Abstract
Background
Thelandros (Pharyngodonidae) is a gastrointestinal nematode parasite with a life cycle including lizards as main hosts. Thelandros chalcidae collected from the large intestine of the Egyptian ocellated skink, Chalcides ocellatus were described and illustrated by light and scanning electron microscopes. Seven out of fifteen (46.66%) of the examined lizards were found to be naturally infected. Also, host intestinal tissues were evaluated from hematoxylin/eosin-stained sections to describe any histopathological changes.
Results
Microscopic examinations revealed that the recovered pharyngodonid species characterized by mouth with triangular opening and surrounded by six simple lips, the cuticle had regular transverse annulations extending from the posterior margin of the lips to the end of the body. Male was cylindrical with distinct truncated posterior end and measured 1.59–1.86 (1.64 ± 0.10) long and 0.29–0.37 (0.32 ± 0.01) in maximum width at the level of mid-body. Female measured 1.72–2.43 (1.85 ± 0.2) long and 0.36–0.49 (0.42 ± 0.01) maximum width at the mid-body level, terminated posteriorly in a short, stout spike. Histological studies observed structural alterations represented by leukocytic infiltration, villi atrophy, and muscularis degeneration. These changes were indicative of inflammatory and degenerative reaction due to Thelandros chalcidae infection.
Conclusion
The present morphological study revealed that the recovered pharyngodonid species was Thelandros chalcidae causing pathological alterations in Chalcides ocellatus intestinal tissues.
Similar content being viewed by others
Background
There are more than 400 species of reptiles occur in Egypt. One of the most abundant and widespread reptiles in Africa and Egypt is the lizard, Chalcides ocellatus (Al-Deen et al., 1995). Unfortunately, little consideration has been given to their helminths community and few records exist in the literature (Al-Deen & Al-Shareef, 1996; Ibrahim & Soliman, 2005). Genus Thelandros was established by Wedl (1861) for T. alatus, a nematode from the intestine of an Egyptian mastigure, Uromastyx aegyptia. The difference between Parapharyngodon (Chatterji, 1933) and Thelandros (Wedl, 1861) was questionable based on morphological similarities. Some authors considered them as synonym (Baylis, 1936; Karve, 1938; García-Calvente, 1948; Skryabin et al., 1951). However, Freitas (1957) considered them as different genera likewise (Adamson, 1981; Baker, 1987; Hering−Hagenbeck et al., 2002; Sharpilo, 1976). Although, previous studies recorded Thelandros sp. infection in wild animals from Egypt (Abdel-Ghaffar et al., 2018, 2020; Ibrahim et al., 2005; Rabie et al., 2012); the taxonomic status of some species is still under discussion and no pathological studies have been undertaken to assess the health impact on their hosts. Therefore, our aim was designed to study the morphology of Thelandros chalcidae and its impact on Chalcides ocellatus.
Materials and Methods
Worm collection
Fifteen Egyptian lizards, Chalcides ocellatus were collected from Abu Rawash region, Giza, Egypt from May to September 2020. Lizards were transported alive to the laboratory, they were euthanized with an overdose of sodium pentobarbital, subsequently necropsied and examined for helminths. The intestinal contents were examined for helminths under a stereomicroscope (OLYMPUS, SZ51). The collected worms were washed several times in saline solution then preserved in 70% ethanol.
Microscopic examination
The recovered nematodes were transferred to lactophenol for clearance, then examined and photographed using light microscope (LEICA DM 750) supplied with a LEICA ICC 50 HD Camera. For scanning electron microscopy, the worms were fixed in a solution of 3% glutaraldehyde, washed in 0.1 M sodium cacodylate buffer (pH 7.4), dehydrated through a graded ethanol series (50%, 60%, 70%, 80%, 90% and 100%), and then dried at 30°c for 30 min using critical point drier “LEICA, EM CPD300”. After complete drying, nematodes were mounted on SEM stubs, coated with gold and examined with JEOL JSM-5200 SEM (Tokyo, Japan) at 25 kV as accelerating voltage. All measurements (mean ± SD, followed by a range in parentheses) are in millimeters (mm).
Histopathological examination
For histological studies, target organ (large intestine) of the Egyptian lizards, Chalcides ocellatus were carefully isolated and washed out in saline water then fixed in 10% buffered formalin. Tissues were dehydrated and processed for paraffin wax embedding. Sections were cut by a rotary microtome and stained with hematoxylin and eosin, then examined for any histopathological lesions and photographed using LEICA DM 750 microscope supplied with a LEICA ICC 50 HD Camera.
Results
The helminthological examination of 15 ocellated skink Chalcides ocellatus revealed natural infection by Thelandros chalcidae with a prevalence of (7/15) 46.66%. The mean intensity of infection was 1–3 parasite per host.
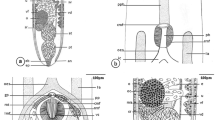
Description (Fig. 1a–i)
a–c, f–h Photomicrograph of T. chalcidiae showing a Whole worm with mouth (M) followed by esophagus (OE) with esophageal bulb (EB) lead to long intestine (IN), cuticle with transverse striations (TS), scale bar = 200 µm. b Anterior extremity showing mouth (M), lips (L), esophagus (OE) and transverse striations (TS), scale bar = 50 µm. c High magnification showing cuticle with transverse striations (TS), scale bar = 5 µm. f Posterior extremity of female worm with tapered tail (T), anus (A), scale bars = 200 µm. g High magnifications of female tail region, scale bars = 50 µm h Posterior extremity of male worm with tail (T), scale bars = 200 µm. d, e, i Scanning electron micrographs of T. chalcidiae d Cephalic region showing mouth (M) with six lips (L), scale bar = 50 µm. e Midbody showing transverse striation (TS) and lateral alae (LA), scale bar = 50 µm. i Truncated posterior extremity of male worm with pointed tail (T), single spicule (SP), caudal papillae (PA) and cloaca (C) scale bar = 50 µm
Generally
The body was robust cylindrical, whitish in colour, tapering at both ends with prominent cuticle striations from cephalic extremity and continuing to posterior end. Oral opening surrounded by 6 small lips. The esophagus was muscular, cylindrical ending by a well-developed bulb which leads to the intestine. Small lateral alae present. Prominent sexual dimorphism.
Male
Small, cylindrical body, distinctly truncated posteriorly and measured 1.59–1.86 (1.64 ± 0.10) mm long and 0.29–0.37 (0.32 ± 0.01) mm in maximum width at the level of mid-body. Oral opening surrounded by 6 small lips. Length of esophagus (including bulb) was 0.52–0.61 (0.57 ± 0.02) mm long and 0.039–0.055 (0.043 ± 0.001) mm wide. Small lateral alae reaching the tail in an auricular form and being widest at the cloacal level. The testes at mid-body, genital cone simple with papillae disposed outside this cone with single spicule measured 0.032–0.037 (0.035 ± 0.001) mm long. Gubernaculum absent. The tail filament was narrow, sharply pointed, inserted dorsally, terminal in position and measured 0.14–0.22 (0.17 ± 0.01) mm in length and 0.028–0.034 (0.03 ± 0.001) mm in width.
Female
Cylindrical body measured 1.72–2.43 (1.85 ± 0.2) mm long and 0.36–0.49 (0.42 ± 0.01) mm maximum width at the mid-body level, tapering anteriorly to blunt point and posteriorly ending in short, stout spike. Oral opening surrounded by 6 small lips. Esophagus was 0.58–0.69 (0.65 ± 0.02) long and 0.041–0.065 (0.045 ± 0.001) mm wide. Nerve ring 0.120–0.143 (0.126 ± 0.01) mm from the anterior end. Vulva located at the mid-body. Ovary amphidelphic, the anterior ovary extended to excretory pore level and the posterior ovary extended posteriorly to anal opening. Uteri divergent. Anus slit like located at 0.27–0.34 (0.3 ± 0.01) mm from the posterior end. The posterior extremity of the body extends like a very short smooth tail measured 0.063–0.11 (0.08 ± 0.01) in length.
Taxonomic summary
Type species: Thelandros chalcidiae (Abdel-Ghaffar et al., 2020) (Family: Pharyngodonidae).
Type-host: Ocellated skink Chalcides ocellatus (Family: Scincidae).
Site of infection: Large intestine.
Type-locality: Abu Rawash area, Giza Egypt.
Prevalence and Intensity of infection: (7/15) 46.66%, 1–3 respectively.
Histopathological findings
Sections of the intestinal tissue revealed cellular infiltration around the embedded worms which peripherally displaced the muscle fibers. Furthermore, moderate fibrosis in connective tissue was evident around this area, and severe lymphocyte infiltration (Fig. 2A, B), erosion of the muscularis layer, lesions associated with severe fibrosis and an inflammatory response reached serosa layer. Finally, the effects were varied and distributed among the four layers of the intestine leading to disintegration of muscularis and serosa layers due to burrowing of the parasite (Fig. 2B, C) as well as degeneration and atrophy of villi, infiltrated inflammatory cells diffused in the lamina propria, hyperplasia of the mucosal glands were observed (Fig. 2D).
Cross sections of intestine of C. ocellatus naturally infected with T. chalcidiae showing: a Parasites penetrate along different intestinal layers. b Embedded worms which displaced the muscle fibers c Disintegration and erosion of muscularis and serosa layers with severe lymphocyte infiltration, d Fibrosis in connective tissue, degeneration and atrophy of villi. Arrowhead: erosion and disintegration, Arrow: burrowing of the parasites, scale bars = 200 µm
Discussion
Oxyurids are parasitic nematodes infecting reptiles that constitute a very interesting group although their knowledge remains scarce (Aho, 1990; Bush, 1990; Martin & Roca, 2004; Sharpilo et al., 2001). Species of genus Thelandros Wedl (1861) infect many reptilian hosts (Bursey & Goldberg, 2005; Dung et al., 2009).
Walton (1941) reviewed the geographical and host distribution of the genus Thelandros and pointed out that many of the known species for this genus were recorded from North Africa. Petter and Quentin (1976) considered Parapharyngodon a synonym of Thelandros. However, Thelandros sp. differentiated from Parapharyngodon sp. based on the posterior end morphology in both sexes (Bursey & Goldberg, 2005) Parapharyngodon sp. males do not have a conical-shaped genital area, or an accessory piece. In contrast, Thelandros sp. males have a prominent genital cone with papillae disposed outside this cone and caudal appendage inserted sub terminally on the body (Bursey & Goldberg, 1999; Bursey et al., 2013). Females of Parapharyngodon sp. generally have a conical shaped tail with a thick pointed end, like a spike. However, Thelandros sp. females have varied tail morphology (Bursey et al., 2013; Ramallo et al., 2016; Velarde-Aguilar et al., 2015).
Bursey and Goldberg (2005) allocated 30 species to the genus Thelandros and stated the main generic features for Thelandros species based on the pattern of the caudal papillae, spicule length and morphology of the anterior cloacal lip in males, location of the vulva and morphology of the posterior end of the female. The current pharyngodonid species revealed main features of genus Thelandros represented by tapering at both ends with prominent cuticle striations from cephalic extremity and continuing to posterior end. Male has a conical-shaped genital area with papillae disposed outside this cone and distinct truncated posterior end with single spicule while female posterior extremity extending in short, stout spike.
Thelandros species herein, resembles T. micipsae Seurat (1917); T. scleratus Travassos (1923) and T. luciusi Hering-Hagenbeck (2001) and T chaicidae Abdel-Ghaffar et al. (2020) by the absence of gubernaculum and caudal papillae surrounded the genital cone and similar to the latter one by presence of long lateral alae reach from the cephalic region to the posterior extremity and male spicule. Also, T. awokoyai Babero and Okpala (1962); T. arequipensis &T. yurensis Calisaya and Cordóva (1997); by the presence of short tail; T. markovi, T. galloti, T. balanchardi Caballero (1968); T. ortleppi Petter (1966); T. samburuensis Bursey and Goldberg (2005) by possessing smooth cloacal lip. However, differ from T. kuntzi Delle (1954); T. luciusi Hering-Hagenbeck (2001) by absence of lateral alae and T. aegypti Rabie et al. (2014) by possessing oral opening without lips and absence of male spicule. Additionally, the recovered parasite sharing T. alatus Ibrahim et al. (2005); T. amburuensis Bursey and Goldberg (2005); Thelandros sp. Hering-Hagenbeck et al. (2002); T. vietnamensis Dung et al. (2009); Thelandros sp. Rabie et al. (2012); T. aegypti Rabie et al. (2014) and T. chalcidae Abdel-Ghaffar et al. (2020) in host type as well as site of infection and the latter three by having the same geographical location.
Oxyurids live in the stomach or intestines of all classes of vertebrates; attach to the mucosa cause a wide range of damages including tissue degradation, hemorrhage, inflammation and mesenteric visceral adhesions (Botella & Esteban, 1995; Mohammed et al., 2017; Naem et al., 2006). The present histological studies revealed numerous pathological alterations in different layers of the large intestine of Chalcides ocellatus infected with T. chaicidae in agreement with previous observations by Schaftenaar et al. (2000) who reported pathological changes in lizard intestines due to nematodes infection. Also, in the present study, complete destruction of intestinal layers represented by erosion of the surface epithelium reaches the muscularis and serosa layers with severe leucocytes infiltration and lesions due to burrowing of the parasite, in consistent with previous reports by Dunn et al. (1983); Botella and Esteban (1995) and Meguid and Eure (1996) who reported that the parasites may occasionally change their site of attachment causing erosion of surface epithelium reaches as deep as the muscularis mucosa results in total loss at the region of attachment. Additionally, histological examination revealed atrophy in intestinal villi, this state may be due to the feeding of parasites on the villi, leading to its disappearance and the lack of surface area of absorption and thus low efficiency of absorption of nutrients and mineral salts (Dunn et al., 1983; Molnar et al., 1994).
Conclusions
Based on morphological and morphometric evidence, the current parasite resembles Thelandros chalcidiae, as well as sharing the same host species, site of infection, and locality record. As a result, we can mark it as Thelandros chalcidiae. Furthermore, for the first time in Egypt, we focused on the pathological effects of T. chalcidiae on Chalcides ocellatus..
Availability of data and materials
Not applicable.
Abbreviations
- A:
-
Anus
- C. ocellatus :
-
Chalcides ocellatus
- C:
-
Cloaca
- EB:
-
Esophageal bulb
- IN:
-
Intestine
- L:
-
Lips
- LA:
-
Lateral alae
- M:
-
Mouth
- OE:
-
Esophagus
- PA:
-
Caudal papillae
- SP:
-
Spicule
- T:
-
Tail
- T. chalcidiae :
-
Thelandros chalcidiae
- TS:
-
Transverse striations
References
Abdel‑Ghaffar, F., Varjabedian, K.G., Al Quraishy, S., Abdel‑Gaber, R., Fol, M., & Talal, N. (2020). Morphological description and phylogenetic assessment of 28S rRNA for Thelandros chalcidiae sp. nov. from Chalcides ocellatus Mol Biol Rep 47:3705–3718. https://doi.org/10.1007/s11033-020-05412-8
Abdel-Ghaffar, F., El-Fayoumi, H., Abdel-Haleem, H., Mohamed, S. A., & Morsy, K. (2018). Description of Thelandros aegypti (Nematoda: Pharyngodonidae) from the Egyptian Spiny-Tailed Lizard, Uromastyx aegyptia (Squamata: Agamidae) in Egypt. Journal of the Egyptian Society of Parasitology, 48(3), 639–644.
Adamson, M. L. (1981). Parapharyngodon osteopili n. sp. (Pharyngodonidae: Oxyuroidea) anda revision of Parapharyngodon and Thelandros. Systematic Parasitology, 3, 105–117. https://doi.org/10.1007/BF00012216
Aho, J. M. (1990). Helminth communities of amphibians and reptiles: Comparative approaches to understanding patterns and processes. In G. W. Esch, A. O. Bush, & J. M. Aho (Eds.), Parasite communities: Patterns and processes (pp. 157–190). Chapman and Hall.
Al-Deen, A., & Al-Shareef, M. F. (1996). Studies on the nematode, Moaciria icosiensis (Seurat, 1917) from Chalcides ocellatus (Forskål, 1775) in Egypt. Journal of the Egyptian Society of Parasitology, 26, 797–802.
Al-Deen, A., Al-Shareef, M. F., & Saber, S. A. (1995). Ecological studies of Chalcides ocellatus (Forskål, 1775) and Hemidactylus turcicus (Linnaeus, 1758) from Egypt with special reference to helminth parasites. Journal of the Egyptian Society of Parasitology, 25, 145–156. https://doi.org/10.1111/j.1442-9993.1997.tb00677.x
Babero, B. B., & Okpala, I. (1962). Parasites of the lizard, Agama colonarum, in Nigeria with description of a new species. Transactions of the American Microscopical Society, 81, 228–237.
Baker, M. (1987). Synopsis of the nematode parasitic in amphibians and reptiles. Memorial University of Newfoundland, Occasional Papers in Biology, 11, 1–325.
Baylis, H. A. (1936). Nematoda. I. Ascaridoidea and Strongyloidea: The fauna of British India. Taylor and Francis, London, UK.
Botella, P., & Esteban, J. G. (1995). Histopathology of the stomach lesion caused by Physaloptera brevivaginata (Nematoda: Physalopteridae) in bats in Spain. Folia Parasitologica, 42, 143–148.
Bursey, C. R., Drake, M., Cole, R., Sterner, M., Pinckney, R., & Sieger, U. (2013). New species of Parapharyngodon (Nematoda: Pharyngodonidae) in Rhinella marina (Anura: Bufonidae) from Grenada, West Indies. Journal of Parasitology, 99, 475–479. https://doi.org/10.1645/GE-3235.1
Bursey, C. R., & Goldberg, S. R. (1999). Parapharyngodon japonicus sp. n. (Nematoda: Pharyngodonidae) from the Japanese clawed salamander, Onychodactylus japonicus (Caudata: Hynobiidae), from Japan. Journal of the Helminthological Society, 66, 180–186.
Bursey, C. R., & Goldberg, S. R. (2005). Two new species of Pharyngodonidae (Nematoda: Oxyuroidea) and other nematodes in Agama caudospina (Squamata: Agamidae) from Kenya, Africa. Journal of Parasitology, 91, 591–599. https://doi.org/10.1645/GE-3421
Bush, A. O. (1990). Helminth communities in avian hosts: Determinants of pattern. In G. W. Bush & J. M. Aho (Eds.), Parasite communities: Patterns and processes (pp. 197–232). Chapman & Hall.
Caballero, R. G. (1968). Contribution à la connaissance des nematodes de sauriens malgaches. Annals of Parasitology, 43, 149–200.
Calisaya, J. L., & Córdova, E. (1997). Tres nuevas especies de Parapharyngodon (Nematoda, Oxiuroidea) parásitas de Tropidurus peruvianus del sur del Perú. Rebiol, 17, 45–55.
Chatterji, R. C. (1933). On a new nematode, Parapharyngodon maplestoni gen. nov., sp. nov., from a Burmese lizard. Annals of Tropical Medicine and Parasitology, 27, 131–134. https://doi.org/10.1080/00034983.1933.11684745
Delle, E. A. (1954). Nematode parasites of Egyptian reptilies. M.Sc thesis in Faculty of Graduate Studies and Research of McGill University.
Dung, B. T., Bursey, C. R., & Goldberg, S. R. (2009). A new species of Thelandros (Nematoda, Oxyuroidea, Pharyngodonidae) in Leiolepis reevesi (Sauria, Agamidae) from Vietnam. Acta Parasitologica, 54, 151–153. https://doi.org/10.2478/s11686-009-0019-1
Dunn, I. J., Russell, L. R., & Adams, J. R. (1983). Caecal histopathology caused by Truttaedacnitis truttae (Nematoda: Cucullanidae) in rainbow trout, Salmo gairdneri. International Journal of Parasitology, 13, 441–445.
Freitas, J. F. T. (1957). Sôbre os gêneros Thelandros Wedl, 1862 e Parapharyngodon Chatterji, 1933, com descrição de Parapharyngodon alvarengai sp. n. (Nematoda, Oxyuroidea). Memorias Do Instituto Oswaldo Cruz, 55, 21–45. https://doi.org/10.1590/S0074-02761957000100003
García-Calvente, I. (1948). Revisión del género Pharyngodon y descripción de nueva species. Revista Ibérica De Parasitología, 8, 367–410.
Hering-Hagenbeck, S. F. B. N. (2001). The metazoan parasite fauna at South African reptiles, with special attention to their nematodes. PhD thesis, Humboldt-University of Berlin.
Hering-Hagenbeck, S. F. B. N., Petter, A. J., & Boomker, J. (2002). Redescription of some Spauligodon spp., and Parapharyngodon spp., and of Skrjabinodon mabyae (Sandground, 1936) Inglis, 1968 (Pharyngodonidae: Oxyuroidea) from insectivorous South African lizards. Onderstepoort Journal of Veterinary Research, 69, 7–29.
Ibrahim, H. M., Fadiel, M. M., & Nair, G. A. (2005). Gastrointestinal Helminthes of the Lizard, Chalcides ocellatus, from Benghazi, Libya. Journal of the Helminthological Society, 79, 35–39. https://doi.org/10.1079/JOH2004258
Ibrahim, M. M., & Soliman, M. F. M. (2005). Factors affecting helminths community structure of the Egyptian lizard Chalcides ocellatus (Forskål, 1775). Parasites, 12, 317–323. https://doi.org/10.1051/parasite/2005124317
Karve, J. N. (1938). Some nematode parasites of lizards. Pages 251–258 in Livro jubilar do professor Lauro Travassos, editadopara commemoraro 25 aniversario de luas acatividades scientificas (1913–1938). Typographia do Instituto Oswaldo Cruz, Rio de Janerio, Brasil.
Martin, J. E., & Roca, V. (2004). Helminth infracommunities of Gallotia caesaris caesaris and Gallotia caesaris gomerae (Sauria: Lacertidae) from the Canary Islands (Eastern Atlantic). Journal of Parasitology, 90(2), 266–270. https://doi.org/10.1645/GE-3198
Meguid, M. A., & Eure, H. E. (1996). Pathobiological associated with the spiruroid nematodes Camallanus oxycephalus and Spinitectus carolini in the intestine of green sunfish, Lepomis cyanellus. Journal of Parasitology, 82, 118–123.
Mohammed, S. Y., Abdelrhman, N. M., Masri, M. A., & Ibrahim, M. Y. (2017). Histopathological changes in the intestines and gonad of grouper fish Epinephelus microdon infected with nematode parasites, Red Sea Coast, Sudan. Red Sea University Journal of Basic and Applied Sciences, 2(2), 350–360.
Molnar, K., Baska, F., Csaba, G., Glavits, R., & Szekely, C. (1994). Pathological and histopathological studies of swim bladder of eels, Anguilla anguilla infected by Anguillicola crass (Nematoda: Dracunculoidea). Diseases of Aquactic Organisms, 15, 41–50.
Naem, S., Farshid, A. A., & Tanhai, M. M. (2006). Pathological findings on natural infection with Physaloptera praeputialis in cats. Veterinarski Arhiv, 76(4), 315–321.
Petter, A. J. (1966). Équilibre des espéces dans les populations de nematodes parasites du colon des tortues terrestres. Mémoir Mus Natl Hist Nat, 39, 1–252.
Petter, A. J., & Quentin, J. C. (1976). CIH Keys to the Nematode Parasites of Vertebrates. No. 4. Keys to the Genera of the Oxyuroidea. Commonwealth Agricultural Bureaux. Farnham Royal, U.K. pp. 30.
Rabie, S. A. H., El-Din, M., Abd El-Latif, M. E. Z., Mohamed, N. I., & Abo Al-Hussin, O. F. (2012). Redescription of Nematodes Pharyngodon mamillatus and Thelandros sp. from Some Reptiles of Qena, Egypt. International Journal of Science and Research 3(11), 1368–1380.
Rabie, S. A., Abd El-Latif, M. E. Z., Mohammed, N. I., & Abo el-Hussien, O. F. (2014). Redescription of two nematode parasites infecting Reptilia in Qena Governorate. Egyptian Academic Journal of Biological Sciences 6(1), 41–50. https://doi.org/10.21608/eajbsz.2014.13492
Ramallo, G., Bursey, C. R., Castillo, G., & Acosta, J. C. (2016). New species of Parapharyngodon (Nematoda: Pharyngodonidae) in Phymaturus spp. (Iguania: Liolaemidae) from Argentina. Acta Parasitologica, 61, 461–465. https://doi.org/10.1515/1p-2016-0062
Schaftenaar, W. G. M., Dorrestein, J. M., Mensink, C. H.,& Cremers, H. J. W. M. (2000). An unusual infestation with Rhabditid nematodes in a green tree monitor lizard (Varanus prasinus): Diagnosis and treatment. In proceeding of the 3rd Scientific Meeting of the European Association of Zoo and Wildlife Veterinarians, Paris, France, pp. 1–4.
Seurat, L. G. (1917). Sur les Oxyuros des Sauriens du Nord Africain. Arch De Zool Exp Dt Gen, 56(9), 401–444.
Sharpilo, V. P., Biserkov, V., Kostadinova, A., Behnke, J. M., & Kuzmin, Y. I. (2001). Helminths of the sand lizard, Lacerta agilis (Reptilia, Lacertidae), in the Palearctic: Faunal diversity and spatial patterns of variation in the composition and structure of component communities. Parasitology 123, 389–400. https://doi.org/10.1017/S0031182001008587.
Sharpilo, C. P. (1976). Parasitic worms of the reptilian fauna of the USSR: Systematics, chorology, biology. Naukova Dumka.
Skryabin, K. I., Shikhobalova, N. P., & Mozgovoy, A. A. (1951). Key to Parasitic Nematodes. Vol.2. Oxyurata and Ascaridata. Izdatel’-stvo Akademii Nauk S.S.S.R., Moscow (English translation by Amerind Publishing Co. Pvt. Ltd., New Delhi India, 1982), Soc Parasitol 48(3):639–644.
Travassos, L. (1923). Informações sobre a fauna helminthological de Mato Grosso. Folha Medica, 4, 29–30.
Velarde-Aguilar, M. G., Mata-Lopez, R., Guillen-Hernandez, S., & Leon-Regagnon, V. (2015). Parapharyngodon n. spp. (Nematoda: Pharyngodonidae) parasites of hylid frogs from Mexico and review of species included in the genus. Journal of Parasitology, 101, 212–230. https://doi.org/10.1645/13-328.1
Walton, A. C. (1941). Distribution of the genus Thelandros (Nematoda: Oxyurida). Proceedings of the Helminthological Society of Washington, 8, 15–18.
Wedl, K. (1861). Zur Helminthenfauna Ägyptens. Akademie Der Wissenschaften. Mathematisch Natur-Wissenschaftliche., 44, 463–482.
Acknowledgements
This work is supported by Faculty of Science, Cairo University, Egypt. Authors extend their appreciations to members of Zoology Department in helping to complete this work.
Funding
None.
Author information
Authors and Affiliations
Contributions
MF and NM have provided guidance during development of idea and prepared different figures required, wrote and revised the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal procedures were carried out according to the regulatory laws regarding experimental Animal Ethics Committee, Faculty of Science, Cairo University, Egypt (CU-IACUC). The informed consent obtained from this study was written and received ethical approval number: (CU/I/S/54/17).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests related to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fol, M.F., Mostafa, N.A. Morphological and histopathological studies of Thelandros chalcidae (Oxyuroidea: Pharyngodonidae) infecting Chalcides ocellatus from Egypt. JoBAZ 82, 61 (2021). https://doi.org/10.1186/s41936-021-00260-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-021-00260-9