Abstract
Background
Wild fish stocks continuously decline in Bangladeshi rivers and oxbow lakes. Small indigenous fish species management solely depends on their population status and conservation. This study aimed to determine the population status of Hyporhamphus limbatus, in Southwestern Bangladesh using conventional and truss morphological characteristics.
Results
A total of 174 fish samples were procured from monsoon season i.e., May to August 2018 the Bhairab River, BR; Kopotakkho River, KR; and Baluhar Baor, BB in Bangladesh. Five meristics, six conventional and twelve truss-based morphometric characters, were considered for stock identification. The Kruskal–Wallis test helped to analyze the meristic characters, while ANOVA, principal component analysis (PCA), discriminant function analysis (DFA), correct classification analysis using DFA, and a UPGMA dendrogram formation were used to investigate the conventional and truss measurements. By the Kruskal–Wallis test, no meristic characters showed significant differences across different populations. Eleven of the 18 morphometric measurements showed significant differences among three populations through the univariate ANOVA. PCA specified the population structure variations and explained 67.438% of the total variance. The first and second discrimination functions accounted for 78.3% and 21.7%, representing 100% of the group variability. Similarly, 85.6% of the grouped cases and 77.6% of cross-validated grouped cases were initially effectively represented by their correct number of individuals. A dendrogram based on morphometric (conventional and truss) displayed three stocks grouped into two clusters, with BR forming a distinct cluster, while KR and BB creating a shared cluster.
Conclusion
In conclusion, such morphological differences are most likely due to their distinctive ancestral origins. This study presents novel reports on the stock assessment of H. limbatus in their natural ecosystem. Furthermore, molecular research and an evaluation of the environmental impact on H. limbatus populations in Bangladesh are strongly recommended.
Similar content being viewed by others
Background
Stock identification is an interdisciplinary research area of fisheries, including genetic makings, biometrics, and life history assessments (Begg & Waldman, 1999; Begg et al., 1999; Cadrin et al., 2014). Stocks are the random fish groups mature enough to self-reproduce with their members of shared life histories (Hilborn & Walters, 2013; Begg et al., 1999; Waldman, 2005). The primary outcomes of management actions, such as determining the stock intricacy of a fish species, are a necessary component of the fisheries management framework (Begg et al., 1999; Croft et al., 2003; Guan et al., 2013). Moreover, a necessary condition for developing effective biodiversity management and conservation is knowledge about the stock structure analysis of a species or population (Turan et al., 2005). Stocks generally differ by various evolutionary factors (i.e., relocation, selection, and genetic drift) and environmental influences that are relatively independent of the connectivity potential and the degree of heterogeneity.
Fish typically display their phenotypic plasticity in two ways during the early stages of development: isometric size variation due to growth and allometric form variation induced by developmental change (Cadrin, 2000). Because of their physiological and environmental requirements, freshwater fish show significant body shape variation, leading to genetic variety and phenotypic plasticity (Eklöv and Svanbäck, 2006). The approaches frequently utilized in stock recognition include meristic and morphometrics (Asadujjaman et al., 2022; Azad et al., 2020; Mahfuj et al., 2021a, 2022), conventional tags (Hall, 2014; Hess et al., 2014), parasites as ordinary tags (MacKenzie & Abaunza, 2014; Mosquera et al., 2003), the chemical composition of otolith (Bickford & Hannigan, 2005; Bouchoucha et al., 2018; Tanner et al., 2016), molecular marker (Ferguson et al., 1995; Hashimoto et al., 2013; Okumuş & Çiftci, 2003), and digital tags (Bain, 2005; Metcalfe & Arnold, 1997; Sippel et al., 2015).
However, the study of morphometric and meristic traits is one of the most widely used and cost-effective strategies among all recognized methods regarding stock identification (Ethin et al., 2019; Mahfuj et al., 2021b, 2022; Mir et al., 2013). The truss-networks created by two or more connected lengths across the body, which finally produced a chronological sequence of related polygons, have been increasingly used to emphasize the intrinsic limitations of standard morphometric techniques (Strauss & Bookstein, 1982).
River fish biodiversity and stocks are rapidly depleting (Atique & An, 2018, 2022; Atique et al., 2019, 2020). Therefore, fisheries stock assessment could indirectly measure the status of ecological health and biological factors threatening the river fish biodiversity (Kim et al., 2019, 2021a, 2021b). The Hyporhamphus limbatus (H. limbatus) belongs to the Beloniformes order and Hemiramphidae family. This species was native to India, Bangladesh, and Mayanmar (Collette & Su, 1986). It lives on freshwater tidal ecosystems and brackish estuaries (Rainboth, 1996). It consumes insects, tiny zooplankton like copepods, rotifers, daphnia, and Moina (Lim et al., 1999). H. limbatus has an elongated, cylindrical, and compressed external morphology with a lower beak, much longer than the upper jaw, and a villiform structure of teeth in many rows on both jaws. It is primarily oviparous, with two annual breeding seasons (Talwar & Jhingran, 1991). Although H. limbatus is listed as Least Concern (LC) species in Bangladesh by Nabi (2015), knowledge of stock identification is scarce in Bangladesh. With minimal progress made recently targeting the length–weight relationship and growth parameters investigations (Hasan et al., 2020), population biology (Kumara & Amarasinghe, 2008) data on stock structure analysis using conventional and truss-based morphometrics is not available that needs to be discussed. Therefore, this study aimed to document the meristic and morphometric (conventional and truss) variation and stock structure of H. Limbatus from two rivers in Southwestern Bangladesh: the Bhairab River and the Kopotakho River in an oxbow lake (Baluhor Baor).
Methods
Sample collection
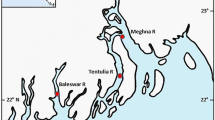
Hundred and seventy four wild H. limbatus were randomly collected from two rivers, viz. Bhairab and Kapotakkho and an oxbow lake, Baluhar Baor, from May to August 2018. Fish samples were collected using gill nets (mesh size 8 mm) with the aid of local fishers. After sampling, the sampled fishes were preserved in an icebox and instantaneously transported to the laboratory for further examination. The sampling location, sample size, length, and collection date are indicated in Fig. 1 and Table 1.
Counting of meristic characteristics
Five meristic characters were counted, viz. number of dorsal fin rays (DFR), number of caudal fin rays (CFR), number of anal fin rays (AFR), number of pelvic fin rays (PelFR), and number of pectoral-fin rays (PecFR) by using needles and glass lens. The same person solely counted this part to avoid any biases.
Digitization of samples
Samples were washed in water flow, drained, and positioned on a smooth, rigid platform with a blank sheet as a background used to calibrate the digital picture coordinates. For identification purposes, each individual was provided with a unique code. The digital images were recorded, which supplied a complete physical appearance and enabled results to be observed if required (Cadrin & Friedland, 1999).
Laboratory procedure
Seven conventional morphometric features were measured with tpsDigV.2.1. (Fig. 2, Table 2). Similarly, the truss framework used for this investigation of H. limbatus was focused on the seven landmarks. The truss system was built by linking the features to form 12 observations (Fig. 3). The truss lengths were extracted from captured snaps using the tpsDig2v2.1 software package (Rohlf, 2006). A box truss of 12 lines connecting these landmarks was generated to represent the fish's basic shape (Strauss & Bookstein, 1982).
Statistical analysis
The size-dependent variations were corrected using an allometric method proposed by Elliott et al. (1995), Madj = M (Ls/Lo)b, where M is the initial dimension, Madj is the size adapted dimension, L0 is the fish's total length, Ls is the general mean of the total length for all fish from all specimens in each observation. Furthermore, b is the slope of the regression of log M on log Lo using all fish from each group, while b is predicted for each feature from the measured data as its slope of the regression of log M on log Lo using all fish from each group. To conclude whether there was a significant difference between the three locations, an ANOVA was performed on 12 morphometric characters. PCA and DFA were used to distinguish the three populations in this study. PCA aids in reducing morphometric data and the duplication of variables (Samaee et al., 2009). The proportion of appropriately classified fish was calculated using the DFA. Cross-validation utilizing correct classification was performed to assess the predictable error rates of the arrangement functions. The SPSS (ver. 22.0) was used to perform all the statistical evaluations on the conventional and truss morphometric data.
Results
Meristic features
Meristic counts were compared (Table 3) among three populations (BR, KR and BB), and no significant changes were detected through the Kruskal–Wallis test (P > 0.05).
None of the validated truss readings exhibited a strong relationship after the log-transformation of TL, signifying that the allometric transformation effectively endured the impacts of body length. The data for both sexes were shared for all following analyses because the morphometric characters of both sexes were not significantly diverse (P > 0.05). In ANOVA, the means of three (SL, EL, and PsOL) of six conventional morphometric characters and eight (3–4, 5–6, 6–7, 7–3, 3–6, 4–6, 3–5, and 2–6) of twelve truss distances were recorded as significantly (P < 0.05) diverse among three study locations (Table 4). The remaining conventional and truss distances were seen as non-significant (P > 0.05).
Principal component analysis (PCA)
A common issue with multivariate analyses in fish morphometric studies is insufficient data. Researchers with hypothetical assessments through PCA and DFA have suggested a ratio of at least 3–3.5 between the number of organisms observed (N) and the variables included (P) in the analysis (Kocovsky et al., 2009). Inadequate N values calculate to acquire covariance leading to erroneous conclusions about group differences (McGarigal et al., 2000). In the present case, all 19 features were placed in this analysis, and the N: P ratio for all 18 truss observations was 9.15. The contribution of variables to principal components (PC) was tested to define which morphometric dimension differentiated best populations. In this regard, Bartlett's Test Sphericity (BTS) and the Kaiser–Meyer–Olkin (KMO) measure were used to see if the data were suitable for PCA. The (BTS) test hypothesizes that the correlation value tends to zero, and the KMO value is significantly high (Nimalathasan, 2009). The KMO statistics range from 0 to 1. According to Kaiser (1974), values larger than 0.5 are acceptable, between 0.50 and 0.69 are medium, 0.7 and 0.79 are good, and between 0.8 and 0.99 are excellent (Field, 2000). In this analysis, the KMO value for the cumulative matrix was 0.77, and the BTS was significant (P < 0.05). Therefore, these outcomes (KMO and BTS) maintained that the tested data was suitable for further factorial analysis.
Six factors with eigenvalues > 1 were identified through PCA on 18 morphometric measurements, accounting for 69.885% of the cumulative variance (Fig. 4). The first factor (PC1) accounted for 25.388% of the variance, while the second (PC2), third (PC3), fourth (PC4), fifth (PC5), and sixth (PC6) accounted for 16.144%, 9.143%, 6.983%, 6.473%, and 5.805%, of variances, respectively (Table 5). The higher loadings were observed in PC1 for EL, PrOL, 1–2, 5–6, 6–7, 3–6, 4–6, 2–6, while in PC2, they were observed for UJL, PrOL, 1–2, 3–4, 6–7, 3–6, 3–5 and 2–6 (Table 5). The characters with eigenvalues crossing one were included in this analysis, while the others were curtailed. The biplot of the morphometric characters unveiled three spaces with an extraordinary intermingling among the three populations in PC1 versus PC2 (Fig. 5).
Discriminate function analysis
Using conventional and truss morphometric dimensions of all populations, standardized canonical discriminant functions reported significant correlations of 0.818 in DF1 and 0.599 in DF2 (Table 6). Two functions (DFs) were created in discriminant function analysis. In the group variability among the populations, the first discriminant function (DF 1) accounted for 78.3% of the total variation, while the second discriminant function (DF 2) accounted for 21.7% (Table 7). The conventional and truss lengths involving the first factor (DF 1) were SL, 2–6, 3–6, 6–7, 7–3, and 7–2. These six distances described the measurement that encompassed the fish's entire body. The second discriminant factor (DF2), on the other hand, accounted for 21.7% of the total variation, where the variables were 4–6, 5–6, EL, 3–4, 3–5, 1–2, PsOL, 4–5, PrOL, MBD, 2–3 and UJL, comprehensively covering the entire fish body. The biplot of DF1 and DF2 explained 100.0% of the total variance among the samples. It revealed the complete isolation of the Bhairab River from the Kopotakho River and Baluhor Baor, and the complete intermingling of Kopotakho River and Baluhor Baor (Fig. 6).
Cluster analysis
A UPGMA dendrogram was drawn using traditional and truss-network-based data for BhR, KR, and BB stocks. The individuals of BhR were clearly distinguished from the KR and BB and formed an out-group in the UPGMA dendrogram. On the contrary, the samples from KR and BB aggregately formed a new sub-cluster with BhR (Fig. 7).
In Table 7, the appropriate percentages of individuals from the three locations are classified based on the original and cross-validation. DFA exhibited 87.33% correct classification of individuals into their original populations, whereas 77.83% showed the cross-validation test results. In the actual classification result, maximum contributions were performed by BR (94.7%) followed by KR (85.0%) and BB (82.3%). Based on the cross-validated data from the classification results, there was clear intermixing, with BR dominating 78.9% of individuals, KR 77.5%, and BB 77.1%, respectively.
Discussion
Although H. limbatus is classified as a Least Concern (LC) species in Bangladesh by Nabi (2015), there is a lack of understanding regarding stock identification in Bangladeshi waters. Analyzing fish stock structure is a valuable technique for controlling naturally occurring populations. Due to the isolation of a population within the environments of a native area, morphological variance is extensive both within and between groups. The current study evaluated the H. limbatus stock identification in two rivers, the Bhairab and Kopotakkho rivers and an oxbow lake called Baluhor in southwest Bangladesh. According to Robinson and Wilson (1996), stock differentiation may result from genetic differences between stocks, which are connected to unique aquatic environments such as fluctuations of temperature, salinity, turbidity, current patterns, and alkalinity as well (Mir et al., 2013; Miyan et al., 2016; Hanif et al., 2019). Nevertheless, identical environmental factors and habitat factors may be the basis of stock similarities.
The number of DFR, CFR, AFR, PelFR and PecFR in H. limbatus did not differ significantly among the fish populations of the three study locations. This indicated that these populations share similar geographical regions and similar ancestors. Though their habitats are entirely separated, and distantly located from each other in the current situation, earlier, their habitats might have originated from the same place. The results of the univariate ANOVA revealed that 7 out of 18 converted morphometric dimensions were significantly diverse in H. limbatus populations. This indicated that the fish have a lot of phenotypic variation. The results of PCA and DFA demonstrated that the BhR partially separated from KR and BB. At the same time, KR and BB showed a greater extent of mixed populations in the biplot result. PCA and DFA are convenient methods to differentiate populations of the same species (Karakousis et al., 1991). In the current study, DFA correctly classified 87.33% (original) and 77.83% (cross-validated) of individuals into their groups, signifying greater intermixing among the populations. Gain et al. (2017) used DFA to find significant morphometric heterogeneity among people of Indian major carp (Cirrhinus mrigala) from a hatchery, an oxbow lake, and a river in southwestern Bangladesh. Mahfuj et al. (2017) also reported similar results for Indian minor carp (Labeo bata) collected from six Bangladeshi rivers. However, the rivers BhR and KR showed partial overlapping in this study, possibly due to the far distances. In contrast, the BB and KR populations showed maximum overlap due to the close distances.
The fish can exhibit more remarkable morphological plasticity in response to environmental modifications (Wimberger, 1992). A higher level of isolation can lead to a significant phenotypic disparity among fish inhabitants within a species, which can be used to separate and manage diverse populations (Turan, 2004). Divergence may arise due to various processes. For instance, home to different spawning regions (Hourston, 1982) or hydrographical elements that prevent or minimize relocation between areas can cause reproductive isolation among fish stocks (Iles & Sinclair, 1982). The failure of management programs to distinguish stock complexity has resulted in spawning activities, resulting in a loss of genetic variation and other ecological problems (Begg et al., 1999).
DFA and UPGMA dendrogram analyses revealed that two independent fish stocks supported the fisheries at these locations. Generally, morphometric variations (conventional and truss) among populations directly serve as the basis for stock discrimination analysis and to elucidate 'phenotypic stocks'. The development of consensus on biologically meaningful understandings is a significant bottleneck in morphometric studies (Cadrin, 2000). Conventional and truss variables on first and second factors characterized eye length (Fig. 2) and body lengths (6–7, 3–6, 2–6, 3–4, and 3–5) (Fig. 3). Fish stocks with healthy individuals have previously been linked to less turbulent water bodies, allowing them to achieve higher velocities with short-term propulsion (Blake, 2004). The water quality parameters of the rivers (BhR and KR) and BB are always in standard condition for their growth. Therefore, we did not investigate the water quality parameters in detail. However, previous reports by Khan et al. (2019) suggested the water quality parameters in the BhR and KR and the adjacent oxbow lakes in Southwestern Bangladesh remain stable throughout the year. Similarly, the DFA results were significantly related to SL, and truss characters such as 2–6, 3–6, 6–7, 7–3, and 7–2, indicating the swimming adaptations primarily influenced the mid portions of the fish body. A parallel report was previously published on Xenentodon cancila (Mahfuj et al., 2019) stocks in Southwestern Bangladesh.
Furthermore, differences in PC1 and DF 1 may be linked to the species feeding behavior, as required for locomotion, foraging, and evasion from a predator (Swain et al., 2005). Webb (1984) demonstrated that body depth adaptations are necessary for best nourishment for periodic and transient swimmers. However, the body shape of fish populations in tropical rivers like the BhR and KR and oxbow lakes like BB could respond well to small indigenous fish species like H. limbatus optimum food availability and productivity. Furthermore, due to the soft labile skin tissues of H. limbatus, the conventional and truss lengths in the belly or stomach regions were categorized in PC1 (Table 5) and DF1 (Table 6), which are entirely dependent on satiation (Chaudhuri et al., 2014). Phytoplankton, zooplankton (copepod and cladoceran), polychaetes, oligochaetes, and aquatic insects are the primary food sources of H. limbatus. They are abundant in tropical freshwater rivers and oxbow lakes (Chaudhuri et al., 2014). However, H. limbatus is a soft-bodied fish containing 80–90% moisture (Bogard et al., 2015); hence landmark positions may not precisely enumerate the proper arrangements of morphometric discrepancies (Cadrin & Friedland, 1999). The chosen morphometric dimensions should reflect important life-history traits in fish species (Cadrin, 2000). On the other hand, the larval and developmental stages of H. limbatus are poorly understood. Inferences based on different methods, such as fatty acid profiles, molecular analysis, and otolith compositions, may deliver more information about the stock structure of this fish species. Recently microsatellite markers have been developed for Buffon's garfish (Zenarchopterus buffonis) in the South China Sea and can be employed to examine their genetic differentiation along with phenotypic traits in H. limbatus. The morphometric features in the contemporary study can draw recommendations for better management and conservation. Hence, there was no shred of evidence of migration routes in this study. For future stock descriptions, large-scale sampling across spatiotemporal scales during the peak breeding period should be deliberated. The temporal changes in morphometric characters caused by stock mixing, feeding, or spawning migration across seasons will be explained by such investigations.
Conclusion
It has been effectively used to study stock separation within a species for different species in freshwater and marine habitats. The truss procedure used in this work showed an apparent aggregation of H. limbatus stocks in three water bodies from southwestern Bangladesh, indicating the need for individual management approaches to preserve the stocks for usage in the future. The results have been corroborated through PCA and DFA analyses. The outcomes of this study will serve as baseline information for stock management of H. limbatus in Southwestern Bangladesh to develop suitable conservation policies and sustainable management practices. Certainly, morphometric data cannot provide all the answers independently. However, aside from genetic factors, morphometric differences between H. limbatus stocks are influenced by exterior elements, including nourishment, habitat, and other ecological dynamics. The current study's findings can be further supported by molecular and biochemical techniques, which would provide more support for the stock structure identified by using the truss analysis in this study. Consequently, detailed research is essential to recognize the importance of these extraneous factors in the morphometric difference in H. limbatus. Furthermore, a rigorous analysis incorporating molecular genetics could candidly validate the current findings.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- BR:
-
Bhairab River
- KR:
-
Kopotakkho River
- BB:
-
Baluhar Baor
- PCA:
-
Principal component analysis
- DFA:
-
Discriminant function analysis
- DF:
-
Discrimination functions
- LC:
-
Least concern
- DFR:
-
Number of dorsal fin rays
- CFR:
-
Number of caudal fin rays
- AFR:
-
Number of anal fin rays
- PelFR:
-
Number of pelvic fin rays
- PecFR:
-
Number of pectoral-fin rays
- BTS:
-
Bartlett's test sphericity
- KMO:
-
Kaiser–Meyer–Olkin
References
Amarasinghe, U. S. (2008). Population dynamics of Hyporhamphus limbatus (Beloniformes, Hemiramphidae) in two lowland reservoirs of Sri Lanka. Sri Lanka Journal of Aquatic Science, 13, 23–38.
Asadujjaman, M., Khan, M. N., Mahfuj, S., Akram, W., Islam, S. I., & Sabbir, W. (2022). Life-history traits of grey mullet, Liza parsia in the Passur River of Southern Bangladesh: Implications for effective management. Bangladesh Journal of Fisheries, 34(1), 53–63.
Atique, U., & An, K. G. (2018). Stream health evaluation using a combined approach of multi-metric chemical pollution and biological integrity models. Water. https://doi.org/10.3390/W10050661
Atique, U., & An, K. G. (2022). Potential risky exotic fish species, their ecological impacts and potential reasons for invasion in Korean aquatic ecosystems. Journal of Ecology and Environment. https://doi.org/10.5141/JEE.22.008
Atique, U., Iqbal, S., Khan, N., Qazi, B., Javeed, A., Anjum, K. M., Haider, M. S., Khan, T. A., Mahmood, S., & Sherzada, S. (2020). Multivariate assessment of water chemistry and metals in a river impacted by tanning industry. Fresenius Environmental Bulletin, 29(04A), 3013–3025.
Atique, U., Lim, B., Yoon, J., & An, K.-G. (2019). Biological health assessments of lotic waters by biotic integrity indices and their relations to water chemistry. Water, 11(3), 436. https://doi.org/10.3390/w11030436
Azad, K. N., Mahfuj, M. S., Iqbal, T., Azad, K. N., & Shafaq, M. A. I. (2020). Differentiation of intraspecific phenotypic plasticity of elongate glassy perchlet, Chanda nama: Insights into landmark-based truss morphometric and meristic variations. Journal of Advanced Veterinary and Animal Research, 7(4), 585–596.
Bain, M. B. (2005). Electronic tags. Stock identification methods (pp. 435–446). Academic Press.
Begg, G. A., Friedland, K. D., & Pearce, J. B. (1999). Stock identification and its role in stock assessment and fisheries management: An overview. Fisheries Research, 43(1–3), 1–8. https://doi.org/10.1016/S0165-7836(99)00062-4
Begg, G. A., & Waldman, J. R. (1999). An holistic approach to fish stock identification. Fisheries Research, 43(1–3), 35–44.
Bickford, N., & Hannigan, R. (2005). Stock identification of walleye via otolith chemistry in the Eleven Point River, Arkansas. North American Journal of Fisheries Management, 25(4), 1542–1549.
Blake, R. W. (2004). Fish functional design and swimming performance. Journal of Fish Biology, 65, 1193–1222. https://doi.org/10.1111/j.0022-1112.2004.00568.x
Bogard, J. R., Thilsted, S. H., Marks, G. C., Wahab, M. A., Hossain, M. A., Jakobsen, J., & Stangoulis, J. (2015). Nutrient composition of important fish species in Bangladesh and potential contribution to recommended nutrient intakes. Journal of Food Composition and Analysis, 42, 120–133. https://doi.org/10.1016/j.jfca.2015.03.002
Bouchoucha, M., Pécheyran, C., Gonzalez, J. L., Lenfant, P., & Darnaude, A. M. (2018). Otolith fingerprints as natural tags to identify juvenile fish life in ports. Estuarine, Coastal and Shelf Science, 212, 210–218.
Cadrin, S. X., Kerr, L. A., & Mariani, S. (2014). Stock Identification Methods: An Overview. In Stock Identification Methods: Applications in Fishery Science: Second Edition (pp. 1–5). https://doi.org/10.1016/B978-0-12-397003-9.00001-1
Cadrin, S. X. (2000). Advances in morphometric identification of fishery stocks. Reviews in Fish Biology and Fisheries, 10(1), 91–112.
Cadrin, S. X., & Friedland, K. D. (1999). The utility of image processing techniques for morphometric analysis and stock identification. Fisheries Research, 43(1–3), 129–139. https://doi.org/10.1016/S0165-7836(99)00070-3
Chaudhuri, A., Mukherjee, S., & Homechaudhuri, S. (2014). Food partitioning among carnivores within feeding guild structure of fishes inhabiting a mudflat ecosystem of Indian Sundarbans. Aquatic Ecology, 48(1), 35–51. https://doi.org/10.1007/s10452-013-9464-x
Collette, B.B., & Su, J. (1986). The halfbeaks (Pisces, Beloniformes, Hemiramphidae) of the far east. In Proceedings of the Academy of Natural Sciences of Philadelphia. 138 (01): pp. 250–302. http://www.jstor.org/stable/4064855
Croft, D. P., Krause, J., Couzin, I. D., & Pitcher, T. J. (2003). When fish shoals meet: Outcomes for evolution and fisheries. Fish and Fisheries, 4(2), 138–146.
Eklöv, P., & Svanbäck, R. (2006). Predation risk influences adaptive morphological variation in fish populations. The American Naturalist, 167(3), 440–452.
Elliott, N. G., Haskard, K., & Koslow, J. A. (1995). Morphometric analysis of orange roughy (Hoplostethus atlanticus) off the continental slope of southern Australia. Journal of Fish Biology, 46(2), 202–220.
Ethin, R., Hossain, M. S., Roy, A., & Rutegwa, M. (2019). Stock identification of minor carp, Cirrhinus reba, Hamilton 1822 through landmark-based morphometric and meristic variations. Fisheries and Aquatic Sciences, 22(1), 1–8.
Ferguson, A. J. B. T., Taggart, J. B., Prodöhl, P. A., McMeel, O., Thompson, C., Stone, C., & Hynes, R. A. (1995). The application of molecular markers to the study and conservation of fish populations, with special reference to Salmo. Journal of Fish Biology, 47, 103–126.
Field, A. (2000). Discovering statistics using SPSS for windows. Sage.
Gain, D., Mahfuj, M. S., Huq, K. A., Islam, S. S., Minar, M. H., Goutham-Bharathi, M. P., & Das, S. K. (2017). Landmark-based morphometric and meristic variations of endangered mrigal Carp, Cirrhinus cirrhosus (Bloch) from wild and hatchery stocks. Sains Malaysiana, 46(5), 695–702. https://doi.org/10.17576/jsm-2017-4605-03
Guan, W., Cao, J., Chen, Y., & Cieri, M. (2013). Impacts of population and fishery spatial structures on fishery stock assessment. Canadian Journal of Fisheries and Aquatic Sciences, 70(8), 1178–1189.
Hall, D. A. (2014). Conventional and radio frequency identification (RFID) tags. Stock identification methods (pp. 365–395). Academic Press.
Hanif, M. A., Siddik, M. A., Islam, M. A., Chaklader, M. R., & Nahar, A. (2019). Multivariate morphometric variability in sardine, Amblygaster clupeoides (Bleeker, 1849), from the Bay of Bengal coast, Bangladesh. The Journal of Basic and Applied Zoology, 80(1), 1–10.
Hasan, M., Al Mamun, A., & Hossain, M. (2020). Length-weight relationships of 12 indigenous fishes and 3 shellfishes from mangrove and floodplain ecosystems in Southwestern Bangladesh. Egyptian Journal of Aquatic Biology and Fisheries, 24(5), 69–76.
Hashimoto, D. T., Prado, F. D. D., Senhorini, J. A., Foresti, F., & Porto-Foresti, F. (2013). Detection of post-F1 fish hybrids in broodstock using molecular markers: Approaches for genetic management in aquaculture. Aquaculture Research, 44(6), 876–884.
Hess, J. E., Whiteaker, J. M., Fryer, J. K., & Narum, S. R. (2014). Monitoring stock-specific abundance, run timing, and straying of Chinook salmon in the Columbia River using genetic stock identification (GSI). North American Journal of Fisheries Management, 34(1), 184–201.
Hilborn, R., & Walters, C. J. (Eds.). (2013). Quantitative fisheries stock assessment: choice, dynamics and uncertainty (pp. 177–178). Springer Science & Business Media.
Hourston, A. S. (1982). Homing by Canada’s west coast herring to management units and divisions as indicated by tag recoveries. Canadian Journal of Fisheries and Aquatic Sciences, 39, 1414–1422.
Iles, T. D., & Sinclair, M. (1982). Atlantic herring stock discreteness and abundance. Science, 215, 627–633.
Kaiser, H. F. (1974). An index of factorial simplicity. Psychometrika, 39, 31–36.
Karakousis, Y., Triantaphyllidis, C., & Economidis, P. S. (1991). Morphological variability among seven populations of brown trout, Salmo trutta L.,in Greece. Journal of Fish Biology, 38(6), 807–817.
Khan, A. S., Hakim, A., Rahman, M., Mandal, B. H., & Ahammed, F. (2019). Seasonal water quality monitoring of the Bhairab River at Noapara industrial area in Bangladesh. SN Applied Sciences, 1(6), 1–8. https://doi.org/10.1007/s42452-019-0583-4
Kim, J.-J., Atique, U., & An, K.-G. (2019). Long-term ecological health assessment of a restored urban stream based on chemical water quality, physical habitat conditions and biological integrity. Water, 11(1), 114. https://doi.org/10.3390/w11010114
Kim, J. Y., Atique, U., & An, K. G. (2021a). Relative abundance and invasion dynamics of alien fish species linked to chemical conditions, ecosystem health, native fish assemblage, and stream order. Water. https://doi.org/10.3390/W13020158
Kim, J.-J., Atique, U., & An, K.-G. (2021b). Key drivers influencing the presence and absence of micropterus salmoides and their effect on native fish communities and biotic integrity. Water, 13(23), 3430. https://doi.org/10.3390/W13233430
Kocovsky, P. M., Adams, J. V., & Bronte, C. R. (2009). The effect of sample size on the stability of principal components analysis of truss-based fish morphometrics. Transactions of the American Fisheries Society, 138(3), 487–496. https://doi.org/10.1577/T08-091.1
Lim, P., Lek, S., Touch, S. T., Mao, S. O., & Chhouk, B. (1999). Diversity and spatial distribution of freshwater fish in Great Lake and Tonle Sap river (Cambodia, Southeast Asia). Aquatic Living Resources, 12(6), 379–386. https://doi.org/10.1016/S0990-7440(99)00107-2
MacKenzie, K., & Abaunza, P. (2014). Parasites as biological tags. Stock identification methods (pp. 185–203). Academic Press.
Mahfuj, M. S., Ahmed, F. F., Hossain, M. F., Islam, S. I., Islam, M. J., Alam, M. A., & Nadia, Z. M. (2022). Stock structure analysis of the endangered Queen Loach, Botia dario (Hamilton 1822) from Five Rivers of Northern Bangladesh by using morphometrics: implications for conservation. Fishes, 7(1), 41.
Mahfuj, M. S., Ashraful, A., Parvez, I., Minar, M. H., & Samad, A. (2017). Morphological variations of Labeo bata populations (Teleostei: Cyprinidae) in six rivers of Bangladesh: a landmark-morphometric contribution. Iranian Journal of Ichthyology, 4(3), 270–280. https://doi.org/10.22034/IJI.V4I3.207
Mahfuj, M. S., Das, S. K., Azad, K. N., Paul, A. K., Hoshan, I., Sultana, S., & Biswas, M. (2021b). Truss network based morphometric and meristic variations among southwestern populations of Macrognathus aculeatus in Bangladesh. Journal of Environmental Biology, 42, 887–894.
Mahfuj, M. S., Islam, S. I., Hanif, M. A., & Mondal, S. (2021a). Stock identification of the Reba carp, Cirrhinus reba: Insight into conventional and truss network-based morphometrics. Egyptian Journal of Aquatic Biology and Fisheries, 25(5), 463–478.
Mahfuj, M. S., Rahman, M. M., Islam, M., Samad, M. A., Paul, A. K., & Adhikary, R. K. (2019). Landmark-based morphometric and meristic variations of freshwater garfish, Xenentodon cancila from four natural stocks of South-Western Bangladesh. Journal of Advanced Veterinary and Animal Research, 6(1), 117. https://doi.org/10.5455/javar.2019.f321
McGarigal, K., Cushman, S., & Stafford, S. (2000). Multivariate statistics for wildlife and ecology research. Springer Verlag.
Metcalfe, J. D., & Arnold, G. P. (1997). Tracking fish with electronic tags. Nature, 387(6634), 665–666.
Mir, J. I., Sarkar, U. K., Dwivedi, A. K., Gusain, O. P., & Jena, J. K. (2013). Stock structure analysis of Labeo rohita (Hamilton, 1822) across the Ganga basin (India) using a truss network system. Journal of Applied Ichthyology, 29(5), 1097–1103. https://doi.org/10.1111/jai.12141
Miyan, K., Khan, M. A., Patel, D. K., Khan, S., & Ansari, N. G. (2016). Truss morphometry and otolith microchemistry reveal stock discrimination in Clarias batrachus (Linnaeus, 1758) inhabiting the Gangetic river system. Fisheries Research, 173, 294–302.
Mosquera, J., De Castro, M., & Go´mez-Gesteira, M. (2003). Parasites as biological tags of fish populations: Advantages and limitations. Comments on Theoretical Biology, 8(1), 69–91.
Nabi, M.R. (2015). Congaturi half-beak, Hyporhamphus limbatus. In IUCN Bangladesh. Red list of bangladesh volume 5: Freshwater fishes (pp. 156). IUCN, International Union for Conservation of Nature, Bangladesh Country Office.
Nimalathasan, B. (2009). Determinants of key performance indicators (KPIs) of private sector banks in Sri Lanka: An application of exploratory factor analysis. The USV Annals of Economics and Public Administration, 9(2), 9–17.
Okumuş, İ, & Çiftci, Y. (2003). Fish population genetics and molecular markers: II-molecular markers and their applications in fisheries and aquaculture. Turkish Journal of Fisheries and Aquatic Sciences, 3, 57–79.
Rainboth, W. J. (1996). Fishes of the cambodian mekong. Food & Agriculture Org.
Robinson, B. W., & Wilson, D. S. (1996). Genetic variation and phenotypic plasticity in a trophically polymorphic population of pumpkinseed sunfish (Lepomis gibbosus). Evolutionary Ecology, 10, 631–652. https://doi.org/10.1007/BF01237711
Rohlf, F.J. (2006) tpsDig2, Version 2. 1. State University of New York, Stony Brook. Retrieved January 29, 2021 from http://life.bio.sunysb.edu/morph.
Samaee, S. M., Patzner, R. A., & Mansour, N. (2009). Morphological differentiation within the population of Siah Mahi, Capoeta capoeta gracilis, (Cyprinidae, Teleostei) in a river of the south Caspian Sea basin: A pilot study. Journal of Applied Ichthyology, 25(5), 583–590.
Sippel, T., Eveson, J. P., Galuardi, B., Lam, C., Hoyle, S., Maunder, M., & Nicol, S. (2015). Using movement data from electronic tags in fisheries stock assessment: a review of models, technology and experimental design. Fisheries Research, 163, 152–160.
Strauss, R. E., & Bookstein, F. L. (1982). The truss: Body form reconstructions in morphometrics. Systematic Biology, 31(2), 113–135.
Swain, D. P., Hutchings, J. A., Foote, C. J., Cadrin, S., & Friedland, K. (2005). Environmental and genetic influences on stock identification characters. In S. X. Cadrin, K. D. Friedland, & J. R. Waldman (Eds.), Stock identification methods: Applications in fishery science (pp. 45–85). Elsevier Academic Press.
Talwar, P. K., & Jhingran, A. G. (1991). Inland fishes of India and adjacent countries (Vol. 2). CRC Press.
Tanner, S. E., Reis-Santos, P., & Cabral, H. N. (2016). Otolith chemistry in stock delineation: A brief overview, current challenges and future prospects. Fisheries Research, 173, 206–213.
Turan, C. (2004). Stock identification of Mediterranean horse mackerel (Trachurus mediterraneus) using morphometric and meristic characters. ICES Journal of Marine Science, 61(5), 774–781.
Turan, C., Yalçin, S., Turan, F., Okur, E., & Akyurt, I. (2005). Morphometric comparisons of African catfish, Clarias gariepinus, populations in Turkey. Folia Zoologica, 54(1/2), 165–172.
Waldman, J. R. (2005). Definition of stocks: an evolving concept. Stock identification methods (pp. 7–16). Academic Press.
Webb, P. W. (1984). Body form, locomotion and foraging in aquatic vertebrates. American Zoologist, 24(1), 107–120.
Wimberger, P. H. (1992). Plasticity of fish body shape: The effects of diet, development, family and age in two species of Geophagus (Pisces: Cichlidae). Biological Journal of the Linnean Society, 45, 197–218.
Acknowledgements
The authors sincerely thank the anonymous local fishers for helping during fish sampling. Thanks to Musfike Meraz, a current student of Environmental Science Discipline Khulna University, Bangladesh, for support in creating sampling sites using ArcGIS.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, SM and UA; methodology, SM, SII, SSJ, UA; software, SM, SSJ; validation, SM, SSJ, UA; formal analysis, SM, MFH, and UA; investigation, SM, UA; resources, SM, SSJ, and UA; data curation, SSJ and MFH; writing, SM, SII and SSJ; review and editing, UA; visualization, SM and UA; supervision, UA; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahfuj, S., Islam, S.I., Jinia, S.S. et al. Stock identification of Congaturi halfbeak (Hyporhamphus limbatus): insight into conventional and truss-based morphometrics. JoBAZ 84, 10 (2023). https://doi.org/10.1186/s41936-023-00329-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-023-00329-7