Abstract
Background
Over the last decade, since clinical trials examining targeted therapeutics for gliomas have failed to demonstrate a meaningful increase in survival, the emphasis has recently been switched toward innovative techniques for modulating the immune response against tumors and their microenvironments (TME). Cancerous cells have eleven hallmarks which make it distinct from normal ones, among which is immune evasion. Immune evasion in glioblastoma helps it evade various treatment modalities.
Summary
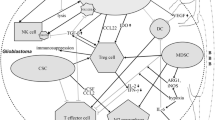
Glioblastoma’s TME is composed of various array of cellular actors, ranging from peripherally derived immune cells to a variety of organ-resident specialized cell types. For example, the blood–brain barrier (BBB) serves as a selective barrier between the systemic circulation and the brain, which effectively separates it from other tissues. It is capable of blocking around 98% of molecules that transport different medications to the target tumor.
Objectives
The purpose of this paper is to offer a concise overview of fundamental immunology and how ‘clever’ gliomas avoid the immune system despite the discovery of immunotherapy for glioma.
Conclusions
Herein, we highlight the complex interplay of the tumor, the TME, and the nearby normal structures makes it difficult to grasp how to approach the tumor itself. Numerous researchers have found that the brain TME is a critical regulator of glioma growth and treatment efficacy.
Similar content being viewed by others
Background
Gliomas are among the most prevalent primary central nervous system (CNS) cancers. A glioma is categorized according to its histogenesis into the standard WHO Grades I–IV [1, 2], with grades III–IV referred to as the high-grade glioma (HGG) [3]. Recently, the World Health Organization (WHO) added phenotypic and genotypic characteristics to the classification [2]. Glioblastoma multiforme (GBM), the most aggressive kind of glioma, has the worst prognosis. Despite the availability of modern multimodal therapy options, the median progression-free survival (PFS) is 7–8 months and a 5-year overall survival (OS) of 9.8% [4].
Resistance to standard treatment in malignant gliomas has been frequently described [5,6,7,8]. Hanahan and Weinberg [9] postulated six hallmarks of cancer in 2000, including the capacity to foster proliferative signals, dodge growth suppressors, stimulate invasion and metastasis, enable replicative immortality, induce angiogenesis, and resist cell death. Years later, additional four hallmarks were proposed to strengthen the theory of cancer biology [10].
Among Hanahan and Weinberg’s eleven traits, the most intriguing is arguably the capacity to prevent immunological breakdown [10]. This exceptional capacity is the result of interplay between the components of the tumor microenvironment (TME). The TME of gliomas is immunosuppressive and is equipped with a variety of survival mechanisms [11]. Understanding the function of TME in glioma immune evasion would benefit both scientists and clinicians, since it will allow for the advancement of contemporary therapies for the management of HGG.
Clinical presentation
Headache is the most common symptom of brain tumors, and in 1–2 in 1000 patients with headache are later diagnosed with a brain tumor [12, 13]. The characteristics vary based on its location, size, and growth rate. Exacerbated on waking up because of supine position, and also precipitated by coughing or Valsava manoeuvre. The likelihood on underlying brain tumor is increased if the headache is increasing in frequency and severity and the later development of other neurological symptoms or signs [12]. Ozawa et al. reported that headache accompanied with progressive weakness or cognitive dysfunction, especially in the frontal lobe, increases the likelihood of a brain tumour 44-fold or 59-fold, respectively [13]. Seizure are second most common presenting symptoms in approximately 20% of patients, followed by progressive weakness (1.5%), and confusion (1.4%) [13].
Prognosis
The typical survival time for adults under the age of 70 who do not receive therapy for high-grade glioma is roughly 3–4.5 months [14]. Then, a biopsy procedure followed by chemotherapy, with or without radiotherapy, boosts survival to approximately 8–10 months on average. Survival rates are 27–31% at 2 years and 7–10% at 5 years when maximal treatment involving debulking surgery and chemoradiotherapy is administered [15].
The median survival time for elderly individuals who receive only the best supportive care is less than 4 months [14, 15]. In patients over 65 years who have undergone a biopsy or resection, hypofractionated irradiation and chemotherapy result in a median survival time of 7–9 months, as opposed to radiation alone [16, 17]. Despite evidence of a survival benefit, the inclusion of adjuvant chemotherapy has no effect on the quality of life of this group [15, 17]. Due to the incurability of glioblastoma, patients should be appropriately advised about the potential impact of adverse effects of treatments on quality of life, in addition to potential survival benefits, particularly elderly patients and those with a poor performance status, who have a particularly poor prognosis [14, 16].
The tumor microenvironment
Cancer cells
A tumor is made up of various different types of cells, and even more so in cancer cells. Early in their development, cancer cells are initially homogeneous only to diversify later. The rise of cell heterogeneity is a result of genetic instability, which favors the formation of distinct cell subpopulations [10, 18].
Immune cells
Macrophages are the most commonly found immune cells in glioma [18], contributing to approximately 30% of tumor mass [19]. Macrophages in a brain tumor may be tissue-resident microglia or bone marrow macrophages (BMDM). Microglia are formed throughout the embryonic development process [20, 21], whereas BMDMs are present when the homeostasis of brain tissue is compromised by a particular pathological state [22]. In the presence of a brain tumor, a compromised blood–brain barrier (BBB) is hypothesized to assist the recruitment of peripherally circulating monocytes into the tumor mass [23]. The tumor-associated macrophages and microglia (TAMs) are composed of these two kinds of macrophages [19]. TAMs are pro-tumorigenic cells that grow in number as tumor grade increases [24, 25]. Despite their origins as macrophages, TAMs generate few pro-inflammatory cytokines and are largely ineffective in stimulating T cells [26].
Dendritic cell
Dendritic cells (DCs) are cells involved in the surveillance of pathogens and in the repair of microenvironmental tissue damage [27]. DCs trigger the immune response by engulfing tumor antigens and presenting them to T- and B-cells [28]. DCs are more prevalent in vascular-rich compartments such as the choroid plexus and meninges rather than in brain parenchyma. This suggests the possibility of peripheral DCs migrating into the CNS via vascular-rich compartments [29, 30]. DCs can gain entry to the brain and spinal cord through afferent lymphatics or venules in the presence of a pathological condition, such as cancers [31]. DCs may detect and present tumor antigens to T cells in the deep cervical lymph nodes to activate coordinated T-cell-mediated responses [31]. DCs release exosomes which express tumor or stimulatory antigens to activate Cytotoxic T cell responses—a feature antagonized by tumor-cell-derived exosomes [32].
Blood–brain barrier (BBB)
Endothelial cells (EC), extracellular matrix (ECM), astrocytes, and pericytes comprise the BBB [33]. The EC is sealed with tight junctions and bordered by an ECM-based basal lamina. On the basal lamina’s outer surface, astrocyte end-feet and pericytes complete the BBB [34]. The BBB’s strict control over the passage of chemicals and cells into and out of the brain [35, 36] may be relaxed under pathologic condition to allow entry to certain immune cells [35, 37]. In gliomas, BBB integrity is weakened due to the tumor’s high metabolic requirement. Such conditions accelerate vasculogenesis, which results in the formation of tortuous arteries [38, 39]. These impaired arteries would inevitably result in hypoxia and an acidic microenvironment. Interestingly, this ostensibly detrimental state promotes tumor development rather than destroying its cells [19, 40, 41]. In addition, the presence of a brain tumor disintegrates astrocytic end-feet and pericytes, resulting in a leaky BBB [42]. The tight junction of the endothelial cell (EC) is known to be compromised in GBM as a result of the activity of vascular endothelial growth factor (VEGF) [43, 44].
Immune evasion
Gliomas weaken the immune system through various means. Gliomas have the capacity to release immunosuppressive substances that have diverse consequences, including the modification of the major histocompatibility complex (MHC) expression, promotion of immature DCs as antigen-presenting cells (APCs) [45], and proliferation of regulatory T (Treg) cells [39, 40]. Using chemokines, glioma also induces T-cell anergy, inhibits natural killer (NK) cells, induces T-cell death, and recruits immunosuppressive T cells [46]. All of these pathways are frequently interconnected, resulting in a vicious cycle that promotes glioma survival.
Immunosuppressive factors
It is well established that gliomas release immunosuppressive substances to inhibit the immune response [45]. It is also known that the secretion of these substances promotes the proliferation of Treg cells. All of the alterations generated by the secreted immunosuppressive substances would later impair the function of cytotoxic T lymphocytes (CTLs) [38, 40, 41]. Interleukin-10 (IL-10), interleukin-6 (IL-6), transforming growth factor (TGF), and prostaglandin E-2 (PGE-2) are the most researched immunosuppressive factors in the glioma microenvironment. Although TAMs are the primary generators of these substances [49,50,51], it has been found that the glioma cells secrete these factors as well [52].
Glioma is also equipped with Glycoprotein A repetition predominant (GARP), a surface molecule known to activate Treg cells, as shown in a study on histopathological specimens of low-grade astrocytomas and glioblastomas. GARP exerts its immunoregulatory function by inducing Treg cells through the help of TGF-ß and also by inhibiting the proliferation of Cytotoxic T cells [53, 54].
Down-regulation of MHC
MHC is a molecule involved in antigen presentation; it is commonly classified into classes I and II, with the former being expressed mostly on nucleated cells and the latter on APCs. Class I MHC binds to internal antigenic peptides and transports them to the cell surface, where they are recognized by CTL [55]. Class I MHC expression was lost in nearly 50% of 47 GBM samples, according to one study [56]. There was a significant positive correlation between HLA class I antigen loss and tumor grade [57]; the analogy is shown in Fig. 1a, andb. Immunosuppressive cytokines such as TGF- and IL-10 are considered to suppress the expression of Class I MHC. These cytokines not only impair antigen presentation, but also induce the expression of the Programmed Death-1 (PD-1) receptor on invading T cells. This receptor would bind to tumor cell-expressed PD-1 ligands (PD-L1) and produce T-cell anergy [58]; an analogous is illustrated in Fig. 1c, andd. The effect of immunosuppressive cytokines and the interaction between PD-1 and its ligand will be discussed in greater detail later in this section. Class II MHC expression is also lowered in glioma-associated microglia (GAM) as a result of the cytokines' impact [59, 60].
Schematic of CTLA-4 activate (a) and blockade (b); steps involved in generation of tumor-specific T cells. The figure shown is a schematic of an APC, with associated cell–cell interactions via CTLA-4/B7. Tumor-associated antigens or neo-antigens are presented by MHC on APCs or tumor cells to T cells with appropriate TCR. CD28 co-activating receptor on T cells binds B7 on APCs. Schematic of PD-1/PD-L1 blockade (c) and activate (d); steps involved in generation of tumor-specific T cells. Anti-PD-1 and PD-L1antibodies are shown
Impaired DC function
DCs derived from myeloid cells are initially uncommitted to antigen presentation. Numerous stimuli would then classify DCs into two phenotypes: type-1 and type-2 polarized effector DCs (cDC1 and cDC2, respectively) [61]. The cDC1s can activate CTLs via Class I MHC, whereas the cDC2s can activate T helper cells via Class II MHC [62, 63]. Various chemokines such as CCL5 and XCL1 recruit cDC1s into the TME [64], where they use the tumor antigen to either stimulate naive CTL at a lymph node [65, 66], or release chemokine to directly recruit CTL into the tumor [67, 68]. However, the presence of TGF-β and PGE-2 in the GBM’s TME converts DCs to a regulatory phenotype, which promotes Treg proliferation instead of CTLs [69]. In addition, it is hypothesized that IL-6 and IL-10 generated by microglia and TAMs [38, 39, 61] impede DC maturation and instead encourage immature DC to proliferate [45]. Apart from their inability to deliver antigen to T cells [71], immature DC produce TGF-β, which contributes to an even more immunosuppressive condition inside the glioma microenvironment [45].
Immunosuppressive TAMs
TAMs as a non-inflammatory cell conforms to the conventional dichotomy of macrophage phenotypes, M1 and M2 [72]. According to this established notion, macrophages may acquire distinct characteristics in response to the stimuli they encounter. Interferon-γ (IFN-γ), lipopolysaccharides (LPS) [73] and granulocyte–macrophage colony stimulating factor (GM-CSF) [74], all induce macrophages to adopt the M1 phenotype, which is important in generating T helper type 1 (Th1) cells [73] and pro-inflammatory cytokines [75].
On the other hand, M2 macrophages promote Th2 lymphocytes, angiogenesis and tumor growth [76]. This trait is related to the low level of IL-12 and IL-23 and a high level of IL-10 and TGF-β. M2-phenotypes are also induced by Macrophage Colony-Stimulating Factor (M-CSF) [74], and IL-34 [75]. M-CSF and IL-34 both stimulate the mitogen-activated protein kinase (MAPK) signaling pathway via the CD115 receptor [75]. The MAPK pathways control a range of processes crucial for cell survival [77]. IL-10 [78] is one of the MAPK pathway’s end products, since it drives Th cell differentiation into Th2 cells, hence increasing the production of anti-inflammatory cytokines [79]. Macrophages within the glioma TME are classified as M2, because they exhibit a high level of anti-inflammatory and pro-angiogenic factors (Fig. 2) [24].
Natural killer (NK) cell inhibition
NK cells are huge granular lymphocytes that, in contrast to CTLs, eradicate virus-infected cells without prior antigen presentation (Fig. 2). NK cells are able to identify “stressed” cells which down-regulate Class I MHC to avoid identification by CTL. This ability is thus vital for anti-tumor immunity [80].
In vivo studies have reported that NK cells are scarce in the brains [81], but may increase in number when the BBB’s integrity is impaired, such as in an autoimmune disease or infection [82,83,84,85,86]. In addition, the presence of CX3CL1, a chemokine generated by neurons, attracts NK cells [87]. The presence of NK cells in GBM revealed that this subgroup of lymphocytes plays a significant role in neoplasm surveillance [88]. TGF-β may inactivate NK cells by downregulating their activating receptors [89,90,91] and ligands, hence lowering their proliferation and convert them into the pro-tumor innate lymphoid cell (ILC)1-like cells [92]. TGF-β is known to suppress the production of the NKG2D receptor, an NK activating receptor [89], whose blockage has been shown to render NK cells incapable of cytotoxicity [93]. Due to its ability to actively release TGF-β, glioma is NKG2D deficient [89].
Under normal circumstances, NK cells express the NKp44 receptor, which can bind to the PDGF-D produced by the majority of GBM cells and generate cytokines that inhibit tumor growth [94]. NKp44 is an activating receptor for NK cells that—together with NKp30, NKp46, and CD16—belongs to the immunoreceptor tyrosine-based activation motif (ITAM) [95, 96]. On the other side, glioma has been shown to express a high level of Galectin [97], a protein family that has been shown to promote tumor angiogenesis [98], cancer cell migration [99], and tumor immune evasion [100]. Galectin-3 was discovered to bind selectively to NKp30 when released from tumor cells, thereby reducing NKp30-mediated cytotoxicity. Therefore, it was postulated that tumors secrete galectin-3 as a unique mechanism for evading NKp30-mediated NK cell immunosurveillance [101]. This hypothesis had previously been tested in vivo, by blocking Galectin-3 with N-acetyllactosamine or an anti-Galectin-3 antibody to restore production of IFN by CTLs [102].
T-cell anergy
GBM has been shown to deplete T cells and desensitize them to the tumor's presence [103]. This theory was based on findings in cases of chronic lymphocytic choriomeningitis virus (LCMV) infection [104, 105], but has now been demonstrated to occur in cancer as well [106]. Numerous inhibitory receptors were up-regulated following chronic antigen exposure [107]. Checkpoint inhibitors block inhibitory signals that regulate lymphocytes; among the up-regulated immune checkpoint inhibitory receptors are the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), PD-1, and PD-L1 (Fig. 2), which have been approved by the FDA as T-cell-based treatment for cancer [108].
PD-1 is a surface receptor which serves as an immunological checkpoint. This receptor is expressed on the surface of activated T cells, NK cells, B lymphocytes, macrophages, DCs, and monocytes [109]. PD-1 suppress immune cells’ inflammatory activities when attached to its ligand, the PD-L1 [110]. Nduom and colleagues examined the expression of PD-L1 in 94 patients and discovered that it was a poor predictive factor for GBM [111]. However, Wang and colleagues used transcriptome data to evaluate 976 glioma samples and discovered that PD-L1 expression was positively linked with higher WHO glioma classification (Fig. 3) [112].
Immunohistochemical detection of gliomas cells profile. This is representative image of a specimen that positively stained with PD-L1 both in glioma patient (a) and primary isolated cell line (b). Original magnification: × 200; scale bar: 50 μm. (Original figure: created by co-author of this article for this article)
The phosphoinositide 3-kinase (PI3K), AKT [113], and mammalian target of rapamycin (mTOR) appear to influence PD-L1 expression (PI3K/AKT/mTOR pathway) [114]. In addition, this pathway is known to modulate various other characteristics of cancer to optimize tumor survival [115]. As indicated by a study on gastrointestinal stromal tumors, PD-1/PD-L1 is thought to promote CD8 + apoptosis [116]. The MAPK signalling pathway is a signalling mechanism that contributes to glioma’s immunosuppressive properties. Recent research has put more attention on the relationship between the PD-1/PD-L1 axis and the MAPK pathway. Stutvoet and colleagues demonstrated that inhibiting the MAPK pathway reduced the induction of PD-L1 protein in lung cancer cells by epidermal growth factor (EGF) and interferon (IFN) [117]. Indeed, IFN-γ released by tumor infiltrating lymphocytes (TIL) is a powerful activator of PD-L1 expression in glioma [108].
Immunosuppresive T-cell recruitment using chemokines
CXCR2 and CXCL8 are two of the most prevalent chemokines in the glioma microenvironment [118]. The upregulation of both chemokine receptors was found to be associated with a bad outcome [118]. GBMs express high levels of CXCR2 which are known mostly for its role in angiogenesis [119]. CXCL8, on the other hand, leads to local and systemic immunosuppression [120] which enables GBM to evade host immunosurveillance. GBM-associated systemic immunosuppression is connected to the increase of immunosuppressive T cells, such as Tregs and myeloid-derived suppressor cells (MDSCs) [121, 122]. MDSCs exert their effect by suppressing T-cell proliferation and activation. MDSCs regulate inflammatory responses in the normal population, therefore, preventing autoimmune illness [123, 124]. CXCL8 expression by GBM has been shown to regulate the entry of MDSCs into the tumor environment via the CXCR2 receptor [125].
Regulatory T-cells (Tregs) and T-cell apoptosis
Numerous studies on many forms of cancer have established that Tregs are involved in immunosuppression [126, 127]. Tregs are a physiological fraction of CD4+ T cells that inhibit the function of T and B cells [128, 129], six different DCs [130,131,132], monocytes or macrophages [132], and NK cells [133, 134]. Functional Tregs express CD4+, CD25+, and Foxp3 [126]. Within the glioma microenvironment, both the number and function of CD4+ T cells are reduced, with an abnormally high proportion of Tregs [135].
A time-dependent increase of Tregs was seen in brain tumors in an in vivo research [136]. Hussain and colleagues isolated and labelled immune cells from human glioblastoma tissue to determine their phenotypes [26]. They discovered that glioma-specific CTL were phenotypically CD8+ and CD25−, indicating that they were inactive. The majority of T cells in a glioma were CD4+, indicating Treg dominance, as demonstrated by positive intracellular staining for Foxp3 [26]. Another study compared GBM and normal brain tissue and discovered that CD4+ CD25+ Foxp3+ Tregs were present only in GBM tissue [137]. The chemokine CXCR2 induces Treg migration into the glioma microenvironment [138].
Tregs have been shown to trigger T-cell death in vitro. T-cells were grown with Tregs for 72 h and apoptosis was demonstrated using transmission electron microscopy [139]. Numerous hypotheses have been advanced to explain how Tregs trigger T-cell death, including inappropriate T-cell activation [140,141,142] and depriving T cells of cytokines [139]. The former method favors aggressive apoptosis, whereas the latter favors quiet apoptosis. The cytokine deprivation-induced apoptosis was discovered preclinically, when pro-survival cytokines shielded T cells against apoptosis. In addition, it was shown that T cells die gradually over 3–4 days rather than instantly as in cytolysis. In addition, an in vitro investigation shown that the concentration of cytokines was lower in cultures containing Tregs than in cultures containing control T cells [139].
Another way for T cells to undergo apoptosis is via the Fas-mediated pathway. GBM expresses the Fas ligand (CD95L) on its surface, which induces T-cell death upon binding to Fas (CD95/APO-1) on T cells [143]. Fas-mediated apoptosis is a well-established concept of cell death. When Fas binds to its ligand, it recruits Fas-associated proteins to DD (FADD). This protein is responsible for the death of cells by recruiting caspase-8 and caspase-10 [144]. Another method of T-cell apoptosis occurs when CD70 on GBM cells interacts with CD27 on T cells. It has been demonstrated that inhibiting this connection partially protects T cells against GBM cell-induced death [145].
Extracellullar matrix
Numerous solid tumors contain abundant extracellular matrix (ECM) molecules, including fibrillar collagens, fibronectin, elastin, and laminins [146]. Up to 60% of the mass of many tumors is composed of extracellular matrix [146]. The tumor cells themselves, but to an even greater extent, cancer-associated fibroblasts (CAFs) are the source of these ECM molecules [147]. CAFs support tumor cells via paracrine stromal cell-derived factor-1 (SDF1) and transforming growth factor beta (TGF) signals, contributing not only to a more malignant tumor phenotype by driving epithelial-to-mesenchymal transition (EMT), but also inducing production of collagen and other ECM molecules [148].
The molecular expression profile can subdivide numerous cancers originating from the same tissue [148]. These molecular subtypes provide a great deal of information regarding the tumor’s metabolism, dysregulation of survival and apoptosis pathways, and the presence or absence of specific proteins [148]. In many cancers, the expression profile of ECM-related genes is also a valuable prognostic factor [148]. In addition to immune suppression markers, high expression of Col3a1, Col4a1, and Col5a2 is associated with a poor prognosis in glioblastoma [148].
Inevitably, the occurrence of metastasis impacts treatment options and therapeutic outcome. EMT is associated with both increased metastasis and chemoresistance. EMT in cancer is associated with the development of stem-cell-like properties [149]. Loss of epithelial polarization, which is linked to anchorage of epithelial layers on a basement membrane, is characteristic of EMT [149]. On top of that, ECM had a role in glioma invasion. Glycosylated chondroitin sulfate proteoglycans (CSPGs), a major component of ECM in the brain contribute to induce glioma invasion.
Exosomes
Exosomes play a vital role in evading immunity and inducing tumor progression. Exosomes, released by DCs, express tumor or stimulatory antigens to activate cytotoxic T cell responses [32]. Previous studies have investigated the critical role of tumor-derived exosomes against immunity. Exosomes, released by impaired DCs, tend to have a greater impact under hypoxia. Exosomes released by hypoxic bone-marrow-derived mesenchymal stem cells (BMSCs) in TEM induce cancer cell invasion and epithelial mesenchymal transition [150]. Exosomes also contribute to the proliferation, invasion, and migration of human umbilical vein endothelial cells in esophageal squamous cell carcinoma under hypoxia [151]. In gliomas, recent study demonstrated that exosomal connexins 43 (Cx43) contributes to glioma angiogenesis mediated by exosomes under hypoxia [152]. Moreover, hypoxic glioblastoma-derived exosomes disrupt the permeability of blood–brain barrier (BBB) [153].
Discussion
In general, errors in a cell’s genome are the cause of the development and formation of neoplastic cells. The tumor’s microenvironment contains a number of factors that promote and sustain its growth. In addition, the resistance to applied therapies is also a result of tumor heterogeneity and its constant alterations [154, 155]. Nonetheless, cancer has developed a number of immune surveillance evasion mechanisms. These include the avoidance of recognition by the down-regulation of MHC, impaired DC function, immunosuppressive TAMs, Natural Killer (NK) cell inhibition, T-cell anergy, immunosuppressive T-Cell recruitment using chemokines, regulatory T-cells (Tregs), T-cell apoptosis and extracellullar matrix. Several of these mechanisms are conducive to progression, the creation of their own environment for cell development, and cell death in their own favorable environment [156,157,158]. Similar to other types of cancer, gliomas weaken the immune system through various pathways. Immunosuppressive ability of glioma plays a vital role for glioma survival. IL-10, IL-6, TGF, and PGE-2 were found to be immunosuppressive factors in the glioma microenvironment. In addition, the presence of GARP, a surface molecule, allows glioma to survive for a longer time by activating Treg cells [53, 112]. On top of that, glioma induced tumor progression by weakening BBB integrity. This will lead to accelerate vasculogenesis and impaired arteries which result in hypoxia and promote tumor development [153, 159]. Glioma also disrupted EC as a result of VEGF [43, 44]. All of these pathways are frequently interconnected, resulting in a vicious cycle that promotes glioma survival and progression. Understanding what occurs within the microenvironment of glioma and which mechanisms are responsible for glioma development and progression will reveal how glioma could protect itself from the immune system.
The concept of immunotherapy for GBM
Decreased MHC expression in GBM frequently correlates with worse prognosis. MHC-I downregulation has previously been attributed to epigenetic and transcriptional dysregulations involved in the stabilization of NFkB, interferon regulatory factors (IRFs), and NOD-like receptor family CARD domain containing protein 5 (NLRC5). These dysregulations are possibly reversible, implying the possibility of reversing MHC-I downregulation in cancer. In addition, STAT3 inhibition, STING activation, chemotherapy, and radiation can all stimulate MHC-I expression [160]. However, there are few trials targeting MHC-I in gliomas.
As previously stated, impaired DC proliferation will further impair CTL function [45]. DC vaccines (DCVs) are a type of immunotherapy that aims to enhance DC activities. DCVs comprised of immunostimulatory APCs created in vitro utilizing CD14 monocytes cultured with GM-CSF and IL-4. In short, DCVs are basically DCs loaded with tumor antigen and injected into the patient [161]. Autologous tumor lysate, cultured tumor cells from surgical specimens, irradiated autologous tumor cells, tumor RNA, or tumor related peptides were utilized as antigens. In a phase II GBM vaccine experiment, Wheeler and colleagues reported that 53% of GBM patients demonstrated a 1.5-fold increase in cytokine response following vaccination. Responders to vaccination have a longer median survival than non-responders (642 days and 430 days) [162]. A large phase III clinical trials is needed to confirm DCV’s efficacy and safety in glioma, as results negating its benefits have also been published [162].
In gliomas, TAM infiltration is dominated by tumor-supportive M2 macrophages. Because TAMs require colony-stimulating factor (CSF) for differentiation and survival, BLZ945, a CSF-1 inhibitor, was utilized to target TAMs in mice GBM models. Inhibition of CSF-1 can decrease the quantity of M2 macrophages, resulting in tumor regression [163]. PLX3397 is a CSF-1 inhibitor that has the ability to cross the BBB and reduce TAMs, thus result in alleviation of tumor invasiveness in mice models of GBM [163]. TAMs-targeted immunotherapy may be useful in the treatment of GBM. However, at the moment this therapeutic modality is still limited to in vivo models [162].
NK cells have significant anti-tumor effects, particularly when CTL function is reduced. Although the number of NK cells in GBMs is deemed low, they retained cytotoxic activity [80]. Enhancing NK cells’ oncolytic capacity might be achieved by counteracting their inhibition, that is through cutting the binding between MHC molecules and killer immunoglobin receptors (KIRs) [95]. Ishikawa and colleagues demonstrated tumor volume decrease using autologous NK cells. In addition, they suggested that this response could be enhanced by combining autologous NK cells with an IL-2 dosage or radiation therapy [164]. Another option is to use allogenic NK cells, which originate from an unrelated donor and are equipped with a KIR receptor that is incapable of recognizing MHC class I molecules. In allogenic NK cells, the KIR receptor does not recognize tumor MHC molecules, resulting in the absence of NK cells inhibition [95].
Anti-CTLA-4 and Anti-PD-1 therapies have primarily been studied in T cells for their direct immunological implications (Fig. 2). Due to their roles as immune checkpoint, therapies targeting CTLA-4 and PD-1 are hypothesized to be able to “free” T cells from inhibition to fight tumor cells. CTLA-4 (CD152) is an inhibitory receptor which downregulates T cell function [165, 166]. This receptor is mainly expressed on Tregs but might be upregulated on other subsets of T cells in pathologic condition, such as cancer. CTLA-4 suppresses the immune system indirectly by inhibiting signals via the co-stimulatory receptor CD28. CTLA-4 reduces immunological responses to weak antigens such as self- and tumor antigens by increasing the activation threshold of T cells [167]. PD-1 binding to PD-L1 is involved predominantly in inhibitory immune signaling. Although the majority of circulating T cells lack PD-1, its expression can be stimulated by exposure to cytokines, such as IL-2, IL-7, IL-15, IL-21, and TGF-β [167].
Neoantigens, which are formed from tumor-specific protein-coding mutations, are immune stimulatory and can operate as bona fide antigens that aid in tumor rejection. T-cell activation and subsequent tumor lysis driven by neoantigen vaccines offers an appealing precision medicine strategy. The process of developing a personalized neoantigen vaccination begins with a comparison of genetic data received from the patient’s peripheral blood mononuclear cells (PBMCs) and excised tumor tissue [168]. Following administration of customized vaccinations, APCs come into contact with the neoantigens contained in the vaccine, thereby initiating the process of neoantigen MHC presentation [169]. Immune responses mediated by T cells are triggered when a certain T cell receptor recognizes a particular neoantigen. In addition, these neoantigen-specific T lymphocytes expand, move toward the tumor site, and subsequently enter the tumor. Immune responses can be found that are CD4 positive (which enhances the immune response) or CD8 positive (which has a cytotoxic effect). Tumor cells that have been eliminated create an adaptive immunological memory response by releasing neoantigens [170].
Adoptive T cell therapy, which entails the selection and development of antigen-specific T cell clones ex vivo, enables the enhancement of antigen-specific immunity without the in vivo restrictions associated with vaccine-based techniques. While some clinical responses have been found in vaccine trials, the amplitude of the induced T cell response has often been small or undetectable and has had a poor correlation with clinical responses. In comparison with vaccination methods, adoptive treatment procedures are capable of circumventing the in vivo restrictions that limit the amplitude and avidity of the targeted response. T cells with a given specificity, function, and affinity for a tumor can be selected in vitro and then expanded to achieve in vivo peripheral blood frequencies that are higher than those achieved by current immunization regimens and are consistent with the levels predicted to be required to mediate tumor elimination in murine tumor therapy models [171]. In DCs, due to their capability to acquire, process, and present antigens to T cells, they are a critical component of immunization. While immature DCs in peripheral tissues acquire antigens readily, antigen presentation typically results in immunological tolerance due to a lack of costimulatory molecules [172]. Immune tolerance is induced via a variety of methods, including T cell deletion and Treg cell growth [173]. DCs laden with antigens that have been activated (mature) induce the differentiation of antigen-specific T cells into effector T cells with distinct roles and cytokine profiles. DC maturation is associated with a variety of cellular changes, including (1) decreased antigen-capture activity, (2) increased expression of surface MHC class II molecules and costimulatory molecules, (3) acquisition of chemokine receptors such as CCR7 that direct their migration, and (4) the ability to secrete various cytokines that regulate T cell differentiation including IL-12 [174].
Current state of immunotherapy for glioma
DCVax-L® has shown a benign safety profile in Phase 3 study, as it has consistently done in prior early stage trials, and in a large group of patients. Study by Liau and colleagues, showed that only 7 of the 331 Intention-to-treat (ITT) patients experienced any grade 3 or 4 adverse events that were at least possibly related to the treatment. With such a safety profile, DCV looks promising and can potentially be combined with a range of other treatments, including immune checkpoint inhibitors and targeted therapies [175].
A review from Kennedy and colleagues shows that TAMs in glioma are a formidable foe, espousing an altered activation state within the local tumor microenvironment characterized by deficiencies in antitumor effector functions, upregulation of potent immunosuppressive mediators, and participation in tumorigenic loops of paracrine signaling [176]. Given the compelling evidence that TAMs contribute significantly to the creation and maintenance of immunosuppression and tumor progression, it is unlikely that clinically effective immunotherapy against malignant gliomas will be achieved until we gain a better understanding of how to influence TAM function in the local tumor microenvironment [176].
Golan and colleagues conclude that immunotherapy with NK cells seems to be a promising strategy for treating GBM patients. Furthermore, the use of techniques that increase direct cell-to-cell contact between GBM cells and NK cells could potentiate the antitumor effect [177].
Liu and colleagues concluded that there is an association between CTLA-4 expression with clinicopathological findings and IDH mutation status in gliomas. Moreover, CTLA-4 was positively correlated with other immune-related proteins in glioma. Additional studies are needed to further explore the molecular mechanisms mediating CTLA-4 expression in gliomas and responses to anti-CTLA-4 therapy [178].
CAR T-cell therapy has become a revolutionary approach for treating hematological malignancies and it has great potential for brain tumors. Land and colleagues discussed the various targets of CAR T-cell therapy, among which is the EGFRvIII [179]. The EGFRvIII is the most common EGFR mutation that occurs in about 45% of GBM patients [179]. In vivo study showed that CAR T-Cell targeting EGFRvIII improved survival of the subject animal, as well as reduced the tumor volume. The subject was mice implanted with EGFRvIII-positive glioblastoma cell line [180].
Limitations and future directions
Multiple therapeutic combination options must be confirmed through clinical research, which would make determining effective therapeutic combinations significantly more difficult and costly as the number of treatments targeting the various aspects of TME increases. To improve high-grade glioma prognosis, novel therapeutics that target multiple TME aspects could be administered alongside standard treatments.
Conclusion
Through a variety of mechanisms, high-grade gliomas are capable of evading immunosurveillance. This extraordinary ability may be one of the reasons behind glioma's poor prognosis despite regular treatments. Therefore, future efforts to develop novel therapeutics that simultaneously target multiple areas of high-grade glioma-TME interaction may yield better results than the current standard. Novel therapeutics that specifically target glioma’s immune evasion mechanisms are among the most fascinating and promising areas of CNS oncology.
Availability of data and materials
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a state of the science review. Neuro Oncol. 2014;16(7):896–913.
Louis D, Perry A, Relfenberger G, von Deimling A, Figarella-Branger D, Cavenee W, et al. The 2016 World Health Organization Classifcation of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803.
De Groot JF. High-grade gliomas. CONTINUUM Lifelong Learning Neurol. 2015;21:332–44.
Walid MS, Smisson HF 3rd, Robinson JSJ. Long-term survival after glioblastoma multiforme. Southern Med J. 2008;101:971–2.
Vasievich EA, Huang L. The suppressive tumor microenvironment: a challenge in cancer immunotherapy. Mol Pharm. 2011;8(3):635–41.
Zhang X, Zhang W, Mao XG, Zhen HN, Cao WD, Hu SJ. Targeting role of glioma stem cells for glioblastoma multiforme. Curr Med Chem. 2013;20(15):1974–84.
Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune microenvironment of gliomas. Lab Investig. 2017;97(5):498–518.
Zhou W, Chen C, Shi Y, Wu Q, Gimple RC, Fang X, et al. Targeting glioma stem cell-derived pericytes disrupts the blood-tumor barrier and improves chemotherapeutic efficacy. Cell Stem Cell. 2017;21(5):591-603.e4.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell [Internet]. 2000;100(1):57–70. https://doi.org/10.1016/S0092-8674(00)81683-9.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Brown NF, Carter TJ, Ottaviani D, Mulholland P. Harnessing the immune system in glioblastoma. Br J Cancer. 2018;119:1171–81.
McKinnon C, Nandhabalan M, Murray SA, Plaha P. Glioblastoma: clinical presentation, diagnosis, and management. BMJ. 2021;374:n1560.
Ozawa M, Brennan PM, Zienius K, Kurian KM, Hollingworth W, Weller D, et al. The usefulness of symptoms alone or combined for general practitioners in considering the diagnosis of a brain tumour: a case-control study using the clinical practice research database (CPRD) (2000–2014). BMJ Open. 2019;9(8): e029686.
Brodbelt A, Greenberg D, Winters T, Williams M, Vernon S, Collins VP. Glioblastoma in England: 2007–2011. Eur J Cancer. 2015;51(4):533–42.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Bjorland LS, Fluge O, Gilje B, Mahesparan R, Farbu E. Treatment approach and survival from glioblastoma: results from a population-based retrospective cohort study from Western Norway. BMJ Open. 2021;11(3): e043208.
Perry JR, Laperriere N, O’Callaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–37.
Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352(6288).
Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–41.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5.
Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–51.
Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–74.
Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–57.
Hambardzumyan D, Gutmann D, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–7.
Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24.
Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses1. Neuro Oncol. 2006;8(3):261–79.
Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272(5258):50–4.
Steinman RM. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–9.
Serot JM, Béné MC, Foliguet B, Faure GC. Monocyte-derived IL-10-secreting dendritic cells in choroid plexus epithelium. J Neuroimmunol. 2000;105(2):115–9.
McMenamin PG, Wealthall RJ, Deverall M, Cooper SJ, Griffin B. Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res. 2003;313(3):259–69.
D’Agostino PM, Gottfried-Blackmore A, Anandasabapathy N, Bulloch K. Brain dendritic cells: biology and pathology. Acta Neuropathol. 2012;124:599–614.
Seo N, Akiyoshi K, Shiku H. Exosome-mediated regulation of tumor immunology. Cancer Sci. 2018;109(10):2998.
Thomsen MS, Routhe LJ, Moos T. The vascular basement membrane in the healthy and pathological brain. J Cerebral Blood Flow Metab. 2017;37:3300–17.
Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412.
Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20:136–44.
Haddad-Tóvolli R, Dragano NRV, Ramalho AFS, Velloso LA. Development and function of the blood-brain barrier in the context of metabolic control. Front Neurosci. 2017;11.
Dombrowski Y, O’Hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci. 2017;20(5):674–80.
Monsky WL, Carreira CM, Tsuzuki Y, Gohongi T, Fukumura D, Jain RK. Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clin Cancer Res. 2002;8(4):1008–13.
Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med. 2009;15(10):1219–23.
Argaw AT, Zhang Y, Snyder BJ, Zhao M-L, Kopp N, Lee SC, et al. IL-1β regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177(8):5574–84.
Engelhardt S, Patkar S, Ogunshola OO. Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia. Br J Pharmacol. 2014;171:1210–30.
Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014;5(1):1–15.
Wen L, Tan Y, Dai S, Zhu Y, Meng T, Yang X, et al. Vegf-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing bbb for glioblastoma-targeting therapy. Drug Deliv. 2017;24(1):1843–55.
Hambardzumyan D, Bergers G. Glioblastoma: defining tumor niches. Trends Cancer. 2015;1:252–65.
Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011;2011:1.
Rolle CE, Sngupta S, Lesniak MS. Mechanism of immune evasion by glioma. In: Yamanaka R, editor. Immunotherapeutic approaches. Landes Bioscience Springer Science + Business Media; 2012. p. 53–76.
Yong Z, Chang L, Mei YX, Yi L. Role and mechanisms of CD4+CD25+ regulatory T cells in the induction and maintenance of transplantation tolerance. Transpl Immunol. 2007;17(2):120–9.
Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62.
Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12(11):1113–25.
Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012;18(1):519–27.
Kirkbride KC, Blobe GC. Inhibiting the TGF-β signalling pathway as a means of cancer immunotherapy. Expert Opin Biol Ther. 2003;3(2):251–61.
Zagzag D, Salnikow K, Chiriboga L, Yee H, Lan L, Ali MA, et al. Downregulation of major histocompatibility complex antigens in invading glioma cells: stealth invasion of the brain. Lab Investig. 2005;85(3):328–41.
Zimmer N, Kim E, Schupp J, Sprang B, Leukel P, Khafaji F, et al. GARP as an immune regulatory molecule in the tumor microenvironment of glioblastoma multiforme. Int J Mol Sci. 2019;20(15):3676.
Sun L, Jin H, Li H. GARP: a surface molecule of regulatory T cells that is involved in the regulatory function and TGF-β releasing. Oncotarget. 2016;7(27):42826.
Hewitt EW. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003;110:163–9.
Facoetti A, Nano R, Zelini P, Morbini P, Benericetti E, Ceroni M, et al. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 2005;11(23):8304–11.
Mehling M, Simon P, Mittelbronn M, Meyermann R, Ferrone S, Weller M, et al. WHO grade associated downregulation of MHC class I antigen-processing machinery components in human astrocytomas: does it reflect a potential immune escape mechanism? Acta Neuropathol. 2007;114(2):111–9.
Massara M, Persico P, Bonavita O, Poeta V, Locati M, Simonelli M, et al. Neutrophils in gliomas. Front Immunol. 2017;8:1349.
Reinhard J, Brösicke N, Theocharidis U, Faissner A. The extracellular matrix niche microenvironment of neural and cancer stem cells in the brain. Int J Biochem Cell Biol. 2016;81:174–83.
Razavi SM, Lee KE, Jin BE, Aujla PS, Gholamin S, Li G. Immune evasion strategies of glioblastoma. Front Surg. 2016; 3.
Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kaliński P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164(9):4507–12.
Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–56.
Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604.
Böttcher JP, Reis e Sousa C. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer. 2018;4:784–92.
Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, et al. Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44(4):924–38.
Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, et al. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016;30(2):324–36.
Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun. 2015;6.
Kastenmüller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity. 2013;38(3):502–13.
Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, et al. Tumor cells convert immature myeloid dendritic cells into TGF-β-secreting cells inducing CD4 +CD25 + regulatory T cell proliferation. J Exp Med. 2005;202(7):919–29.
Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opinion Immunol. 2015;34:22–7.
Santos PM, Butterfield LH. Dendritic cell-based cancer vaccines. J Immunol. 2018;200(2):443–9.
Poh AR, Ernst M. Targeting macrophages in cancer: from bench to bedside. Front Oncol. 2018;8.
Nielsen SR, Schmid MC. Macrophages as key drivers of cancer progression and metastasis. Mediators Inflamm. 2017; 2017.
Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-Macrophage Colony-Stimulating Factor (CSF) and Macrophage CSF-Dependent Macrophage Phenotypes Display Differences in Cytokine Profiles and Transcription Factor Activities: implications for CSF blockade in inflammation. J Immunol. 2007;178(8):5245–52.
Jeannin P, Paolini L, Adam C, Delneste Y. The roles of CSFs on the functional polarization of tumor-associated macrophages. FEBS J. 2018;285:680–99.
Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6.
Morrison DK. MAP kinase pathways. Cold Spring Harb Perspect Biol. 2012;4(11):a011254.
Chang C-F, D’Souza WN, Ch’en IL, Paqges G, Pouyssegur J, Hedrick SM. Polar opposites: Erk direction of CD4 T cell subsets. J Immunol. 2012;189(2):721–31.
Mosmann TR, Kobie JJ, Lee FEH, Quataert SA. T helper cytokine patterns: defined subsets, random expression, and external modulation. Immunol Res. 2009;45(2–3):173–84.
Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128(10):4654–68.
Korin B, Ben-Shaanan TL, Schiller M, Dubovik T, Azulay-Debby H, Boshnak NT, et al. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat Neurosci. 2017;20(9):1300–9.
Lepennetier G, Hracsko Z, Unger M, Van Griensven M, Grummel V, Krumbholz M, et al. Cytokine and immune cell profiling in the cerebrospinal fluid of patients with neuro-inflammatory diseases. J Neuroinflammation. 2019;16(1).
Kastrukoff LF, Lau AS, Takei F, Carbone FR, Scalzo AA. A NK complex-linked locus restricts the spread of herpes simplex virus type 1 in the brains of C57BL/6 mice. Immunol Cell Biol. 2015;93(10):877–84.
Owens GC, Garcia AJ, Mochizuki AY, Chang JW, Reyes SD, Salamon N, et al. Evidence for innate and adaptive immune responses in a cohort of intractable pediatric epilepsy surgery patients. Front Immunol. 2019;10(JAN).
Zhang Y, Gao Z, Wang D, Zhang T, Sun B, Mu L, et al. Accumulation of natural killer cells in ischemic brain tissues and the chemotactic effect of IP-10. J Neuroinflammation. 2014;11.
Liu Q, Sanai N, Jin WN, La Cava A, Van Kaer L, Shi FD. Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nat Neurosci. 2016;19(2):243–52.
Ren F, Zhao Q, Huang L, Zheng Y, Li L, He Q, et al. The R132H mutation in IDH1 promotes the recruitment of NK cells through CX3CL1/CX3CR1 chemotaxis and is correlated with a better prognosis in gliomas. Immunol Cell Biol. 2019;97(5):457–69.
Holl EK, Frazier VN, Landa K, Beasley GM, Hwang ES, Nair SK. Examining peripheral and tumor cellular immunome in patients with cancer. Front Immunol. 2019;10:1767.
Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-β downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol. 2010;12(1):7–13.
Beier CP, Kumar P, Meyer K, Leukel P, Bruttel V, Aschenbrenner I, et al. The cancer stem cell subtype determines immune infiltration of Glioblastoma. Stem Cells Dev. 2012;21(15):2753–61.
Close HJ, Stead LF, Nsengimana J, Reilly KA, Droop A, Wurdak H, et al. Expression profiling of single cells and patient cohorts identifies multiple immunosuppressive pathways and an altered NK cell phenotype in glioblastoma. Clin Exp Immunol. 2020;200(1):33–44.
Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, et al. TGF-β and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129(9):2416–25.
Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9(2):146–54.
Barrow AD, Edeling MA, Trifonov V, Luo J, Goyal P, Bohl B, et al. Natural killer cells control tumor growth by sensing a growth factor. Cell. 2018;172(3):534-548.e19.
Golán I, De La Fuente LR, Costoya JA. NK cell-based glioblastoma immunotherapy. Cancers (Basel). 2018;10(12):522.
Fasbender F, Watzl C. Impedance-based analysis of Natural Killer cell stimulation. Sci Rep. 2018;8(1).
Bresalier R, Yan P, Byrd J, Lotan R, Raz A. Expression of the endogenous galactose-binding protein galectin-3 correlates with the malignant potential of tumors in the central nervous system. Cancer. 1997;80(4):776–87.
Johnson KD, Glinskii OV, Mossine VV, Turk JR, Mawhinney TP, Anthony DC, et al. Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia. 2007;9(8):662–70.
Goetz JG, Joshi B, Lajoie P, Strugnell SS, Scudamore T, Kojic LD, et al. Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine-phosphorylated caveolin-1. J Cell Biol. 2008;180(6):1261–75.
Salatino M, Croci DO, Bianco GA, Ilarregui JM, Toscano MA, Rabinovich GA. Galectin-1 as a potential therapeutic target in autoimmune disorders and cancer. Expert Opin Biol Ther. 2008;8:45–57.
Wang W, Guo H, Geng J, Zheng X, Wei H, Sun R, et al. Tumor-released galectin-3, a soluble inhibitory ligand of human NKp30, plays an important role in tumor escape from NK cell attack. J Biol Chem. 2014;289(48):33311–9.
Demotte N, Wieërs G, Van Der Smissen P, Moser M, Schmidt C, Thielemans K, et al. A galectin-3 ligand corrects the impaired function of human CD4 and CD8 tumor-infiltrating lymphocytes and favors tumor rejection in mice. Cancer Res. 2010;70(19):7476–88.
Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE. T-Cell dysfunction in glioblastoma: applying a new framework. Clin Cancer Res. 2018;24:3792–802.
Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–27.
Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJD, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–13.
Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5(6):677–85.
Woroniecka K, Fecci PE. T-cell exhaustion in glioblastoma. Oncotarget. 2018;9:35287–8.
Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Harrison Farber S, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175–86.
Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44.
Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18:e731–41.
Nduom EK, Wei J, Yaghi NK, Huang N, Kong L-Y, Gabrusiewicz K, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18(2):195–205.
Wang Z, Zhang C, Liu X, Wang Z, Sun L, Li G, et al. Molecular and clinical characterization of PD-L1 expression at transcriptional level via 976 samples of brain glioma. Oncoimmunology. 2016;5(11): e1196310.
Burgering BMT, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602.
Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–56.
O’Donnell JS, Massi D, Teng MWL, Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. 2018;48:91–103.
Zhao R, Song Y, Wang Y, Huang Y, Li Z, Cui Y, et al. PD-1/PD-L1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Prolif. 2019;52(3):e12571.
Stutvoet TS, Kol A, de Vries EGE, de Bruyn M, Fehrmann RSN, Terwisscha van Scheltinga AGT, et al. MAPK pathway activity plays a key role in PD-L1 expression of lung adenocarcinoma cells. J Pathol. 2019;249(1):52–64.
Yang L, Liu Z, Wu R, Yao Q, Gu Z, Liu M. Correlation of C-X-C chemokine receptor 2 upregulation with poor prognosis and recurrence in human glioma. Onco Targets Ther. 2015;8:3203–9.
Acker G, Zollfrank J, Jelgersma C, Nieminen-Kelhä M, Kremenetskaia I, Mueller S, et al. The CXCR2/CXCL2 signalling pathway—an alternative therapeutic approach in high-grade glioma. Eur J Cancer. 2020;126:106–15.
Kumar R, De Mooij T, Peterson TE, Kaptzan T, Johnson AJ, Daniels DJ, et al. Modulating glioma-mediated myeloid-derived suppressor cell development with sulforaphane. PLoS ONE. 2017;12(6):e0179012.
Raychaudhuri B, Ireland PRJ, Ko J, Rini B, Borden EC, Garcia J, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(6):591–9.
Jacobs JFM, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJM, Wesseling P, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol. 2010;225(1–2):195–9.
Cripps JG, Gorham JD. MDSC in autoimmunity. Int Immunopharmacol. 2011;11:789–93.
Bronte V. Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. Eur J Immunol. 2009;39:2670–2.
Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6(237).
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–9.
Liyanage UK, Moore TT, Joo H-G, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–61.
Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4 + CD25 + regulatory T cells. J Immunol. 2005;175(7):4180–3.
Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107(10):3925–32.
Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–12.
Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4 + CD25 + T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172(8):4676–80.
Taams LS, Van Amelsfort JMR, Tiemessen MM, Jacobs KMG, De Jong EC, Akbar AN, et al. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66(3):222–30.
Ralainirina N, Poli A, Michel T, Poos L, Andrès E, Hentges F, et al. Control of NK cell functions by CD4 + CD25 + regulatory T cells. J Leukoc Biol. 2007;81(1):144–53.
Smyth MJ, Teng MWL, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4 + CD25 + T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176(3):1582–7.
Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–302.
Grauer OM, Nierkens S, Bennink E, Toonen LWJ, Boon L, Wesseling P, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglionia immune responses in vivo. Int J Cancer. 2007;121(1):95–105.
El AA, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme1. Neuro Oncol. 2006;8(3):234–43.
Beslow LA, Licht DJ, Smith SE, Storm PB, Heuer GG, Zimmerman RA, et al. Predictors of outcome in childhood intracerebral hemorrhage: a prospective consecutive cohort study. Stroke. 2010;41(2):313–8.
Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–62.
Von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–44.
Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287–96.
Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10(12):1969–80.
Didenko VV, Ngo HN, Minchew C, Baskin DS. Apoptosis of T lymphocytes invading glioblastomas multiforme: a possible tumor defense mechanism. J Neurosurg. 2002;96(3):580–4.
Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Vol. 14, Cell Death and Differentiation. Nature Publishing Group; 2007. p. 400–10.
Chahlavi A, Rayman P, Richmond AL, Biswas K, Zhang R, Vogelbaum M, et al. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res. 2005;65(12):5428–38.
Mammoto T, Jiang A, Jiang E, Panigrahy D, Kieran MW, Mammoto A. Role of collagen matrix in tumor angiogenesis and glioblastoma multiforme progression. Am J Pathol. 2013;183(4):1293–305.
Takai K, Le A, Weaver VM, Werb Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget. 2016;7(50):82889–901.
Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics. 2012;11(4):M111.014647.
Walter C, Davis JT, Mathur J, Pathak A. Physical defects in basement membrane-mimicking collagen-IV matrices trigger cellular EMT and invasion. Integr Biol. 2018;10(6):342–55.
Zhang Y, Zhao Y, Zhang L, Yu W, Wang Y, Chang W. Cellular prion protein as a receptor of toxic amyloid-β42 oligomers is important for Alzheimer’s disease. Front Cell Neurosci. 2019;13(July):1–9.
Mao Y, Wang Y, Dong L, Zhang Y, Zhang Y, Wang C, et al. Hypoxic exosomes facilitate angiogenesis and metastasis in esophageal squamous cell carcinoma through altering the phenotype and transcriptome of endothelial cells. J Exp Clin Cancer Res. 2019;38(1):1–14.
Yang ZJ, Bi QC, Gan LJ, Zhang LL, Wei MJ, Hong T, et al. Exosomes derived from glioma cells under hypoxia promote angiogenesis through up-regulated exosomal connexin 43. Int J Med Sci. 2022;19(7):1205–15.
Zhao Y-H, Pan Z-Y, Wang Z-F, Ma C, Weng H, Li Z-Q. YKL-40 in high-grade glioma: prognostic value of protein versus mRNA expression. Glioma [Internet]. 2018;1(3):104–10. Available from: http://www.jglioma.com/article.asp?issn=2589-6113.
Li I, Nabet BY. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer. 2019;18(1):32.
Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–66.
Ding Y, Tong Z, Jin L, Ye B, Zhou J, Sun Z, et al. An NIR discrete metallacycle constructed from perylene bisimide and tetraphenylethylene fluorophores for imaging-guided cancer radio-chemotherapy. Adv Mater. 2022;34(7): e2106388.
Zhou J, Rao L, Yu G, Cook TR, Chen X, Huang F. Supramolecular cancer nanotheranostics. Chem Soc Rev. 2021;50(4):2839–91.
Zhou J, Yu G, Huang F. Supramolecular chemotherapy based on host–guest molecular recognition: a novel strategy in the battle against cancer with a bright future. Chem Soc Rev. 2017;46(22):7021–53.
Zhang X, Sai B, Wang F, Wang L, Wang Y, Zheng L, et al. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol Cancer. 2019;18(1):1–15.
Cornel AM, Mimpen IL, Nierkens S. MHC Class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers (Basel). 2020;12(7):1760.
Eagles ME, Nassiri F, Badhiwala JH, Suppiah S, Almenawer SA, Zadeh G, et al. Dendritic cell vaccines for high-grade gliomas. Ther Clin Risk Manag. 2018;14:1299–313.
Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68(14):5955–64.
Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–72.
Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24(3b):1861–71.
Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci. 2003;100(14):8372–7.
Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci. 2003;100(8):4712–7.
Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8.
Zhang R, Yuan F, Shu Y, Tian Y, Zhou B, Yi L, et al. Personalized neoantigen-pulsed dendritic cell vaccines show superior immunogenicity to neoantigen-adjuvant vaccines in mouse tumor models. Cancer Immunol Immunother. 2020;69(1):135–45.
Pan R-Y, Chung W-H, Chu M-T, Chen S-J, Chen H-C, Zheng L, et al. Recent development and clinical application of cancer vaccine: targeting neoantigens. J Immunol Res. 2018;2018:1–9.
Londhe VY, Date V. Personalized neoantigen vaccines: a glimmer of hope for glioblastoma. Expert Rev Vaccines. 2020;19(5):407–17.
Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci. 2002;99(25):16168–73.
Banchereau J, Thompson-Snipes L, Zurawski S, Blanck J-P, Cao Y, Clayton S, et al. The differential production of cytokines by human Langerhans cells and dermal CD14+ DCs controls CTL priming. Blood. 2012;119(24):5742–9.
Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–26.
Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39(1):38–48.
Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;1–9.
Kennedy BC, Showers CR, Anderson DE, Anderson L, Canoll P, Bruce JN, et al. Tumor-associated macrophages in glioma: friend or foe? J Oncol. 2013;2013:1.
Zhang C, Burger MC, Jennewein L, Genßler S, Schönfeld K, Zeiner P, et al. ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J Natl Cancer Inst. 2016;108(5):1–12.
Liu F, Huang J, Liu X, Cheng Q, Luo C, Liu Z. CTLA-4 correlates with immune and clinical characteristics of glioma. Cancer Cell Int. 2020;20(1):1–10.
Land CA, Musich PR, Haydar D, Krenciute G, Xie Q. Chimeric antigen receptor T-cell therapy in glioblastoma: charging the T cells to fight. J Transl Med. 2020;18(1):1–13.
Jiang H, Gao H, Kong J, Song B, Wang P, Shi B, et al. Selective targeting of glioblastoma with EGFRvIII/EGFR bitargeted chimeric antigen receptor T cell. Cancer Immunol Res. 2018;6(11):1314–26.
Acknowledgements
Not applicable.
Funding
AF received the Universitas Padjadjaran Academic Leadership Grant, Bandung, Indonesia. RM is an awardee of Indonesia Endowment Fund for Education (Lembaga Pengelola Dana Pendidikan Republik Indonesia) and, therefore, might receive financial rewards for publishing papers on Scopus-indexed journal.
Author information
Authors and Affiliations
Contributions
Author contributions to the study and the manuscript preparation. Conception and design, all authors; writing—original draft, MRA, RM, YH and AF; writing, review and editing, all authors; supervision, IBIH, RIS, JW and AF. Funding acquisition, AF. All authors had full access to the data in the study and take responsibility for the integrity of the data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arifianto, M.R., Meizikri, R., Haq, I.B.I. et al. Emerging hallmark of gliomas microenvironment in evading immunity: a basic concept. Egypt J Neurol Psychiatry Neurosurg 59, 47 (2023). https://doi.org/10.1186/s41983-023-00635-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00635-5