Abstract
Background
The invasive peach fruit fly (PFF), Bactrocera zonata (Saunders) (Diptera: Tephritidae), is a native of Southeast Asia. Entomopathogens like nematodes, bacteria, viruses and fungi have been shown to be effective as a biological control agent against B. zonata. Evaluation the efficacy of different entomopathogenic nematode isolates (EPNs) belonged to the two families (Steinernematidae and Heterorhabditidae); (Steinernema carpocapsae (AII), S. carpocapsae (EGAZ10), Heterorhabditis bacteriophora (HP88) and H. indica (EGAZ2) was carried out against the full-grown larvae of B. zonata under laboratory, semi-field and field conditions.
Results
Data revealed that in all the tested nematode isolates succeeded to reduce the emerging of the PFF, B. zonata compared to controls with significant differences. The LC50 values were 794.3, 1063.2, 1249.8 and 1446.8 IJs/ml, for S. carpocapsae (AII), S. carpocapsae (EGAZ10), H. bacteriophora (HP88) and H. indica (EGAZ2), respectively, at 3 days post treatments. The strain, S. carpocapsae (AII) was effective than S. carpocapsae (EGAZ10). Also, the strain H. bacteriophora (HP88) was more effective than H. indica (EGAZ2). Therefore, the combination between the effective two steinernematid strains (S. carpocapsae (AII) & S. carpocapsae (EGAZ10)) and the two heterorhabditis strains (H. bacteriophora (HP88) & H. indica (EGAZ2)) was efficient in the semi-field experiment. In field condition, the combination of the two efficient strains S. carpocapsae (AII) and H. bacteriophora (HP88) at the concentration of 3000 IJs/ml was more effective in controlling B. zonata causing mortality 97.5%. The Co-Toxicity factor values were − 67.6 for the combination of S. carpocapsae (AII) with S. carpocapsae (EGAZ10) which recorded an antagonistic effect. Also, antagonistic effects were observed for the combined application of H. bacteriophora (HP88) with H. indica (EGAZ2) (− 66.6) in semi-field application; and the same effect was recorded for the combination of S. carpocapsae (AII) with H. bacteriophora (HP88) (− 42.6) in field application.
Conclusion
All EPNs’ experiments showed that the efficacy of foreign nematodes than the local ones. So, combination of the two highly effective imported strains gave satisfied results, especially in the field experiment.
Similar content being viewed by others
Background
The peach fruit fly (PFF), Bactrocera zonata (Saunders, 1841) (Diptera: Tephritidae), is an invasive pest species, native to Southeast Asia. It was first recognized as a new pest of guava and mango in the northern region of Egypt in 1998 (El-Minshawy et al. 1999). According to Hashem et al. (2007), B. zonata has a wide variety of hosts, including fruits and vegetables. It spreads quickly in great numbers throughout Egyptian Governorates. Due to the favorable climate in Egypt, the PFF lives in Egypt and developed into a significant pest over the past ten years and began infesting a variety of fruit hosts, including citrus, mango, peach, fig, guava, apricot, and apple. Additionally, it targets various foods as secondary hosts, including tomato, pepper, and eggplant (Ghanim 2009). Use of insecticides as an only method to control pests in fruits and vegetables has caused environmental pollution and hygienic problems that represent a risk for human and animals (Gallo 2007). In comparison with pesticides, biological management is less cost-effective and less harmful to the environment (Rizvi et al. 2009). According to Dias et al. (2018), natural enemies, parasitoids, predators, and pathogens are frequently used in biological management because they attack pests. Entomopathogens are natural microorganisms that can be found in a variety of ecosystems infecting different stages of the insect hosts. It has been demonstrated that pathogens like nematodes, bacteria, viruses and fungi are viable biological control agents against B. zonata (Bilal et al. 2021).
Rashad et al. (2015) suggested that EPNs and fungi can be used as alternative tools of pesticides for controlling B. zonata after validating protocols of field conditions. Some tephritid species have been observed to be mortally affected by steinernematid and heterorhabditis species. Different phases of B. zonata may be infected by EPNs, according to (Mahmoud et al. 2016).
The objective of this study was to evaluate the efficacy of different EPN isolates: Steinernema carpocapsae (AII), S. carpocapsae (EGAZ10), Heterorhabditis bacteriophora (HP88) and H. indica (EGAZ2) belonged to two families (Steinernematidae and Heterorhabditidae) against the full-grown B. zonata larvae under laboratory, semi-field and field conditions. Also, to evaluate the influence of combination for whether applying two EPN strains at (semi-field and field experiment); it was synergistic, additive or antagonistic effects.
Methods
Insect culture
The stock colony of PFF used in the present study was reared in cages measured (35 × 30 × 30 cm) at the Biological Control Department, Plant Protection Research Institute, Agricultural Research Center (ARC)—Giza, Egypt. Rearing was carried out under greenhouse conditions of 25 ± 2 °C and 55–65% R.H. and the photoperiod ranged 14–16:8–10 L: D. on artificial diet according to (Hosni et al. 2011 and Rashad et al. 2015).
Entomopathogenic Nematodes’ cultures
Source: The Egyptian nematode strains of S. carpocapsae (EGAZ10) and H. Indica (EGAZ2) and the imported nematode species, S. carpocapsae (AII) and H. bacteriophora (HP88) were obtained from Biological Control Department (BCD), Plant Protection Research Institute (PPRI), Agricultural Research Center (ARC), Giza, Egypt.
For mass culturing of the tested nematode species, S. carpocapsae (AII); S. carpocapsae (EGAZ10); H. bacteriophora (HP88) and H. indica (EGAZ2), strains were propagated at the Physiology Laboratory, of PPRI, Giza, Egypt in vivo using larvae of the greater wax moth, Galleria mellonella (Linnaeus, 1758) as a host according to (Shamseldean et al. 2009). Rearing of G. mellonella was acquired from infested bee hives and reared in jars according to Metwally et al. (2012). Fifty last instar larvae of G. mellonella were placed in Petri-dishes; each contains two filter papers moistened with water suspension of nematodes at concentration of (30.000 IJs/2 ml. of distilled water). After 10–15 days of infection, the host cadavers were transferred to nematode-collecting dishes according to White traps method (White 1927) to collect the infective juveniles (IJs). The emerging juveniles of S. carpocapsae (at day 10 post-infection), and, H. bacteriophora (at day 15 post- infection) were harvested daily and then stored in plastic bottles (15 × 15 × 10 cm) containing distilled water at 8–10 and 15 °C until used.
Pathogenicity of the EPN species against B. zonata full-grown larvae under laboratory conditions
Three different species (four isolates) of EPNs: S. carpocapsae (AII), S. carpocapsae (EGAZ10), H. bacteriophora (HP88) and H. Indica (EGAZ2) were suspended in distilled water (d. w.) at the concentrations of 250, 500, 1000, 1500 and 2000 IJs/ml d.w. against B. zonata full-grown larvae. Plastic cups measuring (5.5 × 4 cm) filled with 25 gm. mixed soil (clay 50% + beach sand 50%), supplied with ten B. zonata full-grown larvae, treated with a suspension of nematode species, and then covered with a borer plastic lid. Control experiment was conducted by placing larvae on soil moistened with 1 ml. distilled water only. Four replicates (10 larvae/replicate) were used for each treatment. The mortality was estimated three days post treatments. The experiments were carried out at 25 ± 2 °C and R. H. (60–70% and 20% soil moisture).
Semi-field experiment
The semi-field experiment was performed under trees (uncontrolled conditions) of citrus orchard at ARC- Giza, Egypt, as a preliminary test to examine the effect of the combination of EPN species. The experimental design consisted of six trees for two EPNs treatments and one another tree for control using a total of seven trees. The experiment was applied under trees canopies. The combined nematodes suspension concentrations (combination between the two tested steinernematids or between those species of heterorhabditis) occurred for 1000, 2000, and 4000 IJs/ml. at maximum temperature (Max. T.) (28.85 °C); minimum temperatures (Min. T.) (19.71 °C) and R.H. (81.71%). Climatic conditions data were obtained from (Cairo International Airport Station) weather underground site (https://www.wunderground.com).
Four replicates were conducted for each concentration and distributed under every selected tree of the trial orchards; the accumulative mortality rate was estimated after ten days post treatments. The soil sample was taken using a spoon. The amount of the orchard soil was weighted at a rate of 25 gm and placed in a piece of muslin (to prevent larvae from escaping and attacking insects in the soil, especially ants) supplied with 20 full-grown larvae/replicate. The pieces of muslin were tied with a piece of thread (Fig. 1). The time recorded during the experiment was 5 p.m., the muslin piece containing infected PFF full-grown larvae was buried 5 cm in the soil under the tree canopy and the control treatment was buried without nematodes. The experiment was irrigated every two days by a water hand sprayer in the 0.5 m2 area surrounding each treatment.
Field experiment
Based on the initial screening of the previous four nematode strains, the most efficient steinernematid and heterorhabditis strains were selected for additional evaluations. The experiment was carried out in plastic cans measured (19 × 14 × 8 cm) (Fig. 2) filled with 200 gm soil (moisten soil) under four citrus trees canopies which maximum temperature (30.85 °C), minimum temperature (18.14 °C) and R.H. (88.00%), at the ARC, Giza, Egypt. The combined EPN species (S. carpocapsae (AII) + H. bacteriophora (HP88)) were treated at a concentration of 3000 IJs/ml. The EPNs applied for each test mixed with soil and water, which always kept at 55 ± 5% of the soil weight. In this experiment, every 20 full-grown larvae/replicate were placed in the plastic cans and covered with stockings to prevent escaping larvae and attacking by ants then tied with rubber bands. Part of the bottom of the cans was buried in the soil. Four replicates were used where each treatment placed next to its control. The numbers of dead larvae were recorded after 48 h, and the emergence of adults was recorded after 8 days post treatment.
The dead larvae (Fig. 3) and pupae for laboratory, semi-field and field experiments were incubated in white trap for collecting the infective juveniles of EPNs.
To find out if combining two EPN species would have an antagonistic, additive, or synergistic effect on B. zonata full-grown larvae, laboratory, semi-field and field experiments were carried out.
Combined actions of the nematodes, S. carpocapsae (AII); S. carpocapsae (EGAZ10); H. bacteriophora (HP88); H. indica (EGAZ2); combined the two steinernematid species or heterorhabditis species, and combined S. carpocapsae (AII) + H. bacteriophora (HP88) on B. zonata full-grown larvae were used.
The combined action of the mixtures was expressed as a Co-toxicity factor, estimated by the equation of Mansour et al. (1966) as follows:
where: Observed % mortality is the mortality of individuals treated by the combination. Expected % mortality is the sum of mortalities of each material when used singly. These factors were used to differentiate the results into 3 categories. The interaction result was assessed as, ‘synergism’ when Co-toxicity factor values were greater than or equal to 20; ‘addition’ when Co-toxicity factor values were between − 20 and + 20; and ‘antagonism’ when Co-toxicity factor values were less than or equal to − 20.
Statistical analysis
Obtained data were analyzed according to Finney (1971). Percentage mortality was plotted versus the corresponding concentrations probit lines, and the median lethal concentration LC50 was determined for established regression lines using Ehab soft LDP line software to calculate probit analysis (www.Ehabsoft.com/LDP line). Lower and upper limits were determined when the (g) factor was less than 0.4. Data obtained for significant differences were analyzed using the Analysis of Variance one way (ANOVA) technique, and the means were separated using Fisher’s least significant difference (LSD) test (p = 0.05 level).
Results
Laboratory experiments
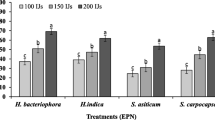
Data in Table 1 showed the mortality percentages of B. zonata full-grown larvae with S. carpocapsae (AII), S. carpocapsae (EGAZ10), H. bacteriophora (HP88) and H. indica (EGAZ2) strains at different concentrations (250, 500, 1000, 1500 and 2000), under laboratory conditions, at 25 ± 2 °C and R.H. (60—70% and 20% soil moisture). The lowest concentration of 250 IJs/ml of each tested strain (S. carpocapsae (AII), S. carpocapsae (EGAZ10), H. bacteriophora (HP88) and H. indica (EGAZ2)) resulted in mortality percentages of 15, 7.5, 10, and 7.5%, respectively, while mortality percentages reached 92.5, 85, 77.5 and 72.5% for the four mentioned EPN strains, respectively, at concentration 2000 IJs/ml. It was noticed that S. carpocapsae (AII) was effective than the other three strains. It is also clear that the mortality percentages increased when concentrations increased. The probit lines in Fig. 4 indicated the pathogenicity at different concentrations of steinernematid and heterorhabditis strains where the LC50 values were 794.3, 1063.2, 1249.8 and 1446.8 IJs/ml for S. carpocapsae (AII), S. carpocapsae (EGAZ10), H. bacteriophora (HP88) and H. indica (EGAZ2), respectively, at 3 days of treatments (Table 2).
Data pointed that steinernematid strains were more effective against B. zonata full-grown larvae than heterorhabditis strains at 25 ± 2 °C and R.H. (60—70% and 20% soil moisture). Also, Table 1 showed that there were significant differences between mortality percentages at different concentrations.
Semi-field experiment
The combined concentrations of 1000, 2000 and 4000 IJs/ml for the two tested steinernematid (S. carpocapsae (AII) & S. carpocapsae (EGAZ10)) or those of heterorhabditis (H. bacteriophora (HP88) & H. indica (EGAZ2)) strains were used, in semi-field conditions at the ARC, Giza, Egypt. Data in Table 3 revealed that the combination of steinernematid strains was more effective than the combination of heterorhabditis strains. Direct relationship between the mortality percentages at different concentrations was recorded. Data in Table 4 revealed an antagonistic effect of the combined steinernematids, S. carpocapsae (AII) and S. carpocapsae (EGAZ10) where Co-toxicity factor value is (− 67.6). Also, H. bacteriophora (HP88) and H. indica (EGAZ2) in combination has a Co-Toxicity factor value (− 66.6). Despite the increase in the proportion of B. zonata death in the combined treatments for all the tested EPNs, the type of interaction was antagonism.
Field experiment
According to the previous trials, the field experiment was conducted. A nematode suspension was used at concentration of 3000 IJs/ml for the combined S. carpocapsae (AII) and H. bacteriophora (HP88) strains, under field conditions of maximum temperature (30.85 °C), minimum temperature (18.14 °C) and R.H. (88%). Results revealed that the combination of the two different strains of EPNs (S. carpocapsae (AII) + H. bacteriophora (HP88)) recorded a mortality percentage of 97.5%. On the other hand, the combined EPNs S. carpocapsae (AII) and H. bacteriophora (HP88) strains recorded an antagonistic effect, where the Co-toxicity factor value was (− 42.6) (Table 4).
Discussion
Results indicated that all tested nematodes had variable modes of bactericidal action in the B. zonata host, resulting in markedly antagonistic effects after strains’ combination. The previous results revealed that steinernematid strains were more effective against B. zonata full-grown larvae than heterorhabditis strains. Also, there were significant differences between mortality percentages at different concentrations when EPNs were separately tested. Combination of the steinernematid or heterorhabditis strains was more effective on the target pest. Data revealed the presence of an antagonistic effect due to the combinations of (S. carpocapsae (AII) + S. carpocapsae (EGAZ10)) or (H. bacteriophora (HP88) + H. indica (EGAZ2)). In the field experiment, results revealed that the combination of the two different strains of EPNs (S. carpocapsae (AII) + H. bacteriophora (HP88)) were more effective and recorded mortality reached 97.5%.
The results agree with those of Abd El-Motaal et al. (2021) who reported that the mortality percentage increased as IJs concentration increased. Rashad et al. (2015) demonstrated that both S. carpocapsae and S. riobrave were superior to H. bacteriophora in contact treatment against B. zonata full-grown larvae; and infectivity among those tested nematode strains was found significantly different. Steinernematid strains, also referred to as "ambushers," were more successful than heterorhabditis strains and more effective against soil-dwelling insects, as reported by Attalla and Eweis (2002). In field tests, Steinernema and Heterorhabditis species have been employed to control pest insects that emerge from fruit and burrow into the soil to pupate, with differing degrees of success. For example, fruit flies Rhagoletis indifferens and Anastrepha ludens (Diptera: Tephritidae) (Toledo et al. 2005) and several other species reviewed by Dolinski and Lacey (2007). Soil properties such as moisture level, pH, organic matter content, texture, and others, affect EPNs dispersal and potential to find a host (Stuart et al. 2015). Third instar larvae of several tephritid flies were reported to be susceptible to EPNs (Langford et al. 2014). Similarly, S. carpocapsae and S. feltiae were found to be more effective species under laboratory, semi-field, and field conditions against the European cherry fruit fly larvae where the mortalities were 88, 78, and 88%, respectively, while no mortality of pupae was observed (Köppler et al. 2004). On the other hand, at field temperature of 30 °C, Heterorhabditis species have a very strong ability to signal their maximal degree of pathogenicity according to El Khoury et al. (2018). These results are different from one insect to another may be due to some reasons such as insect behavior or insect physiological or/and the competition between the used two nematode strains or species. It was reported that combining nematodes species might be more effective in the field, because of the strongest concentration effect and highest propagation rate of the EPNs (O’Callaghan et al. 2014). The effect of mutualistic EPNs Xenorhabdus and Photorhabdus is gram-negative bacteria that can produce several secondary metabolites, including antimicrobial compounds. Otherwise, the nematodes or bacteria themselves make significant contributions to pathogenesis within the insect, poisoning effect and may be due to the presence of some physical or chemical reactions in the insect hemolymph (Lewis and Clarke 2012) and (Chang et al. 2019).
Conclusion
All EPNs experiments showed the efficacy of foreign nematodes than the local ones against B. zonata full-grown larvae and increasing mortality with concentration increase. Also, combination of the two highly effective imported strains gave satisfied results especially in the field experiment.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Abd El- Motaal DS, Nouh GM, Sarhan AA, El-Basha NA, Mandour NS (2021) Utilization of the Entomopathogenic Nematodes Heterorhabditis bacteriophora HP88 and Steinernema feltiae (Filipjev) as biological control agents against the peach fruit fly Bactrocera zonata Saunders (Tephritidae: Diptera). J Appl Plant Prot 10(1):97–102
Attalla FA, Eweis M (2002) Preliminary investigation on the utilization of entomopathogenic nematodes as biological control agents against the peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae). Egypt J Agric Res 80(3):1045–1053
Bilal H, Raza H, Qayyum MA, Ijaz M, Bashir MI, Baig MA, Hassan M (2021) Management of Bactrocera zonata (Diptera: Tephritidae) through application of different tactics: a review. Curr Res Agri Far 2(3):8–16
Chang DZ, Serra L, Lu D, Mortazavi A, Dillman AR (2019) A core set of venom proteins is released by entomopathogenic nematodes in the genus Steinernema. PLoS Pathog 15(5):e1007626. https://doi.org/10.1371/journal.ppat.1007626
Dias NP, Zotti MJ, Montoya P, Carvalho IR, Nava DE (2018) Fruit fly management research: a systematic review of monitoring and control tactics in the world. Crop Prot 112:187–200
Dolinski C, Lacey LA (2007) Microbial control of arthropod pests of tropical tree fruit. Neotrop Entomol 36:161–179
El Khoury Y, Oreste M, Noujeim E, Nemer N, Tarasco E (2018) Effect of temperature on the pathogenicity of Mediterranean native entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) from natural ecosystems. Redia 101:123–127
El-Minshawy AM, El-Eryan MA, and Awad AI (1999) Biological and morphological studies on guava fruit fly, Bactrocera zonata (Diptera: Tephritidae) found recently in Egypt. 8th – P Nat. Conf. of Pests and Diseases of Vegetables and Fruits in Ismailia, Egypt. P. 71–81.
Finney DJ (1971) Probit analyses. Cambridge University Press, New York USA
Gallo J (2007) Integrated plant control: system and management. Encycl Pest Manag 2:279–287
Ghanim NM (2009) Studies on the peach fruit fly, Bactrocera zonata (Saunders) (Diptera :Tephritidae). Ph.D. Thesis, Fac. Agric. Mansura Univ.
Hashem AG, Shehata MN, Abdel-Hafeez TA, Ibrahim SA, El-Kashef KKH (2007) Occurence and distribution of Bactrocera zonata (Saund) in North Sinai. Egypt J Appl Sci 22(10B):682–692
Hosni ME, El-Husseini MM, El-Heneidy AH, Atallah FA (2011) Biological aspects of the peach fruit fly, Bactrocera zonata (Saund.) (Diptera: Tephritidae) and its parasitoid species, Aganaspis daci Weld. (Hymenoptera: Eucoilidae). Egypt J Biol Pest Control 21(2):137–142
Köppler K, Peters A and Vogt H (2004) Basic results in biological control of the European cherry fruit fly, Rhagoletis cerasi L. (Diptera: Tephritidae) with entomopathogenic nematodes
Langford EA, Nielsen UN, Johnson SN, Riegler M (2014) Susceptibility of Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae), to entomopathogenic nematodes. Biol Control 69:34–39
Lewis EE, Campbell J, Griffin C, Kaya H, Peters A (2006) Behavioral ecology of entomopathogenic nematodes. Biol Control 38:66–79
Lewis EE, Clarke DJ (2012) Nematode parasites and entomopathogens. In: Vega FE, Kaya HK (eds) Insect pathology, 2nd ed. Elsevier, pp 395–424
Mahmoud YA, Ebadah IA, Hala MS, Saleh ME (2016) Controlling of larvae, pupae and adults of the peach fruit fly, Bactrocera zonata (Saund.) (Diptera: Tephritidae) with the Entomopathogenic Nematode, Steinernema feltiae. Egypt J Biol Pest Control 26:1
Mansour YA Eldefrawi ME Toppozadz A, Zrid M (1966) Toxicological studies on the Egyptian cotton leaf worm Prodenia litura VI. Potentiation and antagonism of organophosphorus and carbamate insecticides. J. Econ. Entomol., 59(2), 307–311.
Metwally HMS, Hafez GA, Hussein MA, Salem HA, Saleh MME (2012) Low cost artificial diet for rearing the greater wax moth, Galleria mellonella L. (Lepidoptera: Pyralidae) as a host for entomopathogenic nematodes. Egypt J Biol Pest Control 22(1):15–17
O’Callaghan KM, Zenner ANRL, Hartley CJ, Griffin CT (2014) Interference competition in entomopathogenic nematodes: male Steinernema kill members of their own and other species. Int J Parasitol 44:1009–1017
Rashad MM, El-Heneidy AH, Djelouah K, Hassan N, Shairra SA (2015) On the pathogenicity of entomopathogens to the peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae). Egypt J Biol Pest Control 25(3):649–654
Rizvi PQ, Choudhury RA, Ali A (2009) Recent advances in biopesticides. Microbial strategies for crop improvement. Springer, pp 185–203
Shamseldean MM, Hasanain SA, Rezk MZA (2009) Virulence of entomopathogenic nematodes against lepidopterous pests of horticultural crops in Egypt. 4th Conference on recent technologies in Agriculture, pp 74–84
Stuart RJ, Barbercheck ME, Grewal PS (2015) Entomopathogenic nematodes in the soil environment: Distributions, interactions and the influence of biotic and abiotic factors. In: Campos-Harrea R (ed) Nematode pathogenesis of insects and other pests. Springer, Dordrecht, pp 97–137
Toledo J, Ibarra JE, Liedo P, Gomez A, Rasgado MA, Williams T (2005) Infection of Anastrepha ludens (Diptera: Tephritidae) larvae by Heterorhabditis bacteriophora conditions. Biocontrol Sci Technol 15:627–634
White GF (1927) A method for obtaining infective nematode larvae from cultures. Science 66:302–303
Acknowledgements
Not applicable.
Funding
The PLANT-B project supported this work, EU/PRIMA Foundation (grant number 1812).
Author information
Authors and Affiliations
Contributions
All of the authors of this manuscript contributed equally to the design and execution of the experiments described in the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable—the study was conducted on insect species that are abundant in the ecosystem and does not require ethical approval.
Consent for publication
The manuscript has not been published in completely or in part elsewhere.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sallam, R.F., Shalaby, F.F., Hafez, A.A. et al. Efficacy of different entomopathogenic nematode isolates, against the peach fruit fly, Bactrocera zonata (Saund.) (Diptera: Tephritidae). Egypt J Biol Pest Control 34, 12 (2024). https://doi.org/10.1186/s41938-024-00774-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-024-00774-8