correlation with cognitive functions which reflects its sensitivity to pick up even early and very mild cognitive decline. The superiority of spectral relative power in detection of early cognitive decline could be related to its capability to specifically measure the amount of EEG data in each frequency band compared to the background whole frequency slowing that may be detected in later stages of cognitive deterioration.
The relative power changes in PD patients especially with impaired cognition were almost diffuse, however more significant over the frontal and temporal regions. This is consistent with the reported literature that the frontal and temporal regions are mostly involved in cognitive functioning and executive abilities [34].
The significant high relative power in PD patient subgroup with normal cognition compared to controls could be explained as a compensatory effort that correlates with the good cognitive performance. We could not find in the previous literature evidence that supports such a novel finding which may require additional investigation and research studies to confirm this finding and explore its value in different neuropsychiatric diseases.
Our study had some limitations. First, the patient cohort did not include reported cases of dementia which might show the more severe neurophysiological changes in spectral and connectivity measures. Second, there was no analysis of the possible effects of anti-PD medications on the reported neurophysiological changes. Lastly, the cross-sectional design is inferior to longitudinal prospective approach which might explore the progressive neurophysiological changes that coincide with cognitive deterioration and the prognostic value of QEEG biomarkers.
Conclusions
QEEG measures could be used as adjunct to neuropsychological assessment in evaluation of PD-related cognitive decline. Relative power was the most valuable physiological marker to pick up mild cognitive deterioration at an early stage.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FDG-PET:
-
Fluorodeoxyglucose-positron emission tomography
- FFT:
-
Fast Fourier transform
- IQR:
-
Interquartile range
- MCI:
-
Mild cognitive impairment
- MEG:
-
Magnetoencephalography
- MoCA:
-
Montreal Cognitive Assessment
- MRI:
-
Magnetic resonance imaging
- PD:
-
Parkinson’s disease
- PDD:
-
Parkinson disease dementia
- QEEG:
-
Quantitative electroencephalography
- SPSS:
-
Statistical Package for the Social Science Software
- UPDRS:
-
Unified Parkinson’s Disease Rating Scale
References
George JS, Strunk J, Mak-McCully R, Houser M, Poizner H, Aron AR. Dopaminergic therapy in Parkinson’s disease decreases cortical beta band coherence in the resting state and increases cortical beta band power during executive control. Neuroimage Clin. 2013;3:261–70.
Aarsland D. Cognitive impairment in Parkinson’s disease and dementia with Lewy bodies. Parkinson Relat Disord. 2016;22:144–8.
Geraedts VJ, Marinus J, Gouw AA, Mosch A, Stam CJ, van Hilten JJ, et al. Quantitative EEG reflects non-dopaminergic disease severity in Parkinson’s disease. Clin Neurophysiol. 2018;129(8):1748–55.
Caviness JN, Hentz JG, Belden CM, Shill HA, Driver-Dunckley ED, Sabbagh MN, et al. Longitudinal EEG changes correlate with cognitive measure deterioration in Parkinson’s disease. J Parkinson Dis. 2015;5(1):117–24.
Stylianou M, Murphy N, Peraza LR, Graziadio S, Cromarty R, Killen A, et al. Quantitative electroencephalography as a marker of cognitive fluctuations in dementia with Lewy bodies and an aid to differential diagnosis. Clin Neurophysiol. 2018;129(6):1209–20.
Arnaldi D, De Carli F, Famà F, Brugnolo A, Girtler N, Picco A, et al. Prediction of cognitive worsening in de novo Parkinson’s disease: clinical use of biomarkers. Mov Disord. 2017;32(12):1738–47.
Caviness JN, Utianski RL, Hentz JG, Beach TG, Dugger BN, Shill HA, et al. Differential spectral quantitative electroencephalography patterns between control and Parkinson’s disease cohorts. Eur J Neurol. 2016;23(2):387–92.
Cozac VV, Gschwandtner U, Hatz F, Hardmeier M, Rüegg S, Fuhr P. Quantitative EEG and cognitive decline in Parkinson’s disease. Parkinsons Dis. 2016;2016:1–14.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–4.
Fahn S, Marsden C, Calne D, Goldstein M. Fahn S, Elton RL, and members of the UPDRS Development Committee. Unified Parkinson’s disease rating scale. Recent Developments in Parkinson’s Disease.153–63.
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations the Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Mov Disord. 2004;19(9):1020–8.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9.
Abdel RTT, Mohamed ELGM. Montreal Cognitive Assessment Arabic version: Reliability and validity prevalence of mild cognitive impairment among elderly attending geriatric clubs in Cairo. Geriatr Gerontol Int. 2009;9(1):54–61.
Fonseca LC, Tedrus GMAS, Carvas PN, Machado ECFA. Comparison of quantitative EEG between patients with Alzheimer’s disease and those with Parkinson’s disease dementia. Clin Neurophysiol. 2013;124(10):1970–4.
Thatcher RW, North DM, Biver CJ. Development of cortical connections as measured by EEG coherence and phase delays. Hum Brain Mapp. 2008;29(12):1400–15.
Kaiser DA, Sterman MB. Automatic artifact detection, overlapping windows, and state transitions. J Neurother. 2001;4(3):85–92.
Geraedts VJ, Boon LI, Marinus J, Gouw AA, van Hilten JJ, Stam CJ, et al. Clinical correlates of quantitative EEG in Parkinson disease: a systematic review. Neurology. 2018;91(19):871–83.
Caviness JN, Hentz JG, Evidente VG, Driver-Dunckley E, Samanta J, Mahant P, et al. Both early and late cognitive dysfunction affects the electroencephalogram in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(6):348–54.
Fonseca LC, Tedrus G, Letro GH, Bossoni AS. Dementia, mild cognitive impairment and quantitative EEG in patients with Parkinson’s disease. Clin EEG Neurosci. 2009;40(3):168–72.
Ponsen MM, Stam CJ, Bosboom JLW, Berendse HW, Hillebrand A. A three dimensional anatomical view of oscillatory resting-state activity and functional connectivity in Parkinson’s disease related dementia: An MEG study using atlas-based beamforming. Neuroimage Clin. 2013;2:95–102.
Novak K, Chase BA, Narayanan J, Indic P, Markopoulou K. Quantitative electroencephalography as a biomarker for cognitive dysfunction in Parkinson’s disease. Front Aging Neurosci. 2021. https://doi.org/10.3389/fnagi.2021.804991.
Elkholy MM, Aboubakr HH, Abd ElMonem NA, Soliman RH, Masoud MM. Quantitative EEG spectral power ratio as cognitive biomarker for patients with Parkinson disease. Egypt J Med Res. 2022;3(1):313–22. https://doi.org/10.21608/ejmr.2022.222940.
Chaturvedi M, Hatz F, Gschwandtner U, Bogaarts JG, Meyer A, Fuhr P, et al. Quantitative EEG (QEEG) measures differentiate Parkinson’s disease (PD) patients from healthy controls (HC). Front Aging Neurosci. 2017;9:3.
Gu Y, Chen J, Lu Y, Pan S. Integrative frequency power of EEG correlates with progression of mild cognitive impairment to dementia in Parkinson’s disease. Clin EEG Neurosci. 2016;47(2):113–7.
Stoffers D, Bosboom JLW, Deijen JB, Wolters EC, Stam CJ, Berendse HW. Increased cortico-cortical functional connectivity in early-stage Parkinson’s disease: an MEG study. Neuroimage. 2008;41(2):212–22.
Utianski RL, Caviness JN, van Straaten ECW, Beach TG, Dugger BN, Shill HA, et al. Graph theory network function in Parkinson’s disease assessed with electroencephalography. Clin Neurophysiol. 2016;127(5):2228–36.
Halliday GM, McCann H. The progression of pathology in Parkinson’s disease. Ann N Y Acad Sci. 2010;1184(1):188–95.
Selnes P, Aarsland D, Bjørnerud A, Gjerstad L, Wallin A, Hessen E, et al. Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. J Alzheimers Dis. 2013;33(3):723–36.
Christopher L, Strafella AP. Neuroimaging of brain changes associated with cognitive impairment in Parkinson’s disease. J Neuropsychol. 2013;7(2):225–40.
Aarsland D. Cognitive impairment in Parkinson’s disease and dementia with Lewy bodies. Parkinsonism Relat Disord. 2016;22:S144–8.
Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008;70:1470–7.
Steriade M, Gloor P, Llinas RR, Da Silva FHL, Mesulam MM. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr Clin Neurophysiol. 1990;76(6):481–508.
Kozelka JW, Pedley TA. Beta and mu rhythms. J Clin Neurophysiol. 1990;7(2):191–208.
Dubbelink KTEO, Stoffers D, Deijen JB, Twisk JWR, Stam CJ, Berendse HW. Cognitive decline in Parkinson’s disease is associated with slowing of resting-state brain activity: a longitudinal study. Neurobiol Aging. 2013;34(2):408–18.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
RS pioneered the idea of research. HA and MM recruited the patients, collected and revised the clinical data. NA revised the data and participated in study designs and coordination. ME collected and analyzed the EEG data, performed statistical analysis and drafted the manuscript. All authors read and approve the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved from the local ethical committee of faculty of Medicine, Beni-Suef University (Approval number: FMBSUREC/05032019) and in accordance with the principles of Helsinki Declaration and an informed written consent was obtained from all participants before enrollment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Global average peak frequency of the patients and controls. Figure S2. Global average coherence of the patients and controls. Figure S3. Global average phase lag degree of the patients and controls.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elkholy, M.M., Aboubakr, H.H., Abd ElMonem, N.A. et al. EEG spectral and connectivity parameters as cognitive biomarkers in Parkinson disease. Egypt J Neurol Psychiatry Neurosurg 59, 54 (2023). https://doi.org/10.1186/s41983-023-00656-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00656-0