Abstract
Background
Apple decline diseases, responsible for seedlings root and collar rot in nurseries, are an important disease. Different fungal and bacterial antagonists were evaluated to control Pythium ultimum associated with this serious disease using in vitro and in vivo greenhouse assays.
Results
The in vitro test of ten Aspergillus spp. and ten Trichoderma harzianum isolates showed their efficacy to reduce the radial growth of P. ultimum. The isolates, A. niger A10, A. candidus A5, T. harzianum Tr9 and Tr10, were the most effective with a high inhibition percent that exceeded 50%. The in vivo test of the four most effective antagonists and a strain of Bacillus subtilis (B) showed that the combination of the two Aspergillus isolates (A5 and A10) gave the best result with a decrease in root browning index by 55.55%. Results showed also the efficacy of all tested antagonists and their combinations on the sanitary state index of the inoculated plants except the combination between T. harzianum (Tr10) and B. subtilis (B). The two treatments, T. harzianum (Tr10) and B. subtilis (B), significantly improved the height of inoculated plants by 173.19 and 191.3%, respectively. Regarding the efficacy of antagonists on apple seedlings root weight, A. niger (A10) was the only treatment that significantly increased this parameter by 363.17%.
Conclusions
A. niger A10, A. candidus A5, T. harzianum Tr9 and Tr10 exhibited the highest value of in vitro inhibition to growth of P. ultimum. The combination of A. niger A10 and A. candidus A5 was the most effective in vivo treatment in reducing the disease severity index. However, T. harzianum (Tr10), B. subtilis (B) and A. niger (A10) revealed to be able to stimulate the apple seedlings growth.
Similar content being viewed by others
Background
Apple (Malus domestica) crop occupies an important place in the world, in terms of total fruit yield within the fruit industry (Yuan et al. 2018). However, the development of this crop has various phytosanitary problems such as seedlings decline disease (Mazzola and Manici 2012). Apple decline disease is a biological phenomenon caused by soil-borne agents like some species of fungi (Rhizoctonia, Fusarium and Cylindrocarpon), oomycetes (Pythium, Phytopythium and Phytophthora) and the nematodes (Pratylenchus) that attack the roots of apple trees (Tewoldemedhin et al. 2011).
Investigations conducted in nurseries revealed that the roots of nursery seedlings were infested by several apple orchards decline agents like Fusarium, Pythium and Phytopythium species. The association of apple orchards declines causative agents with nursery trees suggested that these could function as potential apple orchards inoculum sources that might limit post-plant tree growth (Moein et al. 2019).
The apple tree plants protection against these pathogens could be managed by the application of fungicides. In fact, some fungicides such as fosetyl-Al and metalaxyl have excellent systemic activity against several diseases caused by oomycetes species (Mannai and Boughalleb-M’Hamdi 2021). However, economic and environmental pressures to reduce the reliance on chemicals have led to a renewed interest to the use of pathogens such as bacteria and antagonistic fungi (Whipps and Lumsden 1991). Among biological control agents, Trichoderma, Aspergillus and Bacillus species are the most widely used antagonists for controlling plant diseases (Mannai and Boughalleb-M’Hamdi 2022).
Trichoderma spp. have been reported to be eco-friendly biological control agent for managing plant diseases, which enable the use of chemical fungicides to be minimized (Puyam 2016). Trichoderma species are abundant in all types of soil and are considered as potential antagonistic agents against parasitic soil-borne microorganisms (Shahid et al. 2014). Trichoderma species can produce extracellular enzymes and antifungal antibiotics (Barúa et al. 2019). They may also be competitors of fungal pathogens for space and nutrients, through rhizosphere competence (Cardoza et al. 2005).
Various species of the genus, Aspergillus have been recognized as a rich source of biologically active secondary metabolites (El-Sayed and Ali 2020). High diversity of secondary active metabolites by Aspergillus spp. could be attributed to their versatility of growing in a wide range of temperature, pH and osmolarity (Lubertozzi and Keasling 2009).
The bacterial genus, Bacillus, is one of the most frequently occurring endophytes that have been used as a biocontrol agent (Devi et al. 2022). The ability of Bacillus species to produce endospores renders them resistant to severe environmental conditions, making them a good choice for biocontrol agent. The antagonistic activity of Bacillus may be due to the production of siderophore and extracellular metabolites (Miljaković et al. 2020).
Therefore, the objectives of this study were: (1) to evaluate the in vitro antifungal potential of Aspergillus and Trichoderma isolates against the mycelial growth of P. ultimum associated with apple seedlings decline and (2) to test the ability of these antagonists with B. subtilis used individually or in combination to manage the disease severity and to enhance growth of infected apple plants.
Methods
Pathogen used
One isolate of P. ultimum (GenBank Accession no. MH260594) was used in this study. It was obtained from apple seedling nurseries infected by decline diseases in Tunisia and proved as a causative agent of this disease (Mannai 2019).
Antagonists tested
The antagonistic fungal and bacterial strains used in this study were isolated from Tunisian fruit trees nurseries (Table 1). Healthy samples of apple and peach roots were washed under tap water to remove adhering soil and cut aseptically into small pieces of 3 to 5 mm in length, followed by dipping in a solution of sodium hypochlorite (2%) for 2 min. Then, these pieces were rinsed with sterile distilled water and air dried in a laminar flow hood. When completely dried, samples were plated onto PDA medium (Potato-Dextrose-Agar). The plates were then incubated in the dark at 28 °C and checked daily for colony growth. Colonies that developed from the root segments were then transferred to PDA plates and purified by single-spore method using Water Agar (2%) medium. The identification of the collected antagonists isolates was performed after 7 days of incubation of each colony on PDA medium at 28 °C, based on morphological criteria as described by Siddiquee (2017) and Shah et al. (2019) for Trichoderma isolates and Diba et al. (2007) for Aspergillus isolates. The Bacillus strain was identified by morphological and biochemical analysis (Furuya et al. 2011).
Effect of selected antagonists on mycelial growth of Pythium ultimum associated with apple seedlings decline
Antifungal activities of the fungal antagonists on radial mycelial growth of the P. ultimum isolate were determined by dual confrontation technique performed in 90-mm Petri dishes containing PDA according to Mannai and Boughalleb-M’hamdi (2022). Agar plugs (6 mm in diameter) cut from pathogen cultures were placed each opposite to those of tested fungal antagonists. The control cultures were subcultured with a plug of the pathogen, and the antagonist plug was replaced by a plug of PDA medium. Three repetitions were used for each individual treatment. The incubation was performed at 25 °C for five days, and the experiment was repeated twice.
The inhibition percentage of P. ultimum mycelial growth was calculated according to the following formula:
where T is the average colony radius in the presence of the antagonist fungus and C is the average radius of the control colonies.
Effect of antagonists on the severity of the disease
The methodology of Mannai and Boughalleb-M’Hamdi (2022) was followed with some modifications. Two isolates of Trichoderma (T9 and T10), two isolates of Aspergillus (A5 and A10) and one B. subtilis (B) strain were used. To prepare the inoculum of each antagonist treatment, some agar plugs of the antagonist were incubated, for one week, in an Erlenmeyer containing 200 ml of PDB (Potato Dextrose Broth) medium, with stirring (120 rpm). The obtained suspensions were adjusted to 106 spores/ml for fungal species and 106 cells/ml for B. subtilis strain. The treatment was carried out at two dates: 1 and 30 days from the beginning of the experiment (50 ml/plant). The isolates of antagonists were applied solo and in combination.
The P. ultimum inoculum was prepared by inoculating 10 agar plugs onto a flask (500 ml) containing sand-oat (200 g of sand, 20 g of oat and 30 ml of distilled water, which had been autoclaved twice at 120 °C for 20 min). For the control, the pathogen mycelial plugs were replaced by PDA plugs. The flasks were incubated for 1 week at 25 °C and shaken every 2 days to ensure homogenous colonization (Strauss and Labuschagne 1995). After incubation, the sand-oat inoculum was added around apple seedlings roots to the third upper potting mix at the rate of 1% (v/v), on the 14th day from the beginning of the experiment. Two controls were included in the assays, negative control (untreated and not inoculated) and positive one (inoculated and not treated).
For each treatment, three plants were separately placed in 23-cm-diameter plastic pots containing a treated mixture of peat and sand (in 2:1 v/v). The experiment was conducted according to a completely randomized design, with three repetitions per elementary treatment. The seedlings were harvested after three months. Four parameters were recorded: the sanitary state index, the seedlings height, the root weight and the root browning index.
The sanitary state index rated onto 0–5 scale (0 = healthy seedlings; 1 = moderate discoloration of plant leaves (≤ 25%); 2 = moderate discoloration and falling leaves (≤ 50%); 3 = moderate discoloration of plant collar, stem and leaves (≤ 75%); 4 = extensive discoloration of plant collar and stem with falling leaves (> 75%); and 5 = dead plant) (Santini et al. 2006). The root browning index was rated according to a 0–5 scale: (0 = no obvious symptoms; 1 = moderate discoloration of root tissue; 2 = moderate discoloration of tissue with some lesion; 3 = extensive discoloration of tissue; 4 = extensive discoloration of tissue with girdling lesions; and 5 = dead plant) (Tewoldemedhin et al. 2011).
Results
Effect of antagonists on Pythium ultimummycelial growth
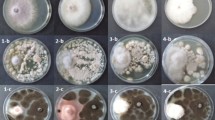
All Aspergillus spp. and T. harzianum isolates reduced the radial growth of the apple seedlings decline agent P. ultimum in comparison with the relative control. The A. niger A10 was the most effective (72.07%), followed by A. candidusA5 that reduced this pathogen by 53.15%. The results showed also that T. harzianum Tr9 and Tr10 were the most effective with a high inhibition percent of the pathogen mycelial growth with an inhibition percent more than 80%, 5 days post-incubation at 25 °C (Fig. 1 and Table 2). The four antagonists A5, A10, Tr9 and Tr10 were chosen for the in vivo test because they were the most effective in vitro (Fig. 1 and Table2).
Effect of antagonists on the severity of apple seedlings decline disease induced by Pythium ultimum
Variance analysis of the root browning index recorded three months after inoculation by P. ultimum showed a significant difference (p ≤ 0.05) between different treatments and the two controls. Indeed, the only treatment that significantly reduced this parameter was the combination of the two Aspergillus isolates (A5 and A10). It gave the best result with a decrease in root browning index by 55.55% (Table 3 and Fig. 2).
The results showed alsoa highly significant efficacy (p ≤ 0.001) of all antagonists tested and their combinations on the vigor status of the vegetative part of the inoculated plants except the combination between T. harzianum (Tr10) and B. subtilis (B). Nevertheless, the test of these last antagonists each alone improved the seedlings vigor status by 55.67%. The improvement in this parameter was 44.33% for A. nigerA10, T. harzianumTr9 and the combination of A. candidus and A. niger (A5 + A10) and 55.67% for A. candidus A5, T. harzianum Tr10, B. subtilis B and the combination of the two isolates of T. harzianumTr9 + Tr10 (Table 3).
The two treatments of T. harzianum Tr10 and B. subtilis B significantly improved the height of inoculated plants by 173.19 and 191.3%, respectively (Table 3). Regarding the root weight, the antagonist A. niger A10 was the only treatment that significantly increased this parameter by 363.17% on inoculated apple seedlings. The other treatments revealed to be ineffective to improve this parameter (Fig. 3 and Table 3).
Discussion
The approach of control used in the present study is the biological control by means of different antagonistic agents. The isolates of T.harzianum Tr9 and Tr10, native to the Kasserine region, were the most effective in vitro against P. ultimum. The in vivo test showed that these isolates and their combinations reduced the health status index severity of apple plants inoculated with P. ultimum. Tr10 also significantly improved the height of apple trees inoculated with P. ultimum. Several previous studies have shown that Trichoderma spp. are among the most studied a biological fungal agent marketed as biopesticides (Yassin et al. 2021). Furthermore, Green et al.(2001) explained the efficient biological control using T. harzianum by its ability to compete with P. ultimum for substrates from the seed coat and infected root tissues. Recently, Elshahawy and El-Mohamedy (2019) reported that in the greenhouse experiment, the combined inoculation of five Trichoderma isolates suppressed damping-off induced by P. aphanidermatum and increased the survival of tomato plants by 74.5%. A recent study in Tunisia showed that in dual culture assay, T. harzianum inhibited P. ultimum radial growth by 18.54% with drastic changes in pathogen hyphae expressed as strong lysis, formation of mycelial cords and mycoparasitism (Mannai et al. 2020). The evaluation of post-emergence damping-off suppression ability proved that T. harzianum had significantly improved the pepper plant height by 22.22% over pathogen-inoculated and untreated control (Mannai et al. 2020). The evaluation of pre-emergence damping-off suppression ability showed that pepper seeds treated with T. harzianum conidial suspensions gave 60% less pre-emergence damping-off infections caused by P. ultimum, compared to the positive control (Mannai et al. 2020). In addition, the use of Trichoderma spp. in agriculture can offer many benefits such as colonization of the rhizosphere allowing rapid establishment in stable microbial communities of the rhizosphere, control of pathogens using various mechanisms, improving plant vigor and stimulating growth root (Harman et al. 2004).
The strain of B. subtilis tested in vivo was also very effective against P. ultimum. In fact, this antagonist reduced the vigor status index severity and increased the height of the apple plants inoculated with P. ultimum. The present findings are also in agreement with previous studies reporting that Bacillus sp. was an important microbial antagonist of pathogens. It improved plant growth and reduced fungal pathogens in apple orchards infested with dieback disease (replantation) (Van Schoor and Bezuidenhout 2014).
The in vitro test showed that the isolates A5 (A. candidus) and A10 (A. niger) were among the most effective. Furthermore, A. niger (A10) reduced the severity of P. ultimum on apple trees. The use of A5, A10 and their combination exhibited good result by reducing the health status index severity caused by P. ultimum. This result is in agreement with many studies reporting that several Aspergillus species were able to of produce a number of bioactive secondary metabolites (El-Sayed and Ali 2020). In addition to their antagonistic capacity, several members of this genus have demonstrated their ability to confer plant diseases resistance and other known benefits such as soil suppression (Urja and Meenu 2010). A. flavipes was identified as a strong inhibitor for growth of various oomycetes species (El-Sayed and Ali 2020). Furthermore, a recent investigation showed the efficacy of Aspergillus species for the radial growth reduction of F. oxysporum, F. solani, P. ultimum and Phytophthora citrophthora associated with peach seedling decline in Tunisian nurseries. It revealed also that A. niger improved peach plants height compared to the control inoculated with P. ultimum by 40.49% (Mannai and Boughalleb‐M’Hamdi 2022).
The in vivo assays showed also that the combination of A. candidus and A. niger isolates (A5 and A10) decreased the root browning index and improved the seedlings vigor status. The combination of T. harzianum isolates Tr9 + Tr10 improved the seedlings vigor status. These results are in agreement with those of Meyer and Roberts (2002) who reported that a combinatory approach has also the potential to resolve problems that occur with individual biocontrol agents. Numerous studies reported that the performance in suppression of pathogens or disease increased by combinations of different biocontrol agents (Roberts et al. 2005). However, the present study showed also the inefficacy of the combination between T. harzianum (Tr10) and B. subtilis (B) to reduce the decline severity index. Nevertheless, the test of these last antagonists each alone improved significantly the seedlings vigor status. This may have been due to an incompatible reaction amongst strains (Thilagavathi et al. 2007). There are many studies about the combinations of antagonists that resulted to decrease the performance relative to individual applications of these biological control agents (Mannai and Boughalleb‐M’Hamdi 2022). Several researchers indicated that for increased disease suppression, the combined strains in biocontrol preparations must be compatible (Roberts et al. 2005).
Conclusions
The in vitro test showed that Aspergillus niger A10, A. candidus A5, T. harzianum Tr9 and Tr10 were the most effective bioagent against P. ultimum. The in vivo test proved the efficacy of the combination of A. niger A10 and A. candidus A5 that reduced the disease severity index and T. harzianum (Tr10), B. subtilis (B) and A. niger (A10) that stimulated the apple seedlings growth.
Availability of data and materials
All data are available in the manuscript.
Abbreviations
- A. candidus :
-
Aspergillus candidus
- A. niger :
-
Aspergillus niger
- ANOVA:
-
Analysis of variance
- B. subtilis :
-
Bacillus subtilis
- P. ultimum :
-
Pythium ultimum
- T. harzianum :
-
Trichoderma harzianum
- SPSS:
-
Statistical Package for the Social Sciences software
- SNK:
-
Student–Newman–Keul’s
References
Barúa JE, Cruz M, Pedro N, Cautain B, Hermosa R, Cardoza RE, Gutiérrez S, Monte E, Vicente F, Collado IG (2019) Synthesis of trichodermin derivatives and their antimicrobial and cytotoxic activities. Molecules 24:3811. https://doi.org/10.3390/molecules24203811
Cardoza RE, Hermosa MR, Vizcaíno JA, Sanz L, Monte E, Gutiérrez S (2005) Secondary metabolites produced by Trichoderma and their importance in the biocontrol process. Res Signpost Indian 661:207
Devi NO, Devi RKT, Debbarma M, Hajong M, Thokchom S (2022) Effect of endophytic Bacillus and arbuscular mycorrhiza fungi (AMF) against Fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici, Egypt. J Biol Pest Control 32:1. https://doi.org/10.1186/s41938-021-00499-y
Diba K, Kordbacheh P, Mirhendi SH, Rezaie S, Mahmoudi M (2007) Identification of Aspergillus species using morphological characteristics. Pak J Med Sci 23(6):867–872
El-Sayed AS, Ali GS (2020) Aspergillus flavipes is a novel efficient biocontrol agent of Phytophthora parasitica. Biol Control 140:104072. https://doi.org/10.1016/j.biocontrol.2019.104072
Elshahawy IE, El-Mohamedy RS (2019) Biological control of Pythium damping-off and root-rot diseases of tomato using Trichoderma isolates employed alone or in combination. J Plant Pathol 101:597–608
Furuya S, Mochizuki M, Aoki Y, Kobayashi H, Takayanagi T, Shimizu M, Suzuki S (2011) Isolation and characterization of Bacillus subtilis KS1 for the biocontrol of grapevine fungal diseases. Biocontrol Sci Technol 21:705–720. https://doi.org/10.1080/09583157.2011.574208
Green HN, Heiberg K, Lejbølle JDF (2001) The use of a GUS transformant of Trichoderma harzianum, strain T3a, to study metabolic activity in the spermosphere and rhizosphere related to biocontrol of Pythium damping-off and root rot. Eur J Plant Pathol 107:349–359
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species—opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Lubertozzi D, Keasling JD (2009) Developing Aspergillus as a host for heterologous expression. Biotechnol Adv 27:53–75
Mannai S, Boughalleb-M’Hamdi N (2021) In vitro and in vivo effects of some chemical fungicides against Pythium ultimum and Phytophthora citrophthora associated with peach seedlings decline. Nov Res Microbiol J 5(6):1431–1446. https://doi.org/10.21608/nrmj.2021.207166
Mannai S, Boughalleb-M’Hamdi N (2022) In vitro and in planta potential effect of some indigenous antagonists against Fusarium and pythiaceous species associated with peach seedlings decline. Egypt J Biol Pest Control 32:60. https://doi.org/10.1186/s41938-022-00540-8
Mannai S, Jabnoun-Khiareddine H, Nasraoui B, Daami-Remadi M (2020) Biocontrol of Pythium damping-off on pepper (Capsicum annuum) with selected fungal and rhizobacterial agents. Int J Phytopathol 09(01):29–42. https://doi.org/10.33687/phytopath.009.01.3083
Mazzola M, Manici M (2012) Apple replant disease: role of microbial ecology in cause and control. Annu Rev Phytopathol 50:45–65
Meyer SLF, Roberts DP (2002) Combinations of biocontrol agents for management of plant-parasitic nematodes and soil borne plant-pathogenic fungi. J Nematol 34:1–8
Miljaković D, Marinković J, Balešević-Tubić S (2020) The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 8(7):1037
Moein S, Mazzola M, NtusheloNS McLeodA (2019) Apple nursery trees and irrigation water as potential external inoculum sources of apple replant disease in South Africa. Eur J Plant Pathol 153:1131–1147. https://doi.org/10.1007/s10658-018-01631-9
Puyam A (2016) Advent of Trichoderma as a bio-control agent—a review. J Appl Nat Sci 8(2):1100–1109
Roberts DP, Lohrkea SM, Meyerb SLF, Buyera JS, Bowers JH, Bakerd CJ, Lie W, Souzaf JT, Lewis JA, Chung S (2005) Biocontrol agents applied individually and in combination for suppression of soil borne diseases of cucumber. Crop Prot 24:141–155
Santini A, Biancalani F, Biancalani F, Barzanti GP, Capretti P (2006) Pathogenicity of four Phytophthora species on wild cherry and Italian alder seedlings. J Phytopathol 154:163–167
Shahid M, Srivastava M, Singh A, Kumar V, Rastogi S, Pathak N, Srivastava A (2014) Comparative study of biological agents, Trichoderma harzianum (Th-Azad) and Trichoderma viride (01PP) for controlling wilt disease in pigeon pea. J Microbial Biochem Technol 6:110–115
Strauss J, Labuschagne N (1995) Pathogenicity of Fusarium solani isolates on citrus roots and evaluation of different inoculum types. Toegepaste Plantwetenskap 9:48–52
Tewoldemedhin YT, Mazzola M, Spies CFJ, McLeod A (2011) Characterization of fungi (Fusarium and Rhizoctonia) and oomycetes (Phytophthora and Pythium) associated with apple orchards in South Africa. Eur J Plant Pathol 130:215–229. https://doi.org/10.1007/s10658-011-9747-9
Thilagavathi R, Saravanakumar D, Ragupathi N, Samiyappan R (2007) A combination of biocontrol agents improves the management of dry root rot (Macrophomina phaseolina) in greengram. Phytopathol Mediterr 46:157–167
Van Schoor L, Bezuidenhout K (2014) Potential use of compost extract and Bacillus inoculants in combination with compost in managing apple replant disease. S A Fruit J 13:62–67
Whipps JM, Lumsden RD (1991) Biological control of Pythium species. Biocontrol Sci Technol 1:75–90
Yassin MT, Mostafa AA, Al-Askar AA (2021) In vitro antagonistic activity of Trichoderma harzianum and T. viride strains compared to carbendazim fungicide against the fungal phytopathogens of Sorghum bicolor (L.) Moench. Egypt J Biol Pest Control 31:118
Yuan B, Zhan J, Chen C (2018) Evolution of a development model for fruit industry against background of an aging population: intensive or extensive adjustment? Sustainability 10:49. https://doi.org/10.3390/su10010049
Mannai S (2019) Apple and peach seedlings dieback in nurseries: diagnosis, morphological and molecular characterization of causative agents and management methods. High Institute of Agronomy of Chott Mariem, Tunisia. Doctoral thesis, Agronomic Sciences, p 226
Shah MM, Sharif U, Rufai BT (2019) Trichoderma—the most widely used fungicide. Introductory chapter: identification and isolation of Trichoderma spp. Their significance in agriculture, human health, industrial and environmental application. https://doi.org/10.5772/intechopen.83528
Siddiquee S (2017) Morphology-based characterization of Trichoderma species. Practical handbook of the biology and molecular diversity of trichoderma species from tropical regions, pp 41–73. https://doi.org/10.1007/978-3-319-64946-7_4
Acknowledgements
This study was financed by Plant projects, ‘Institution de la Recherche et de l’Enseignement Supérieur Agricoles (IRESA)’; Ministry of Agriculture, and also by L21AGR05, University of Sousse, Tunisia.
Funding
This study was financed by Plant projects, ‘Institution de la Recherche et de l’Enseignement Supérieur Agricoles (IRESA)’; Ministry of Agriculture, and also by L21AGR05, University of Sousse, Tunisia.
Author information
Authors and Affiliations
Contributions
NBM and SM designed the research and conducted surveys, sampling and analyses. SM wrote and NBM revised the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mannai, S., Boughalleb-M’Hamdi, N. Evaluation of Trichoderma harzianum, Bacillus subtilis and Aspergillus species efficacy in controlling Pythium ultimum associated with apple seedlings decline in nurseries and their growth promotion effect. Egypt J Biol Pest Control 33, 59 (2023). https://doi.org/10.1186/s41938-023-00705-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-023-00705-z