Abstract
Background
Vascular malformation of the spine accounted for 3–4% of all intradural lesions. Spinal arteriovenous malformation (AVM) is often missed because of overlapping symptoms with other pathology and ambiguous imaging. Here, we report a conus medullary AVM that mimics intramedullary tumours either from clinical findings or MR imaging.
Case presentation
We report a 24-year-old man with left foot monoparesis, paresthesia, and intermittent claudication for the last 3 months. Magnetic resonance imaging revealed a strongly enhanced intramedullary lesion with a hypointense signal on T1-weighted images and a hyperintense signal on T2-weighted images without flow void, suggesting an intramedullary tumour of ependymoma. Left-sided hemilaminectomy was performed, revealing an AVM on conus medullary. Microsurgical resection was performed by subsequently ligating the arterial feeder and draining vein using a temporary clip. Improvement of neurological status without postoperative sequelae was noted.
Conclusions
Because of the similarity in epidemiology, symptoms, clinical progression, and imaging, suspicion of spinal AVM should remain. This case highlights that appropriate and meticulous surgical resection can preserve the patient's neurological function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Spinal arteriovenous malformation (AVM) is a type of vascular malformation of the spine composed of nidus with draining vein and feeding artery, accounting for 3–4% of all intradural spinal cord tumours [1,2,3]. As part of intramedullary AVM subclassification, conus medullary AVM is less frequent [1]. Despite advances in neuroimaging technology, vascular lesion diagnosis is often missed, because the symptoms usually overlap with more frequent cases of myelopathy, such as neoplasms and other spinal degenerative diseases [4]. In patients with progressive myelopathy symptoms, Magnetic Resonance Imaging (MRI) is the primary diagnostic modality. However, this examination often provides ambiguous or even normal imaging in cases of vascular lesions. Thus, the diagnosis of spinal AVM is overlooked [4, 5].
Current therapeutic approaches to spinal AVM include close observation, endovascular embolisation, microsurgery resection, stereotactic radiosurgery, or a combination of those mentioned above [1, 6]. If left untreated, spinal AVM could cause significant and progressive neurological disability in a short time [7]. Here, we report a conus medullary AVM mimicking intramedullary tumour that was opted to be resected intraoperatively while preserving the patient's neurological function.
Case presentation
A 24-year-old man with an uneventful clinical history was referred to our outpatient clinic with complaints of weakness on the left foot 3 months prior. The patient initially complained of a tingling sensation in the left foot, followed by progreesive weakness on the left sole. Pain on the left foot was also aggravated upon walking but abated after resting. The complaints varied throughout the day, sometimes interfering with walking (Modified McCormick Scale 2) [8]. No history of trauma was reported.
Neurological examination revealed reduced lower extremity muscular strength (MMT4) on the left extensor digitorum pedis, extensor halucis longus, and gastrocnemius, accompanied by respective muscle atrophy. Normal pinprick, light touch, and proprioceptive sensation were noted. Diminished Achilles tendon reflex (+ 1) on the left foot was found without any pathological reflexes. Sphincter examinations revealed normal findings.
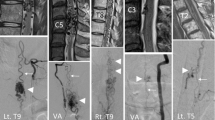
The whole spine MRI (Siemens 3 T Skyra VD13, Siemens Healthineers, Erlangen, Germany) revealed an intramedullary mass at Thoracal 11th and 12th level with a low-intensity feature on T1-weighted and high intensity on T2-weighted images that strongly enhanced after gadolinium injection with significant cord swelling. No serpiginous areas of the signal void were observed. Based on these findings, we suspected an intramedullary tumour of ependymoma. Vascular malformation diagnosis was not considered at this point; thus, no further imaging and angiography was carried out (Fig. 1).
After preoperative evaluation, surgery was performed to achieve surgical decompression by maximally preserving the functional status. We performed a left-sided hemilaminectomy, revealing tortuous vessels on the dorsal surface of the conus medullary. Based on the purpose of decompressing the medulla, we decided to proceed with the surgery.
We subsequently ligated the arterial feeder and draining vein with temporary aneurysm clips. Upon careful inspection, the nidus was found adhered to the nearby radix. Thus, pial resection of the AVM nidus along the conus and nerve roots was done. The nidus and attached radix were extirpated en bloc. The feeding arteries were cut, and the draining veins were coagulated. Post-resection evaluation showed enough decompression of the nerve root (Fig. 2).
The surgery and the postoperative course were uneventful. The patient was discharged within four days of surgery. After one month of follow-up, the patient’s complaints were resolved entirely (Modified McCormick Scale 1) [8]. Upon neurological examination, we found improved lower extremity muscular strength (MMT5) on the left extensor digitorum pedis, extensor halucis longus, and gastrocnemius.
A follow-up MRI showed a tortuous flow void lesion suggesting a small residual mass on the ventral part of the conus. Due to limited resources, we did not further perform MR angiography and digital subtraction angiography. The patient is currently under close observation (Fig. 3).
Discussion
According to Kim and Spetzler’s classification [10], spinal vascular malformation consists of a spinal vascular tumour (hemangioblastoma and cavernous malformation), a spinal aneurysm, and arteriovenous fistula (AVF) [1, 9,10,11]. Spinal AVM is responsible for 20–30% of spinal vascular malformations [6, 7, 12, 13]. Intramedullary AVM was the most common (40.9%), followed by conus medullary (31.8%), metameric (18.2%), and extradural AVM (9.1%) [1]. Because of its infrequent incidence, misdiagnosis often occurs due to atypical clinical symptoms and a lack of understanding about conus medullary AVM [1, 6, 9, 14]. Additional information such as age, gender, clinical course, imaging, and location may aid in differentiating vascular lesions from other lesions. [15]
Rangel-Castilla and colleagues conducted a study on 110 patients with spinal AVF and AVM, and found that women and men had the same distribution (51.8% men and 48.2% women) [1]. According to Lad and colleagues[6], Singh and colleagues [2], Ozpinar and colleagues [3], and Park and colleagues [14], the incidence was significantly higher in men—consistent with our study. Regarding age, the average age of spinal AVM patients was 45–64 (with a range of 18 months–to 81 years) [1,2,3, 6]. For cases of intramedullary tumours, ependymomas are the most common entity, with a peak incidence in the fourth and fifth decades, predominantly occurring in men [15, 16]. In this study, we presented a case of a 24-year-old man with conus medullary AVM, which does not fit into the classical age group for spinal AVM or ependymomas.
The clinical course of AVM and ependymoma are similar and cannot be differentiated [4, 5]. Intramedullary AVM lesion has an unremitting neurological worsening, with 50% of all patients being severely disabled in three years, and only less than 10% could walk without assistance [1]. As in AVM, spinal ependymoma also has a slowly-progressive clinical course with an average time between presentation and diagnosis of more than two years [4].
Multiple theories may explain the pathophysiology of how neurological symptoms may occur from AVM. Abnormal blood flow from the artery to the nidus and vein without going through the capillary causes pressure from the artery to be transmitted directly through the vein. This phenomenon will lead to stagnation of blood flow resulting in impaired spinal cord venous drainage and venous hypertension. Venous hypertension is then sent to all valveless intrinsic veins in the spinal cord, resulting in a decrease in the arteriovenous pressure gradient in the spinal cord, decreased tissue perfusion, and progressive deterioration of spinal cord function [1, 14, 17,18,19].
Another known pathophysiology of neurological deficit in spinal AVM is mass effect, haemorrhage, or vascular steal phenomenon. The above mechanism will cause symptoms, such as pain, sensory deficit, motoric abnormality, or myelopathy [2, 3, 6]. Our patient presented with the classical presentation of left foot monoparesis, paresthesia, and intermittent claudication. Venous hypertension and rapid redistribution of blood supply leading to the formation of ischemic areas, known as the “steal phenomenon”, may explain how neurogenic claudication occurred in our patient [2, 17,18,19].
A thorough examination is essential in differentiating spinal AVM from other lesions and planning a suitable therapeutic option. MRI is the primary diagnostic modality for patients with progressive myelopathy symptoms. Spinal AVM findings on the MRI include hyperintensity signals on T2, reflecting venous hypertension and ischemia, enlargement of the lower spinal cord segment, abnormal intramedullary signal before and contrast administration, and the presence of abnormal serpiginous blood vessels [19]. However, findings such as signal abnormalities and cord enlargement are non-specific. These findings may arise not only from vascular lesions but also from neoplasms and infections. Moreover, the flow-void phenomenon may not be seen on T2 if the flow in the engorged vein is very slow or if the imaging plane is parallel to the flow direction [20]. Therefore, a normal MRI does not exclude AVM, and angiography should be followed if a spinal vascular lesion is suspected [14, 21].
Intramedullary ependymoma appears as an enhancing centrally located lesion with an isointense signal on T1-weighted imaging and a hyperintense signal on T2-weighted imaging. Due to the similarity, spinal AVM may be misdiagnosed as an intramedullary tumour [4, 5, 15, 22]. The whole spine MR imaging in this study revealed an enhancing intramedullary lesion with a hypointense signal on T1-weighted images and hyperintense signal on T2-weighted images at 11th to 12th thoracic vertebrae with spinal cord swelling and no flow void, suggesting intramedullary tumour of ependymoma. Vascular malformation was not suspected at this point.
The gold standard examination for spinal AVM is Digital Subtraction Angiography (DSA). This examination could detect and characterized vascular malformation [3, 6, 18, 19]. Unfortunately, we did not perform angiography in this study because spinal AVM was not suspected in the initial diagnosis and spinal DSA is not yet available in our center.
In this case, conus medullary AVM was diagnosed intraoperatively. Nevertheless, appropriate surgical treatment could still be done. The management principles of AVM are to stop the flow of dilated feeding arteries, excision of the nidus, strip off the dilated perimedullary veins, and obliterate arterial/venous aneurysms while maintaining blood flow to the spinal cord and preserving neurological function. This can be achieved by occlusion of the feeding vessel alone or occlusion and resection [1, 6, 9, 14]. Current therapeutic options are endovascular embolisation, microsurgery resection, stereotactic radiosurgery, and a combination of all above with or without using somatosensory and motor-evoked potential monitoring or indocyanine green fluorescent angiography [1, 6, 23].
Before selecting the proper management for vascular malformation, several essential considerations that need to be evaluated regarding the type and location of the lesion, vascular structure, inflow and outflow tract, hemodynamic characteristics, and surgeon’s preference [1, 9, 14]. This study identified a conus medullary AVM with multiple feeding arteries, nidus, and complex venous drainage attaching to the radix. Endovascular embolisation is growing as a treatment option. In 56% of cases, this therapy was safe and was used as the sole therapy in 9.1% [23]. However, not every AVM can be treated with embolisation alone [1, 9]. Even if spinal AVM was suspected earlier, endovascular embolisation was not suitable in this case, given the highly eloquent surrounding tissue and the complexity of the AVM [1].
Here, we performed a left-sided hemilaminectomy on Thoracal 11th and 12th levels, followed by microsurgery resection. Hemilaminectomy was chosen because the approach provides adequate exposure with less trauma to the posterior column [24]. To achieve the goal of AVM management, we coagulated the feeding arteries, draining veins, and resected the nidus. Some part of the nidus attaching to the radix was intentionally left to avoid risking the patient's neurological function.
The previous study mentioned significant improvement in neurological function after AVM surgery. Good outcome was found in 70–95% of all cases, with 11 out of 16 patients with conus medullary AVM (68.7%) experiencing improvement. This could be explained by the decompression effect after AVM resection, subdural or intraparenchymal haemorrhage evacuation, syrinx drainage, and spinal cord untethering [1, 2, 7]. Despite the unforeseen diagnosis, the patient tolerated the surgery well and improved motoric function without any postoperative sequelae upon follow-up.
The recommended follow-up examination is MRI and spinal angiography [9]. Since spinal angiography is unavailable in our center, we only performed MRI, which revealed a small residual mass on the ventral conus intentionally left to preserve neurological function. Although the recurrence and regrowth risk of adult spinal AVM is low [25, 26], this patient still needs long-term surveillance, since total resection was not achieved. In addition, conus medullary AVM has the highest recurrence rate due to a large number of feeding arteries. [11]
The main limitation of this study is that we did not perform pre- and post-surgical angiography. Although an MRI examination is adequate in planning the surgery, a DSA could better characterize the vascular malformation structure, feeding arteries, venous drainage, and hemodynamics [14, 19, 21, 27]. Long-term surveillance is also essential in determining whether or not there is a regrowth of the lesion, which could affect neurological function.
Conclusions
Because of the similarity in epidemiology, symptoms, clinical progression, and imaging, suspicion of spinal AVM should remain. Myelopathy and claudication may occur due to ischemia caused by the stealing phenomenon of the AVM, venous hypertension, mass effect, and haemorrhage. There is a steep learning curve to changing the surgical plan intraoperative. Regardless, this case highlights that appropriate and meticulous surgical resection can preserve the patient's neurological function.
Intraoperative image finding (A) a left-sided hemilaminectomy on Thoracal 11th and 12th level, showing (B) tortuous vessels on the dorsal surface of the conus medullary, (C) arterial feeder and draining vein were temporarily clipped, (D) post-resection evaluation showed enough decompression of the nerve root
Availability of data and materials
The authors declare that important data supporting the findings of this study are available within the article. Further data are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Abbreviations
- AVM:
-
Arteriorvenous malformation
- MRI:
-
Magnetic resonance imaging
- AVF:
-
Arteriovenous fistula
- DSA:
-
Digital subtraction angiography
References
Rangel-Castilla L, Russin JJ, Zaidi HA, Martinez-del-Campo E, Park MS, Albuquerque FC, et al. Contemporary management of spinal AVFs and AVMs: lessons learned from 110 cases. Neurosurg Focus FOC. 2014;37(3):E14.
Singh B, Behari S, Jaiswal AK, Sahu RN, Mehrotra A, Mohan BM, et al. Spinal arteriovenous malformations: is surgery indicated? Asian J Neurosurg. 2016;11(2):134–42.
Ozpinar A, Weiner GM, Ducruet AF. Chapter 14—Epidemiology, clinical presentation, diagnostic evaluation, and prognosis of spinal arteriovenous malformations. In: Spetzler RF, Moon K, Almefty ROBTH of CN, editors. Arteriovenous and cavernous malformations. Elsevier; 2017. p. 145–52.
Oh S, Bae C, Ahn JS, Rhim S. Spinal intradural ventral arteriovenous fistula mimicking an intramedullary ependymoma. Kor J Spine. 2010;7:107–10.
Campos W, Silva B, Guasti J. Spinal intradural arteriovenous fistula mimicking intramedullary tumor and associated with a giant intracranial aneurysm. Arq Bras Neurocir. 2011;30:199.
Lad SP, Santarelli JG, Patil CG, Steinberg GK, Boakye M. National trends in spinal arteriovenous malformations: a review. Neurosurg Focus FOC. 2009;26(1):1–5.
Varshneya K, Pendharkar AV, Azad TD, Ratliff JK, Veeravagu A. A descriptive analysis of spinal cord arteriovenous malformations: clinical features, outcomes, and trends in management. World Neurosurg. 2019;131:e579–85.
Bakhshi SK, Waqas M, Shakaib B, Enam SA. Management and outcomes of intramedullary spinal cord tumors: a single center experience from a developing country. Surg Neurol Int. 2016;7(24):S617–22.
Zozulya YP, Slin’ko EI, Al-Qashqish II. Spinal arteriovenous malformations: new classification and surgical treatment. Neurosurg Focus FOC. 2006;20(5):1–17.
Kim LJ, Spetzler RF. Classification and surgical management of spinal arteriovenous lesions: arteriovenous fistulae and arteriovenous malformations. Neurosurgery. 2006;59(suppl_5):S3-195-S3-201.
Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg Spine. 2002;96(2):145–56.
Hur JW, Cho TH, Park DH, Lee JB, Park JY, Chung YG. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J Spinal Cord Med. 2016;39(6):655–64.
Lee YJ, Terbrugge KG, Saliou G, Krings T. Clinical features and outcomes of spinal cord arteriovenous malformations. Stroke. 2014;45(9):2606–12.
Park JE, Koo HW, Liu H, Jung SC, Park D, Suh DC. Clinical characteristics and treatment outcomes of spinal arteriovenous malformations. Clin Neuroradiol. 2018;28(1):39–46.
Abul-Kasim K, Thurnher MM, McKeever P, Sundgren PC. Intradural spinal tumors: current classification and MRI features. Neuroradiology. 2008;50(4):301–14.
Wostrack M, Ringel F, Eicker S, Jägersberg M, Schaller K, Kerschbaumer J, et al. Spinal ependymoma in adults: a multicenter investigation of surgical outcome and progression-free survival. J Neurosurg Spine. 2018;28:1–9.
Madsen JR, Heros RC. Spinal arteriovenous malformations and neurogenic claudication. Report of two cases. J Neurosurg. 1988;68(5):793–7.
Mull M, Nijenhuis RJ, Backes WH, Krings T, Wilmink JT, Thron A. Value and limitations of contrast-enhanced MR angiography in spinal arteriovenous malformations and dural arteriovenous fistulas. Am J Neuroradiol. 2007;28(7):1249 LP – 1258.
Kataoka H, Miyamoto S, Nagata I, Ueba T, Hashimoto N. Venous congestion is a major cause of neurological deterioration in spinal arteriovenous malformations. Neurosurgery. 2001;48(6):1224–30.
Doppman JL, Di Chiro G, Dwyer AJ, Frank JL, Oldfield EH. Magnetic resonance imaging of spinal arteriovenous malformations. J Neurosurg. 1987;66(6):830–4.
Koenig E, Thron A, Schrader V, Dichgans J. Spinal arteriovenous malformations and fistulae: clinical, neuroradiological and neurophysiological findings. J Neurol. 1989;236(5):260–6.
Roccatagliata L, Centanaro F, Castellan L. Venous congestive myelopathy in spinal dural arteriovenous fistula mimicking neoplasia. Neurol Sci. 2007;28(4):212–5.
Xu DS, Sun H, Spetzler RF. Chapter 15—Spinal arteriovenous malformations: surgical management. In: Spetzler RF, Moon K, Almefty ROBTH of CN, editors. Arteriovenous and cavernous malformations. Elsevier; 2017. p. 153–60.
Awyono S, Mardhika PE, Mahadewa TGB, Wido A, Gaol HL, Wijaya IP. Unilateral laminectomy approach for total resection of intradural extramedullary spinal tumor. Neurol Spinale Med Chir. 2019;2(2):23–6.
Patchana T, Savla P, Taka TM, Ghanchi H, Wiginton J 4th, Schiraldi M, et al. Spinal arteriovenous malformation: case report and review of the literature. Cureus. 2020;12(11):e11614–e11614.
Freudenstein D, Duffner F, Ernemann U, Rachinger J, Grote EH. Recurrence of a cerebral arteriovenous malformation after surgical excision. Cerebrovasc Dis. 2001;11(1):59–64.
Ebner FH, Roser F, Acioly MA, Schoeber W, Tatagiba M. Intramedullary lesions of the conus medullaris: differential diagnosis and surgical management. Neurosurg Rev. 2008;32(3):287.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
MM contributed to writing and interpreting patient data. TGBM was the first one to come up with the idea and was a significant contributor to writing the manuscript. SA and DTP contributed to writing and interpreting patient data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Since this is a case report, there is no need for ethical approval. Written informed consent was obtained from the patient to publish this case report and accompanying images.
Consent for publication
Written informed consent was obtained from the patient to publish this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monica, M., Mahadewa, T.G.B., Awyono, S. et al. Conus medullary arteriovenous malformation mimicking intramedullary tumor: a case report. Egypt J Neurol Psychiatry Neurosurg 58, 126 (2022). https://doi.org/10.1186/s41983-022-00553-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-022-00553-y