Abstract
Background
Traumatic acute subdural haematoma occurs in about 10–20% of patients with severe head injuries. This study aims to investigate the relation between outcome and the age, Glasgow Coma Scale on admission as well as haematoma thickness upon admission CAT scan.
This is a prospective observational clinical trial study of 39 patients with isolated traumatic acute subdural haematomas treated with conservative or surgical procedures during a one-year study period.
Results
There was a statistically significant relation between Glasgow Outcome Score and both age of the patients and Glasgow Coma Scale upon admission. However, there was a non-statistically significant relationship between Glasgow Outcome Score and haematoma thickness upon admission CAT scan.
Conclusions
Age of the patients with traumatic acute subdural haematoma as well as Glasgow Coma Scale upon admission are essential predictors of the outcome.
Clinical trial registration details: Name of the registry: Traumatic Acute Subdural Haematoma: Management and Outcome. Trial registration number: NCT03971240. Date of registration: June 3, 2019. URL of trial registry record: https://clinicaltrials.gov/ct2/show/record/NCT03971240?term=Mohamed+Ahmed+Alghriany&draw=2&rank=1.
Similar content being viewed by others
Background
Traumatic acute subdural haematoma occurs in about 10–20% of patients with severe head injuries [1]. The presentation of traumatic acute subdural haematoma varies depending on its pathogenesis and the degree of concomitant brain injury, and TASH may be associated with severe axonal injury, brain contusion or other haematomas such as intracerebral or epidural haematoma [2].
In patients with TASH, non-operative treatment may be applied in stable patients or those with improvement in consciousness during the period from injury time until evaluation time at the hospital. In addition to patients with haematoma with a thickness of less than 10 mm and a midline shift of less than 5 mm [3].
Surgery is indicated if haematoma thickness is greater than 10 mm or if the midline shift is greater than 5 mm [4].
The aim of surgery is to protect against brain herniation as well as secondary ischaemic brain injury [5].
The selection of surgical procedures depends on the surgeon’s experience, the neurological status of patients, the duration of the deterioration, the pre-operative neuroimaging findings, and degree of intraoperative brain swelling [4].
Clinical evaluation of acute subdural haematoma depends mainly on the calculation of the Glasgow Coma Scale (GCS) [5].
Glasgow Coma Scale is the main method for the evaluation of conscious level in patients with head injury [5].
The GCS is divided into 3 categories: eye opening (E), motor response (M), and verbal response (V). The score is determined by the sum of the score in each of the 3 categories, with a maximum score of 15 and a minimum score of 3 [6].
According to GCS, head injury is classified as: mild head injury (GCS of 13–15), moderate head injury (GCS of 9–12), and severe head Injury (GCS of 8 or less) [7].
TASH patients with severe brain injury (GCS less than 8) were found in 35–80% of cases [3].
The Glasgow Outcome Score (GOS) is a scale to evaluate the outcome of patients with brain injury [6].
The Glasgow Outcome Score (GOS) is categorized into grades: grade 1 for death, grade 2 for persistent vegetative state, grade 3 for severe disability, grade 4 for mild disability and grade 5 for low disability, considering that 2–3 with a poor outcome and 4–5 with a favourable outcome. [6].
Many factors have been identified to correlate with the outcome of patients with severe head injuries, including age, GCS and CT findings [8].
It has been reported that patients older than 65 years are statistically correlated with worse outcomes [9].
There is a statistically significant correlation between GCS and the outcome (the lower the GCS, the worst the outcome). However 8% of patients in spite of their low GCS (3–5), achieved good functional outcome and this means that they got benefit from aggressive surgery [1, 10].
Methods
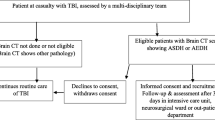
This is a prospective observational clinical trial study of patients with isolated traumatic acute subdural haematomas throughout a one-year study period from 01/09/2019 to 31/08/2020, conducted at Assiut university Hospital, Trauma unit. All ages are included in the study.
Any kind of head trauma that will cause traumatic acute subdural haematoma non-surgical-treated patients’ inclusion criteria are patients with a small haematoma (less than 1 cm thickness with CT and asymptomatic, patients who were clinically stable (no change in GCS) or improved during observation (elevation in GCS).
We attempted to maintain adequate cerebral perfusion by maintaining a mean arterial pressure between 80 and 110 mmHg.
In some cases of severe injuries, agitation or altered mental status, intubation was administrated to achieve adequate sedation, and etomidate was used for induction along with Propofol and fentanyl.
The short-term elevation of the head of patients with hyperventilation leads to vasoconstriction and a reduction in intracranial pressure.
In hypotensive patients, we used a hypertonic solution, while in hypertensive patients, mannitol was infused.
Sometimes antiepileptic drugs (phenytoin or levetiracetam) was given as a prophylaxis against seizures.
Surgically treated patients inclusion criteria are patients with ASDH thickness of 10 mm or more upon admission with a CT scan, patients with ASDH and midline shift 5 mm or more with a CT scan, patients with a GCS score less than 9 with ASDH thickness of less than 10 mm and a midline shift of less than 5 mm underwent surgical evacuation of the haematoma if the GCS score decreased between the time of injury and hospital admission by 2 or more points on the GCS and/or the patient presented with signs of herniation (changes in pupillary size and reactivity or changes in vital functions).
Exclusion criteria are patients with blood diseases or defective coagulation, CT demonstrates the presence of other associated intracranial haematomas (intraventricular, intracerebral, or epidural).
Short-term follow-up For 4–6 weeks from admission to discharge from hospital, long-term follow-up for 6 months after discharge from the hospital. Follow-up assessment was conducted clinically by GCS recording and the Glasgow Outcome Score (GOS) at discharge time and radiographically by CT findings.
All patients were included based on inclusion criteria after an informed consent was obtained from patients or patient attendants including Full history taking, general examination and neurological examination, neuroimaging including CT scanning of the brain and cervical spine to exclude any kind of cervical spine trauma.
The operative technique used was a very large decompressive craniectomy had obvious benefit and was done for all patients except one in whom we did craniotomy.
One large U-shaped dura flap was done, based on the superior sagittal sinus, with a relieving incision over the anterior and posterior temporal lobe. The dural edges were held with stay sutures.
The subdural haematoma was obvious on opening the dura. It was removed rapidly with gentle suction, taking care not to injure the underlying cortex, which may be contused and haemorrhagic. Obvious cortical bleeding sites was coagulated with bipolar diathermy, with oxidized cellulose gauze helping to control areas of contused oozing cortex.
In most cases of severe intraoperative brain swelling, we did duroplasty using precranial or fascia lata graft from the thigh, not replacing the craniectomy flap then achieving rapid scalp closure.
The patient was transferred to the intensive care unit postoperatively for controlled ventilation and management of intracranial pressure, and routine CT brain was performed within 24 h. The CAT scan device was Siemens® 90 up 38, 32 slice images. Normovolemia and normotension were therapeutic goals.
Attention was directed towards electrolyte disturbance and patient’s hydration and towards other injuries as required.
Anticonvulsant prophylaxis was indicated for the first week. Postoperative antibiotic prophylaxis was commonly used.
Patients with neurological deficits were treated according to the cause of the deficit. If the cause is recollection of haematoma or postoperative hydrocephalus that needed another surgical intervention, we provided the proper surgical treatment. Neurological deficits due to brain contusions and/or lacerations were treated medically in the ICU.
Data collected included age, GCS upon admission, haematoma thickness upon admission CT and outcome by GOS.
Statistical analysis of data was conducted using SPSS version 20. Data were expressed in proportion to categorical variables and mean with or without standard deviation of continuous variables and for comparing two independent groups Student’s T and Chi-square test were applied based on the type of variables.
Results
This is a prospective observational hospital-based study that included 39 patients with isolated traumatic acute subdural haematoma (TASH) admitted and managed in the trauma unit of Assiut university hospital in the period of 01/09/2019 to 31/08/2020 with follow-up period up to 6 weeks of admission.
The ages ranged from 9 to 75 years. The mean age was 38.025 years. Patients from 9 to 30 years were 16 patients (41%), from 31 to 55 were 16 patients (41%) and from 56 to 75 were 7 patients (18%).
There was a high significant relation between age of the patients and GOS (p value 0.003). Death occurred in 100% of patients between the ages of 56 and 75 years old, 44% in patients between 31 and 55 and 25% in patients between 9 and 30 years (Table 1a and b).
Four patients (10.25%) had GCS greater than or equal to 13 (mild brain injury). GCS between 9 and 12 was found in 15 patients (38.46%) with moderate brain injury. 20 patients (51.28%) had GCS upon admission less than or equal to 8 (severe brain injury).
There was a highly statistically significant relation between the GOS of patients and GCS upon admission (p value 0.006). Death happened in 60% of patients with GCS less than or equal to 8, while 33.3% of patients with GCS between 9 and 12 died, and no patient died with GCS greater or equal to 13 (Table 2a and b).
There were 17 patients (43.58%) with haematoma thickness less than 10 mm, while there were 22 patients (56.41%) with haematoma thickness more than or equal to 10 mm.
There was a non-statistically significant relationship between haematoma thickness in admission CT and GOS (p value 0.220) (Table 3a and b).
Conservative treatment applied to 14 patients (35.89%), early surgery to 22 patients (56.41%) and delayed surgery for 3 patients (7.9%) with a GCS reduction by ≥ 2 points from GCS admission and/ or changes in the reactivity and equality of pupil or in vital signs.
The number of deceased patients in this study was 17 patients with a mortality rate of 43.5% compared with 22 patient who survived with a survival rate of 56.5%.
Discussion
Traumatic acute subdural haematoma (TASH) is one of the lethal causes following severe head injuries. Traumatic acute subdural haematoma occurs in about 10–20% of patients with severe head injuries [11].
The haematomas commonly have a high mortality rate (between 40 and 60%). This high rate of mortality may be due to the haematoma, primary brain injury at the moment of impact, and subsequent changes due to hypotension and hypoxia [12].
Isolated TASH means it is not associated with other haematomas like epidural, subarachnoid or intracerebral ones. It only occurs in 30–40% of TASH [3].
In the current paper, we reported and studied 39 patients with isolated TASH who were admitted and managed in the trauma Unit of Assiut university Hospital over a period of one year (from 1/9/2019 to 31/8/2020).
Age of patients ranged from 9 to 75 years. The mean age was 38.025 years. Patients from 9 to 30 years were 17 (41%), from 31 to 55 were 16 (41%) and from 56 to 75 years were 7 patients (18%). There were 28 male (71.79%) and 11 females (28.20%). We reported that death occurred at the age of 9–30 years were 4, poor outcome (GOS 2, 3) in 4 and favourable outcome (GOS 4, 5) were in 9 patients. In the age group from 31 to 55 years, death was in 7, poor outcome in 7 and favourable in 2 patients.
There was a highly significant correlation (p-value 0.003) between the age of patient and GOS, as poor outcome was found in patients with a mean age of 47.18 years while favourable outcome was reported in patients with a mean age 26.27 years. Our findings are in agreement with those of Hanif et al. [13] as they reported significant mortality in older patients (50% over 70 years of age, 25.6% between 40 and 70 years, and 26% below 40 years).
Our findings are in agreement with those of Ono and colleagues [14], who found a statistically significant correlation between age and patient outcome.
Amato and colleagues [15] in his study of 28 patients with isolated TASH concluded the same findings. Yılmaz and colleagues [16] in their study of 93 patients with TASH who underwent decompressive craniectomy, they found that older age were statistically significant with mortality rate (p-value 0.007). However, Chen et al. [17] said that age has no statistically significant association with functional outcomes.
Glasgow Coma Scale (GCS) is the most important indicator that reflects the degree of brain injury and reflects the patient's neurological status on admission and during follow-up [18].
In the present study, upon admission, 4 patients (10.25%) had GCS greater or equal to 13 (mild brain injury), GCS between 9 and 12 was found in 15 patients (38.46%) with moderate brain injury while 20 patients (51.28%) had GCS less than or equal to 8 (severe brain injury). Among the 20 patients with GCS less or equal 8, death occurred in 12 patients (60%) while poor outcome was reported in 6 patients (30%) and favourable outcome was found only in 2 patients (10%). Among 15 patients with GCS 9 to 12 with moderate brain injury, death happened in 5 patients (33.3%), poor outcome in 5 patients (33.3%), while favourable outcome was reported in 5 patients (33.3%).
In 4 patients with GCS 13–15 (mild brain injury), no death reported, and all 4 patients had a favourable outcome (100%).
In the current study, there was a highly statistically significant correlation between patients' GOS outcome and GCS upon admission (p-value 0.006). Aykut and colleagues [19] reported that among 57 patients with GCS 3–8, 40 died (70.2%) and a favourable outcome was found in 4 patients (7%), whereas in 35 patients with GCS 9–12, death occurred in 19 (54.3) and favourable recovery was reported in 19 patients (28.5%). Among 21 patients with GCS 13–15, death was reported in 5 (23.8%), while a favourable recovery was found in 16 patients (76.2%). Moreover, they reported a strong correlation between GCS score in admission and outcome. These findings agree with the findings of the current study. Phuenpathom and colleagues [20] concluded that GSC score is one of the most critical factors predicting the outcome in patients with TASH. However, Chin et al. [17] demonstrated that higher GCS score did not affect the outcome, which can be attributed to the exclusion criteria in their study, as they excluded patients with GCS 3 or 4 combined with bilateral pupil dilation. These reasons reduced the impact of the GCS score on the outcome of their study.
CT is the main diagnostic aid in TASH. Classic SDHs usually presented in one or two brain areas like parietal or temporoparietal area, however it can be spread over an entire hemisphere. In the early phase (hyperacute phase), TASH may be isodense as it is still liquid. Then, it coagulates and condenses during the acute phase and shows a typical hyperdense crescent-appearance in CT [21].
In the current study, out of 39 patients with isolated TASH, the thickness of the haematoma in 17 (43.58%) patients was less than 10 mm, while the thickness of the haematoma in 22 patients (56.41%) was equal or more than 10 mm.
In 22 patients with a haematoma thickness upon CT admission equal to or more than 10 mm, 10 (45.5%) died, 8 (36.4%) had a poor outcome, while favourable outcome was reported in 4 (18.2%) patients. However, there was a non-statistically significant relationship between admission CT and GOS (p value 0.220).
This may be attributed to those accidents with acceleration and deceleration effect lead to displacement of the neural tissue and damage that not commensurate with CT finding upon admission.
Of the 39 patients with TASH, 14 (35.89%) were treated conservatively. We based the selection of these patients on that haematoma thickness during admission with CT less than 10 mm or patients who were clinically stable or improved during observation. Five of these 14 (35.71%) died during the follow-up period (up to 6 weeks of admission). We reported 3 patients who subsequently needed surgery, nonetheless, due to the statistical analysis they were included with patients who were surgically treated.
Conclusions
Acute traumatic subdural haematoma is associated with high mortality rate which may be attributed to haematoma and/or primary brain injury. Patients with TASH and low GCS may get benefit from the aggressive surgical intervention. The age of patients as well as GCS upon admission are important predictors of the outcome and can be used as a prognostic factor.
Availability of data and materials
All the data and results of the statistical analysis are available with the authors and ready to be shared with approved personnel upon demand.
Abbreviations
- ASDH:
-
Acute subdural haematoma
- CT:
-
Computed tomography
- GCS:
-
Glasgow Coma Scale
- GOS:
-
Glasgow Outcome Score
- MRI:
-
Magnetic resonance image
- TASH:
-
Traumatic acute subdural haematoma
References
Karibe H, Hayashi T, Hirano T, Kameyama M, Nakagawa A, Tominaga T. Surgical management of traumatic acute subdural hematoma in adults: a review. Neurol Med Chir (Tokyo). 2014;54(11):887–94.
Gusmão SNS, Pittella JEH. Acute subdural hematoma and diffuse axonal injury in fatal road traffic accident victims: a clinico-pathological study of 15 patients. Arq Neuropsiquiatr. 2003;61(3B):746–50.
Giuliani SG, Maria Cremonini A, Cenni P, Zappi D, Taylor F, Nasi MT. CT prognostic factors in acute subdural haematomas: the value of the ’worst’ CT scan. Br J Neurosurg. 2000;14(2):110–6.
Shima K, Aruga T, Onuma T, Shigemori M. Members of the Japanese Guidelines Committee on the Management of Severe Head Injury, and the Japan Society of Neurotraumatology. JSNT-guidelines for the management of severe head injury (abridged edition). Asian J Neurosurg. 2010;5(1):15–23.
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(suppl 3):S2-16.
Jennett B, Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet. 1975;305(7905):480–4.
Gwinnutt CL, Driscoll PA. Advanced trauma life support. Eur J Anaesthesiol EJA. 1996;13(2):95–101.
Signorini DF, Andrews PJD, Jones PA, Wardlaw JM, Miller JD. Predicting survival using simple clinical variables: a case study in traumatic brain injury. J Neurol Neurosurg Psychiatry. 1999;66(1):20–5.
Wilberger JE, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg. 1991;74(2):212–8.
Karibe H, Kameyama M, Kawase M, Hirano T, Kawaguchi T, Tominaga T. Usefulness and limitation of trephination as an early treatment option for severe traumatic head injury: an analysis of Japan Neurotrauma Data Bank Project 2009. Neurotraumatology. 2013;36:30–6.
Massaro F, Lanotte M, Faccani G, Triolo C. One hundred and twenty-seven cases of acute subdural haematoma operated on. Acta Neurochir (Wien). 1996;138(2):185–91.
Chabok SY, Safaie M, Moghadam AD, Behzadnia H, KhaliliRad M, Larimi SR. Acute subdural hematoma: a comparative study of 2 types of operative techniques. Neurosurg Q. 2011;21(2):103–6.
Hanif S, Abodunde O, Ali Z, Pidgeon C. Age related outcome in acute subdural haematoma following traumatic head injury. Ir Med J. 2009;102(8):255.
Ono J, Yamaura A, Kubota M, Okimura Y, Isobe K. Outcome prediction in severe head injury: analyses of clinical prognostic factors. J Clin Neurosci. 2001;8(2):120–3.
D'Amato L, Piazza O, Alliata L, Sabia G, Zito G, Frassanito L, et al. Prognosis of isolated acute post-traumatic subdural haematoma. J Neurosurg Sci. 2007;51(3):107–11.
Yılmaz İ, Ertem DH, Kılıç M, Altaş K, Mirhasilova M, Ozdemir B, et al. Factors associated with mortality in acute subdural hematoma: Is decompressive craniectomy effective? Turkish J Trauma Emerg Surg. 2019;25(2):147–53.
Chen S-H, Sun J-M, Fang W-K. The impact of time from injury to surgery in functional recovery of traumatic acute subdural hematoma. BMC Neurol. 2020;20:1–6.
Marmarou A, Lu J, Butcher I, McHugh GS, Murray GD, Steyerberg EW, et al. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: an IMPACT analysis. J Neurotrauma. 2007;24(2):270–80.
Karasu A, Civelek E, Aras Y, Sabanci PA, Cansever T, Yanar H, et al. Analyses of clinical prognostic factors in operated traumatic acute subdural hematomas. Ulus travma acil cerrahi derg. 2010;16(3):233–6.
Phuenpathom N, Choomuang M, Ratanalert S. Outcome and outcome prediction in acute subdural hematoma. Surg Neurol. 1993;40(1):22–5.
Koegel C, McCallum R, Greenhill M, García DL, Kohli A, Mallon M. Imaging of traumatic intracranial hemorrhage. J Am Osteopat Coll Radiol. 2018;8(3):13–20.
Acknowledgements
Not applicable.
Funding
No external funding for the design or implementation of the study, collection, analysis, and interpretation of the data or writing the manuscript was received by the authors.
Author information
Authors and Affiliations
Contributions
All the authors have contributed to the study design, implementation, data and results analysis, writing, and editing of the manuscript. The authors have agreed to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
This research was performed after the authorization of the ethical committee of the Faculty of Medicine, Assiut University, with committee reference number 17100813. This research included human patients; therefore, a written informed consent was taken from all the participants, or their legal guardians as required by the ethical committee recommendations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ragaee, M.A., Mahmoud, R.N., Alghriany, M.A. et al. Isolated traumatic acute subdural haematoma: outcome in relation to age, Glasgow Coma Scale, and haematoma thickness. Egypt J Neurol Psychiatry Neurosurg 57, 156 (2021). https://doi.org/10.1186/s41983-021-00410-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-021-00410-4