Abstract
Background
The genus Acletoxenus (tribe Gitonini, subfamily Steganinae, family Drosophilidae) is a small widespread genus, comprising only four species worldwide, namely: Acletoxenus indicus Malloch, 1929, A. quadristriatus Duda, 1936, A. meijerei Duda, 1924 in addition to A. formosus (Leow, 1864), the species of the present study. The larvae of Acletoxenus spp. are known as predators of whiteflies.
Results
The genus Acletoxenus and its predaceous species A. formosus (Leow, 1864) are recorded in the present study for the first time from Egypt. This species was found associated with the immature stages of the glasshouse whitefly, Trialeurodes vaporariorum Westwood, 1856 (Hemiptera: Aleyrodidae) feeding on the castor bean plant, Ricinus communis L. which has been grown at the Plant Protection Institute, Agricultural Research Center, Giza, Egypt. The morphological diagnoses, in situ predatory behavior in the field and ex situ biological remarks in the laboratory were discussed.
Conclusion
The predaceous drosophilid fly, Acletoxenus formosus (Leow, 1864), is recorded herein with its genus for the first time from Egypt as the second representative of the tribe Gitonini (subfamilies Steganinae, family Drosophilidae) in the country. The recording of this species in Egypt is of a great interest as it will encourage and lead to further research on different biological aspects. This drosophilid is a beneficial fly as its larvae are predators of immature stages of whiteflies (family Aleyrodidae) and it could be used as a potential biological control agent.
Similar content being viewed by others
Background
The family Drosophilidae is a diverse family in the superfamily Ephydroidea (Yassin 2013), encompassing more than 4000 species worldwide, classified in 76 genera (Pape et al. 2011) and two subfamilies, Steganinae and Drosophilinae (Bächli 2022), of which the Drosophilinae is the most diversified subfamily with more than 3200 species (Gottschalk et al. 2008).
In Egypt, the family Drosophilidae was represented by 16 species, of which 15 species are classified in the subfamily Drosophilinae. However, the subfamily Steganinae, to which the present species belongs, was represented in Egypt by only one species, Cacoxenus perspicax (Knab, 1914), in tribe Gitonini (El-Hawagry et al. 2018). The present species, Acletoxenus formosus (Leow, 1864), is recorded herein with its genus for the first time from Egypt as the second representative of the tribe Gitonini in the country.
Acletoxenus is a small widespread genus (Grimaldi 1988), with only four species worldwide, namely: A. indicus Malloch, 1929, A. quadristriatus Duda, 1936, A. meijerei Duda, 1924 in addition to A. formosus (Leow, 1864), the species of the present study (Bächli et al. 2004).
The majority of vinegar flies (family Drosophilidae) are primarily consumers of microorganisms like bacteria and yeasts which linked with the initial stages of fruit and plant rotting material and are considered as serious primary pests of many of these fruits (Schmitz et al. 2007). Contrarily, the Acletoxenus spp. are beneficial as their larvae are predators of whiteflies (family Aleyrodidae, order Hemiptera) (Wong et al. 2017) and they could be considered as potential biological control agents for whiteflies. Many studies discussed life history and behavior of the Acletoxenus larvae as natural enemies of whitefly immature stages and the possible use of them in the biological control; e.g., Ulusoy and Ülgentürk (2003) and Yu et al. (2012). Larvae of all species of the genus Acletoxenus seem to have similar habits (Knab 1914).
The exotic species A. formosus was always found in low abundance in man-made habitats, mostly in urban gardens. The larvae of this species are associated with colonies of their whitefly prey in cultivated areas (Rego et al. 2017).
The aim of the present study is to provide some information on the recent invasion of A. formosus in Egypt, with some field observations and biological remarks.
Methods
The present drosophilid species was observed for the first time associated with the immature stages of glasshouse whitefly T. vaporariorum feeding on the castor bean plant, Ricinus communis L. grown at Plant Protection Research Institute, Giza, Egypt. In situ observation of the drosophilid fly behavior was carried out daily for three weeks in December 2021 by the first author. The observations continued weekly until the whitefly dense infestation ended at the end of February 2022.
Some leaves of castor bean plant R. communis densely infested with immature stages of glasshouse whitefly T. vaporariorum and encompassing many drosofilid eggs, larvae and pupae were later transferred to the laboratory to rear the drosophilid fly to the adult stage for identification. The whitefly-infested leaves were maintained in Petri dishes in an air-conditioned laboratory at 25 °C. The leaves were placed in the dishes on a moist piece of tissue paper.
The drosophilid fly species was identified using d'Assis Fonseca (1965) and Bächli et al. (2004), and the whitefly species was identified by the experts in Classification Research Department, Plant Protection Research Institute, Agricultural Research Centre, Ministry of Agriculture, Giza, Egypt.
The following literature was consulted for the morphological diagnosis: original description (Leow 1864; Malloch 1929; Bock 1982; Bächli et al. 2004; Wong et al. 2017). Terminology of the morphological diagnoses follows Cumming and Wood (2017).
Some studies were consulted to understand the behavior and biology of A. formosus; e.g., Clausen and Berry (1932), Yu et al. (2012) and Wong et al. (2017). Colored photographs were taken using a Nikon D5300 camera.
Results
Subfamily STEGANINAE
Tribe GITONINI
Genus Acletoxenus Frauenfeld
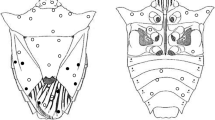
Acletoxenus formosus (Leow, 1864) (Fig. 1).
Gitona formosus Loew, 1864: 366. Type locality: Europe (mainly Germany and Poland).
Acletoxenus syrphoides Frauenfeld, 1868: 152. Type locality: Austria and Italy.
Distribution: AU: Australia (Victoria); PA: Algeria, Austria, Canary Is., Egypt (first record), England, Germany, Israel, Italy, Poland, Syria, Turkey, Wales.
Egyptian localities: Lower Nile Valley: Dokki, Giza.
Dates of collection: December 2021 and January 2022.
Specimens examined: 1♂, 2♀♀, Giza: Dokki; I. 2022; Nada leg. (EFC); 2♂♂, 1♀, the same data (PPDD).
Diagnosis
Small flies (Fig. 1), 1.9–2.2 mm in length. Eyes very large; frons narrow, nearly parallel, pale yellowish-brown, with ocellar triangle blackish; ocellar setae absent; postocellar setae minute; all orbital setae long, i.e., proclinate orbital setae are not noticeably shorter than the anterior reclinate setae; face pale yellowish-brown; gena narrow, usually inconspicuous; occiput black; scape and pedicel whitish, flagellum yellow; arista micropubescent, dark brown to black. Mesonotum entirely black with lateral margins white to yellowish-white; humeral callus and pleura white to pale yellowish-white, with a large blackish marking especially on katepisternum, anatergite and subscutellum; scutellum yellowish-white to yellow. Wings with costa exceeding the apex of vein R2+3; cells bm and dm confluent. Legs without preapical and apical setae. Abdomen pubescent, entirely yellowish with black markings on tergites; males usually have 1st to 3rd abdominal tergites with basal broad black markings, while the 4th tergite with a well-defined black triangular to rounded marking medially at basal margin and a black marking laterally; 3 small ill-defined blackish spots may present on 5th tergite; markings on other tergites are not consistent and usually in the form of reduced spots; females have markings not as extensive as in males and somewhat reduced.
Remarks: A. formosus. and A. meijerei, both having the proclinate orbital setae, are not noticeably shorter than the anterior reclinate setae but they can be differentiated by mesonotum which is almost entirely black with white to yellowish-white lateral margins in A. formosus, while mesonotum in A. meijerei has two broad dark vittae, which are more or less confluent behind the suture and do not extend to the hind margin (Malloch 1929; Bock 1982).
Field observations
The predatory behavior of A. formosus larvae was observed daily in the field (in situ) for three weeks (starting from December 5, 2021). The larvae of the fly were observed predating on the immature stages of glasshouse whitefly T. vaporariorum, feeding on the leaves of castor bean plant, R. communis (Fig. 2). The leaves of the plant were found densely infested with immature stages of the whitefly T. vaporariorum (Fig. 2b, c). Infested leaves were checked carefully to locate the drosophilid larvae. Weakly sclerotized larvae were found active during the day moving slowly forward or backward through peristaltic contractions of their abdominal segments and raising their pseudocephalons and swinging them from one side to the other to seek their prey of stationary whitefly immature stages. If no prey was found, the mouth hooks were used to anchor the pseudocephalons of the larva in the plant leaf, and then the abdominal segments of the larva were noticed to move forward via contraction. However, it was observed that if the larva finds its prey, it stabs the prey (a whitefly immature stage) using its mouth hooks and imbibe its content. The larva was also observed to secrete a mucus material by which it glues the exoskeleton of empty whitefly immature stages, whitefly eggs and wax to its body. The drosophilid larvae were cream colored in early instars; then, they turned to green in final instars (Figs. 2d, e, 3a, b). The last larval instar pupated within the last larval exoskeleton and the pupa was glued via a flattened ventral surface to leaf surfaces (Fig. 3c–e).
Daily observations were stopped after 3 weeks; however, weekly rapid observations were continued. It was found that the dense infestation of castor bean plant with glasshouse whitefly and the predacious behavior of the drosophilid fly continued until the end of February 2022.
Biological remarks
The whitefly-infested leaves of castor bean plant were observed in the laboratory (ex situ observation). The leaves were encompassing different stages the drosofilid fly associated with the whitefly immature stages. We tracked some eggs of the fly which thought to be laid recently. Eggs (about 0.4 mm in length) were white and firmly attached to the leaves’ surfaces. Eggs began to hatch after two days and cream-colored larvae (about 0.7 mm in length) emerged. The larva grew and turned to greenish in the final 3rd instar (3–4 mm in length). The all larval stage lasted for 7–10 days.
The larva was noticed to glue the exoskeleton of empty whitefly immature stages, whitefly eggs and wax to its body by a mucus material it secretes, then pupation took place within the last larval exoskeleton, so the greenish color remained in the pupa (about 3.5 mm in length). The pupal stage lasted for 9–11 days and finally adults emerged and lived for about 3 days.
We did not try to continue rearing the fly as our main purpose was merely to identify the predacious fly.
Discussion
The present species, A. formosus, is recorded herein with its genus for the first time from Egypt as the second representative of the tribe Gitonini (subfamilies Steganinae) in the country. Nevertheless, the number of species in this subfamily and in the family Drosophilidae as a whole is still relatively few in Egypt (17 species), as no taxonomic studies on this family in particular have been carried out in Egypt before and the majority of drosophilid species recorded in Egypt were listed in one faunal study (Bächli et al. 1982), so taxonomic studies on the Egyptian Drosophilidae are greatly needed (El-Hawagry et al. 2018).
The present observations coincide with many other previous studies in other countries in the fact that A. formosus is a natural enemy of the immature stages of whiteflies (family Aleyrodidae) and there is a possible use of them in the biological control. Wong et al. (2017) and Bou Hasan and Ibrahim (2018) are two of these studies. The whiteflies generally prefer hot climates and the majority of species have been described in tropical and warm areas (Bink-Moenen and Mound 1990). Whiteflies are phytophagous pests causing damage to many plants by feeding directly on these plants as adults and immature stages (sap-suckers) or indirectly by the production of honeydew by the immature stages (Beitia and Hernández-Suárez 2014). A. formosus was recorded from Europe preying upon the whitefly, Siphoninus phillyreae (Haliday) (ash whitefly) on Crataegus plants and upon the whitefly, Aleurotuba jelinekii (Frauenfeld) on Viburnum plants (Knab 1914). Larvae of A. formosus were recorded also from the UK feeding on the whitefly Siphoninus immaculatus (Heeger) on Hedera helix (common ivy) and on the cabbage whitefly Aleyrodes proletella (Linnaeus) (Halstead 2011). Bou Hasan and Ibrahim (2018) also recorded A. formosus from Syria as a predator of the cabbage whitefly. Larvae of both A. formosus and A. indicus were recorded from the USA preying upon the ash whitefly on Pyrus calleryana (Bradford pears) (Hackney et al. 1997).
The recording of this species in Egypt is of a great interest as it will encourage and lead to further research on different biological aspects. This drosophilid is a beneficial fly as its larvae are predators of immature stages of whiteflies (family Aleyrodidae) and it could be used as a potential biological control agent. As it is difficult to establish laboratory culture, research is necessary in this aspect in order to achieve successful biological control.
Conclusions
The predaceous drosophilid fly A. formosus is recorded herein with its genus for the first time from Egypt as the second representative of the tribe Gitonini (subfamilies Steganinae, family Drosophilidae) in the country. This drosophilid is a beneficial fly as its larvae are predators of immature stages of whiteflies (family Aleyrodidae) and it could be used as a potential biological control agent.
Availability of data and materials
Data supporting the conclusions of this article are presented in the main manuscript.
Abbreviations
- AU:
-
Australasian
- EFC:
-
Collection of Entomology Department, Faculty of Science, Cairo University, Giza, Egypt (Efflatoun’s collection)
- Is.:
-
Islands
- PA:
-
Palaearctic
- PPDD:
-
Ministry of Agriculture Collection, Plant Protection Research Institute, Dokki, Giza, Egypt
References
Bächli G (2022) TaxoDros: the database on taxonomy of Drosophilidae. https://www.taxodros.uzh.ch/. Accessed 1 Mar 2022
Bächli G, Burla H, Jungen H (1982) Drosophila subobscura in Egypt and its probable derivation. Genetica 59:3–7. https://doi.org/10.1007/BF00130809
Bächli G, Vilela CR, Escher SA, Saura A (2004) The Drosophilidae (Diptera) of Fennoscandia and Denmark. In: Fauna Entomologica Scandinavica, vol 39. Brill Academic Publishers, Leiden, p 362
Beitia FJ, Hernández-Suárez E (2014) Whiteflies management. Chapter 7. In: Tello MJC, Camacho FF (eds) Organisms for the control of pathogens in protected crops. Cultural practices for sustainable agriculture. Fundación Cajamar, El ejido, pp 191–223
Bink-Moenen RM, Mound LA (1990) Whiteflies: diversity, biosystematics and evolutionary patterns. In: Gerling D (ed) Whiteflies: their bionomics. Pest status and Management, Intercept Ltd, UK, p 348
Bock IR (1982) Drosophilidae of Australia. V. Remaining genera and synopsis (Insecta: Diptera). Aust J Zool Suppl Ser 89:1–164. https://doi.org/10.1071/AJZS089
Bou Hasan WJ, Ibrahim A (2018) Biological characteristics and the predation efficacy of Acletoxenus formosus (Loew, 1864) as a predator of the whitefly of cabbage, Aleyrodes proletella (L.) under laboratory conditions. Arab J Plant Prot 36(2):135–140
Clausen CP, Berry PA (1932) The citrus blackfly in Asia, and the importation of its natural enemies into tropical America. Technical Bulletin No 320. US Department of Agriculture. Washington, p 58
Cumming JM, Wood DM (2017) Adult morphology and terminology. In: Kirk-Spriggs H, Sinclair BJ (eds) Manual of Afrotropical Diptera. Vol. 1. Introductory Chapters and Keys to Diptera Families. Suricata 4. South African National Biodiversity Institute, Pretoria, South Africa, pp 89–133
d’Assis Fonseca ECM (1965) A short key to the British Drosophilidae (Diptera) including a new species of Amiota. Trans Soc Br Entomol 16:233–244
Duda O (1924) Beitrag zur Systematik der Drosophiliden unter besonderer Berücksichtigung der paläarktischen u. orientalischen Arten (Dipteren). Arch Naturgesch 90A(3):172–234
Duda O (1936) Weitere neue afrikanische und orientalische akalyptrate Musciden (Dipt.) des British Museum. Ann Mag Nat Hist 18(10):337–351
El-Hawagry MS, Zatwarnicki T, Ebrahim AM (2018) Catalogue of the Egyptian Ephydroidea (Diptera: Schizophora: Acalyptratae). Zootaxa 4444(3):201–246. https://doi.org/10.11646/zootaxa.4444.3.1
von Frauenfeld GR (1868) Zoologische Miscellen. XIV & XV. Verh Zool Bot Ges Wien 18:147–165
Gottschalk MS, Hofmann PRP, Valente VLS (2008) Diptera, Drosophilidae: historical occurrence in Brazil. Check List 4(4):485–518
Grimaldi DA (1988) 8. Relicts in the Drosophilidae (Diptera). In: Liebherr JK (ed) Zoogeography of Caribbean insects: 183–213. Cornell University Press, Ithaca, p 285. https://doi.org/10.7591/9781501746017-010
Hackney GD, Kidd KA, McDonald RC, Robbins NS (1997) Ash whitefly biological control in North Carolina. Ash whitefly biological control in North Carolina. NCDA Beneficial Insect Lab Annual Report of Activities, pp 33–35
Halstead AJ (2011) [Acletoxenus formosus (Loew)] Diptera section of the BENHS Annual Exhibition, held 13 November 2010. Br J Entomol Nat Hist 24:162
Knab F (1914) Drosophilidae with parasitic larvae. Insecutor Inscit Menst 2(1):165–169
Loew H (1864) Gitona formosa, eine neue deutsche Art. Wien Entomol Monatsch 8:366–368
Malloch JR (1929) Exotic Muscaridae (Diptera). XXV Ann Mag Nat Hist 3(10):545–564
Pape T, Blagoderov V, Mostovski MB (2011) Order Diptera Linnaeus, 1758. In: Zhang ZQ (ed) Animal biodiversity: an outline of higher–level classification and survey of taxonomic richness. Zootaxa 3148:222–229
Rego C, Aguiar AMF, Cravo D, Boieiro M (2017) Invasive fruit flies (Diptera: Drosophilidae) meet in a biodiversity hotspot. J Entomol Res Soc 19(1):61–69
Schmitz HJ, Valente VLS, Hofmann PRP (2007) Taxonomic survey of Drosophilidae (Diptera) from mangrove forests of Santa Catarina Island, southern Brazil. Neotrop Entomol 36:53–64. https://doi.org/10.1590/S1519-566X2007000100007
Ulusoy MR, Ülgentürk S (2003) The natural enemies of whiteflies (Hemiptera: Aleyrodidae) in southern Anatolia. Zool Middle East 28:119–124
Westwood JO (1856) The new Aleyrodes of the greenhouse. Gard Chron 1856:852
Wong J, Foo M, Tan HTW, Meier R (2017) Whitefly predation and extensive mesonotum color polymorphism in an Acletoxenus population from Singapore (Diptera: Drosophilidae). ZooKeys 725:49–69. https://doi.org/10.3897/zookeys.725.13675
Yassin A (2013) Phylogenetic classification of the Drosophilidae Rondani (Diptera): the role of morphology in the postgenomic era. Syst Entomol 38(2):349–364. https://doi.org/10.1111/j.1365-3113.2012.00665.x
Yu G, Wu L, Lu J, Chen H (2012) Discovery of a predaceous drosophilid Acletoxenus indicus Malloch in South China, with descriptions of the taxonomic, ecological and molecular characters (Diptera: Drosophilidae). J Nat Hist 46(5–6):349–354. https://doi.org/10.1080/00222933.2011.639466
Acknowledgements
We would like to thank the research teams in Department of Piercing and Sucking Insects and Department of Classification Research, Plant Protection Research Institute, Agricultural Research Centre, Ministry of Agriculture, Dokki, Egypt, for their invaluable assistance throughout the present study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MN observed and reared the fly, photographed the infested leaves. AE participated in identifying the predaceous fly. ME participated in identifying the predaceous fly, photographed the fly and drafted the manuscript. All authors have participated in the study design and coordination and interpreted the data. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nada, M.S.E., Ebrahim, A.M.E. & El-Hawagry, M.S.A. First record of the genus Acletoxenus (Diptera: Drosophilidae) and its predaceous species A. formosus (Leow, 1864) in Egypt, with some field observations and biological remarks. Egypt J Biol Pest Control 32, 68 (2022). https://doi.org/10.1186/s41938-022-00567-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-022-00567-x